Syntheses,Crystal Structures and Magnetic Properties of Cyanide-and Phenolate-Bridged Two-Dimensional M(Ⅱ)-Mn(Ⅱ)(M=Ru and Os)Complexes
2017-07-05ZHANGLiFangXULuJIYuJieNIZhongHai
ZHANG Li-FangXU LuJI Yu-JieNI Zhong-Hai
(School of Chemical Engineering and Technology,China University of Mining and Technology,Xuzhou,Jiangsu 221116,China)
Syntheses,Crystal Structures and Magnetic Properties of Cyanide-and Phenolate-Bridged Two-Dimensional M(Ⅱ)-Mn(Ⅱ)(M=Ru and Os)Complexes
ZHANG Li-FangXU LuJI Yu-JieNI Zhong-Hai*
(School of Chemical Engineering and Technology,China University of Mining and Technology,Xuzhou,Jiangsu 221116,China)
Two new M(Ⅱ)-Mn(Ⅱ)complexes{[Mn(Ⅱ)(salen)]4[Mn(Ⅱ)(salen)(H2O)]2[M(Ⅱ)(CN)6]}(ClO4)2·2H2O(M=Ru(1) and Os(2))(salen2-=N,N′-ethylenebis(salicylideneaminato)dianion)have been synthesized based on[Mn(Ⅱ)(salen)]+segment and hexacyanide-containing building blocks[M(Ⅱ)(CN)6]4-.Single crystal X-ray diffraction analyses show that both complexes are isostructurally two-dimensional(2D)structures in which cyanide-bridged heptanuclear [Mn(Ⅱ)6M(Ⅱ)]2+units are linked together by double phenolate bridges.Magnetic studies show that they are abnormal antiferromagnetic through the double phenolate bridges with the magnetic coupling constant J=-0.340cm-1for 1 and-0.561 cm-1for 2 based on the spin exchange Hamiltonian Hˆ=-2JMnMnSˆMn1SˆMn2.CCDC:1447244,1;1447245,2.
dimetallic complex;cyanide-bridged;phenolate-bridged;antiferromagnetic coupling
0 Introduction
The design and synthesis of new coordination complexeswithexcellentmagnetic,opticaland adsorptive properties have received much attention in the past several decades[1-16].Up to date,a large number of complexes with different topological structures have beenobtainedthroughtherationaldesignand selection of metal ions,bridging groups and ligands. Among the many famous bridging groups,cyanide group plays an important role because the topological structuresofcyanide-bridgedcomplexescanberelatively readily controlled and anticipated through changing the number and position of cyanide group and the charge number of the cyanide-containing blocks.Although there are several tens of cyanidecontaining building blocks for cyanide-bridged complexes,the central metal ions are mainly focused on the 3d metal ions such as Fe(Ⅱ),Fe(Ⅱ),Cr(Ⅵ)or Ni(Ⅱ)et al[17-25].The cyanide-bridged complexes based on cyanide-containing building blocks containing 4d and 5d metal ions are relatively limited[1,5,8,10-11,15].
Corresponding to cyanide-containing metal ions, the selection of another functional metal centers is also very important for the assembly of interesting functional complexes.High-spin[Mn(Ⅱ)(salen)]+segment and its derivatives as known magnetic carriers have been extensively employed for new magnetic materials[12-16,26-28], and a variety of polynuclear,1D chains[11,15],2D networks[2,4,29-30]complexes which exhibit ferro-,antiferro-, ferri-or metamagetic behaviors have been reported. Recently,we synthesized two new 2D cyanide-and phenolate-bridged M(Ⅱ)-Mn(Ⅱ)complexes based on [Mn(Ⅱ)(salen)]+segment and[M(Ⅱ)(CN)6]4-(M=Ru and Os)building blocks(Scheme 1).Herein,we report the synthesis,crystal structures and magnetic properties of two new complexes{[Mn(Ⅱ)(salen)]4[Mn(Ⅱ)(salen) (H2O)]2[M(Ⅱ)(CN)6]}(ClO4)2·2H2O(M=Ru(1)and Os(2), salen2-=N,N′-ethylenebis(salicylideneaminato)dianion).
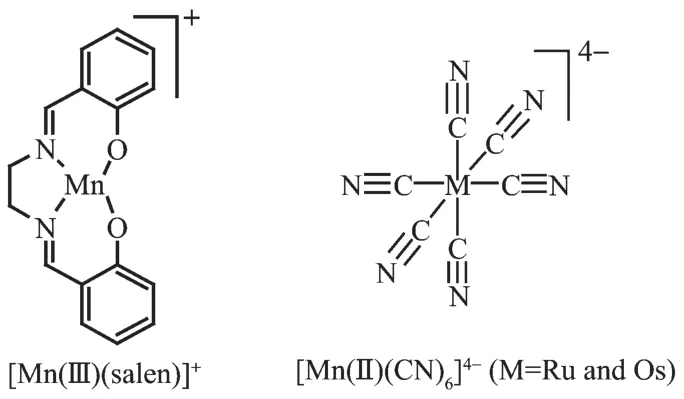
Scheme 1Building blocks for complexes 1 and 2
1 Experimental
1.1 Materials and physical measurements
Elemental analyses(C,H and N)were carried out on an Elementar Vario El.The infrared spectra of solid samples on KBr pellets were recorded on a Nicolet 7199B FT-IR spectrophotometer in the regions of 4 000~400 cm-1.The powder XRD data were measured on a Bruker D8 Advance X-ray diffractometer equipped with a Cu Kα radiation(λ=0.154 18 nm, cathode voltage=40 kV,cathode current=30 mA,2θ= 5°~50°).Variable-temperature magnetic susceptibilities of powder samples were measured on a Quantum Design MPMS SQUID magnetometer in the applied field of 1 000 Oe.The experimental susceptibilities were corrected for the diamagnetism estimated based on Pascal′s constants.
All of the reactions were carried out under an air atmosphere,and all chemicals and solvents used in the synthesis were reagent grade without further purification.[Mn(salen)]ClO4was available from our previous work[31].K4[Ru(CN)6]and K4[Os(CN)6]were prepared by the literature methods[32].
Caution!KCN is hypertoxic and hazardous. Perchlorate salts of metal complexes with organic ligands are potentially explosive.They should be handled in small quantities with care.
1.2 Preparation of complexes 1 and 2
The two complexes were prepared using one similar procedure.Therefore,only the synthesis of complex 1 was detailed as a typical representative.A methanol/MeCN(1∶1,V/V,10 mL)solution of[Mn (salen)]ClO4(0.4 mmol)was carefully layered onto a methanol/H2O(1∶4,V/V,10 mL)solution of K4[Ru (CN)6](0.1 mmol).After the mixture stood for a few weeks in a dark room,dark brown block single crystals suitable for X-ray diffraction were obtained.Then they were filtered,washed with 1∶1(V/V)methanol-water and dried at room temperature.
Complex 1:Yield:58%.Anal.Calcd.for C102H92N18O24Cl2Mn6Ru(%):C,49.85;H,3.75;N,10.26.Found (%):C,49.62;H,3.84;N,10.05.Main IR frequencies (KBr disk,cm-1):2 050(m,νC≡N),1 087(m,νClO4).
Complex 2:Yield:60%.Anal.Calcd.for C102H92N18O24Cl2Mn6Os(%):C,48.10;H,3.62;N,9.90.Found (%):C,47.86;H,3.74;N,9.80.Main IR frequencies (KBr disk,cm-1):2 038(m,νC≡N),1 087(m,νClO4).
1.3 Single-crystal X-ray diffraction data collection and structure refinement
Single-crystal X-ray diffraction data of complexes1 and 2 were collected on Bruker APEXⅡCCD diffractometer equipped with a graphite-monochromatized Mo Kα radiation(λ=0.071 073 nm).The structures were solved by the direct method and refined by full-matrix leastsquares(SHELX-2014)on F2[33]. Hydrogen atoms were added geometrically and refined using a riding model.Images were created by using DIAMOND program.There is no hydrogen bond acceptor for one hydrogen atom of coordinated water molecule in two complexes,which may be due to steric effect of relatively large salen2-ligand as well as the release of some solvent molecules.The crystal data for the two complexes are given in Table 1.
CCDC:1447244,1;1447245,2.
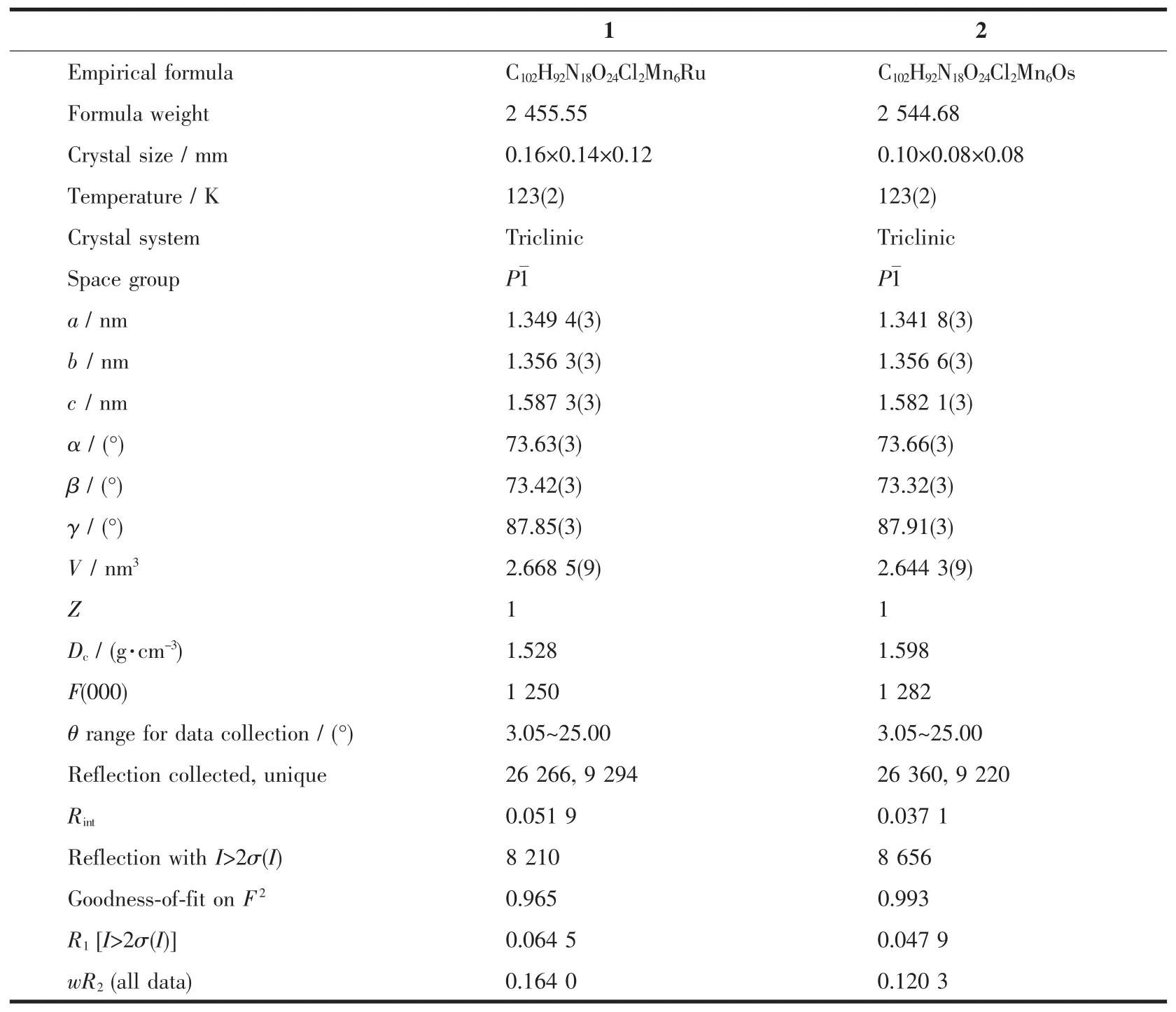
Table 1Crystal data and structure refinement parameters for complexes 1 and 2
2 Results and discussion
2.1 Crystal structures of complexes 1 and 2
The X-ray crystal analysis reveals that both complexes crystallize in the triclinic P1 space group and they are isostructural.Firstly,a[M(Ⅱ)(CN)6]4-anion connects axially six[Mn(Ⅱ)(salen)]+cations through its six cyanide bridges to form centrosymmetric heptanuclear[Mn(Ⅱ)6M(Ⅱ)]2+cationic units as depicted in Fig.1.Then,the heptanuclear[Mn(Ⅱ)6M(Ⅱ)]2+cationic units are connected together by double phenolate bridges from four sides,giving two-dimensional(2D) layer-like structures(Fig.2).The measured XRD patterns on powder samples of complex 1 and 2 are consistent well with the calculated data based on their single crystal structures(Fig.3),indicating the two samples have high purity.Selected bond distances and angles of the two complexes are listed in Table 2.
For the cyanide-containing metal centers,the coordination environments of the two complexes are the same and relatively simple.The central Ru(Ⅱ)or Os(Ⅱ)metal ions is coordinated by six carbon atoms of cyanide groups.The M-C bond lengths are very similar(0.204 2(5)~0.204 6(5)nm)although the twometal centers are different periodic elements of the same family,which can be compared to the bond parameters of their building blocks[M(Ⅱ)(CN)6]4-[32]. The M-C≡N linkages are almost collinear with the angles from 176.7(4)°to 177.7(4)°,which are similar toother3dmetalcyanide-containingbuilding blocks[17-25].The intramolecular Mn(Ⅱ)-M(Ⅱ)separations through the cyanide bridges locate within 0.504 4~0.518 2 nm.
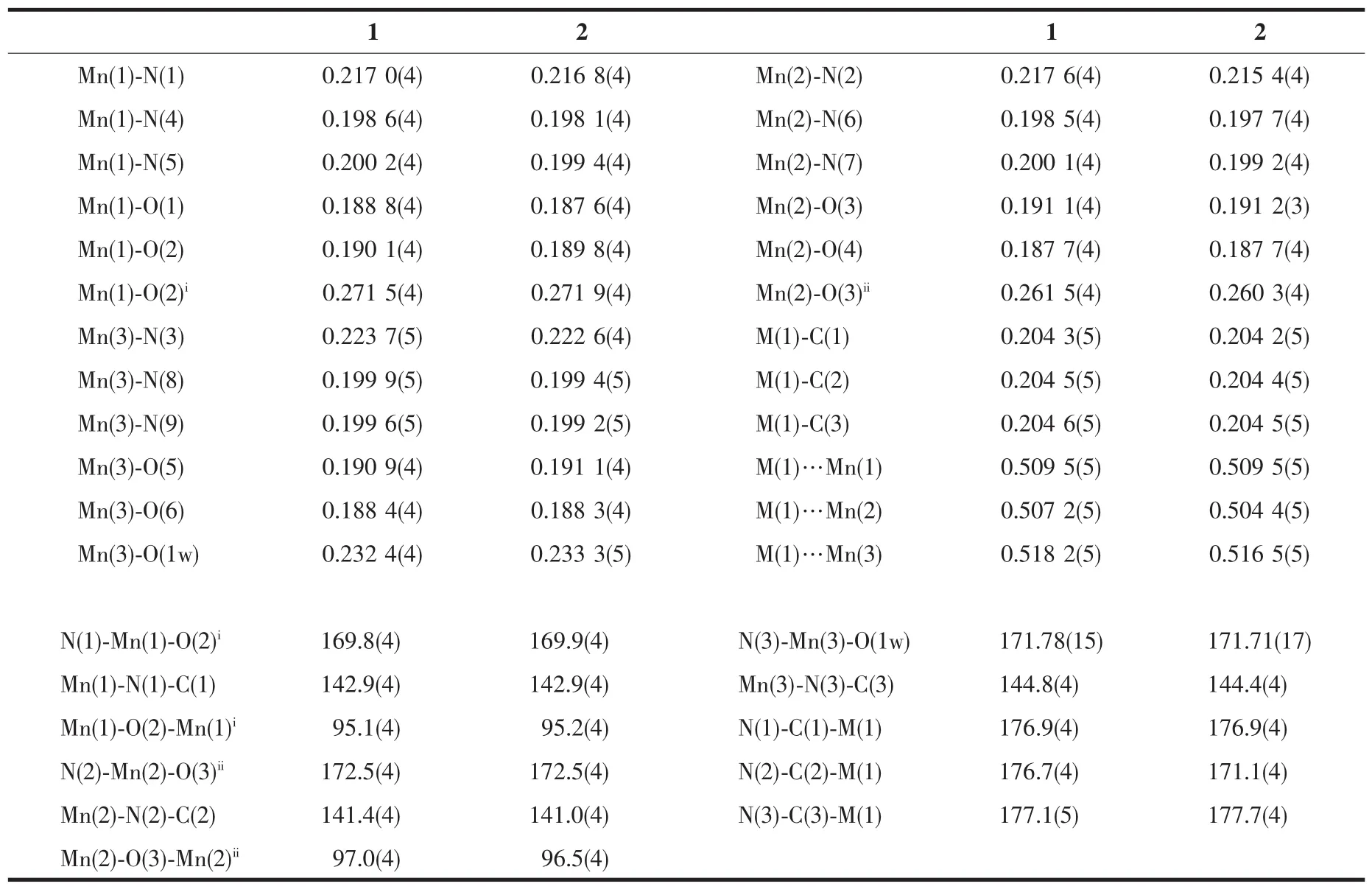
Table 2Selected bond distances(nm)and angles(°)of complexes 1 and 2
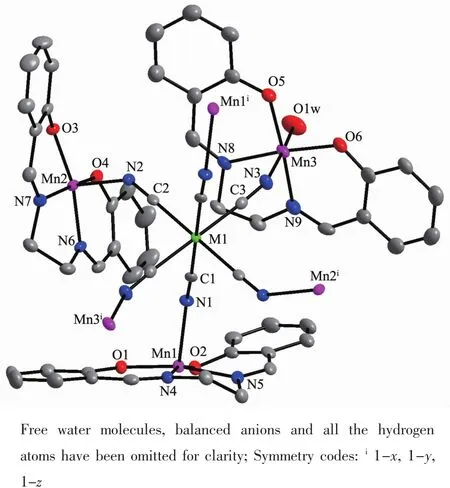
Fig.1Molecular structures of complexes with 30% probability ellipsoids(M=Ru(1)and Os(2))
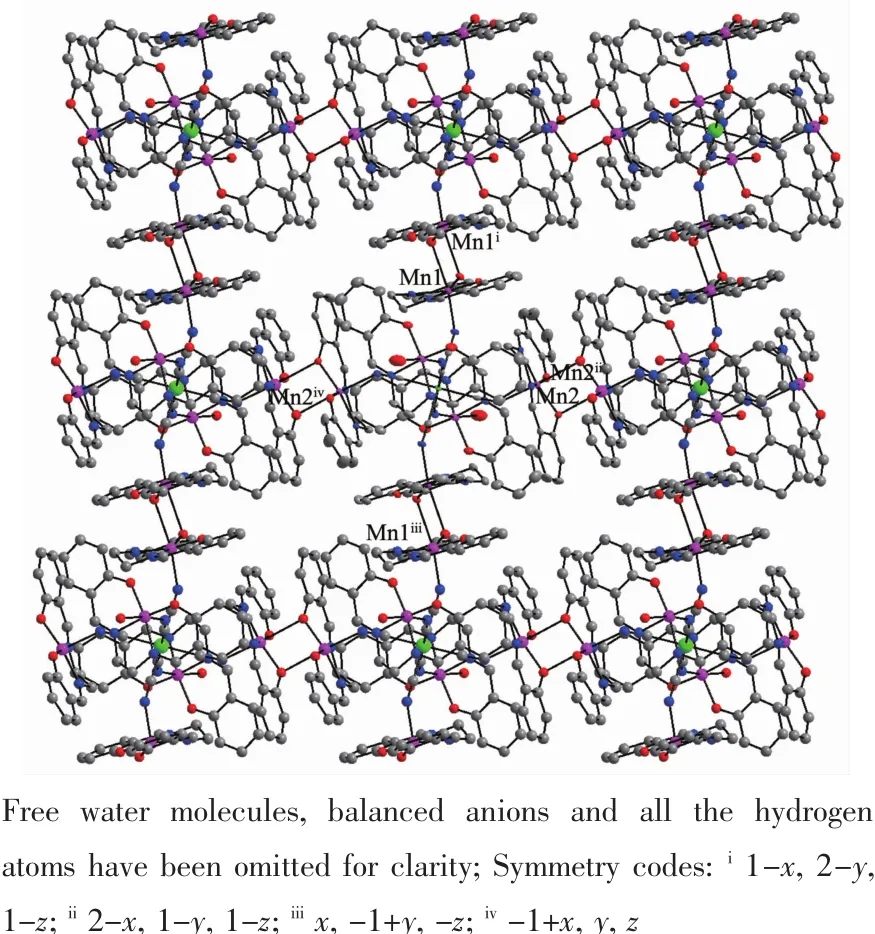
Fig.22D layer-like structures of complexes 1 and 2
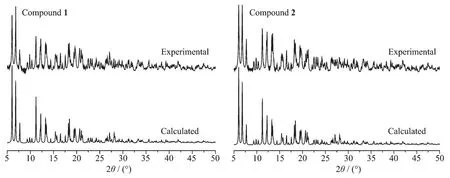
Fig.3XRD patterns for powder samples of complexes 1 and 2
There are three independent Mn(Ⅱ)ions in complexes 1 and 2.Mn(1)and Mn(2)ions have the same coordination environments.The Mn(1)and Mn(2) ions severally form[Mn(Ⅱ)(NC)(salen)(μ-Ophenolate)2Mn(Ⅱ)(NC)(salen)]linkages by double phenolate bridges between the neighboring[Mn(Ⅱ)6M(Ⅱ)]2+units,giving one-dimensional(1D)chains,respectively,along the a and b directions.Then complexes 1 and 2 lead to 2D layer-like structures,which can be widely observed among the[Mn(Ⅱ)(salen)]+Schiff base derivatives[2,4,27,29-30]. Mn(1)and Mn(2)centers are surrounded by N2O2atoms set from tetradentate salen2-ligand in the equatorial plane,a N atom from the cyanide bridge and a O atom from the neighboring[Mn(Ⅱ)(salen)]+moiety in twoaxialpositions,givingrisetoanelongated octahedral coordination geometry.The Jahn-Teller elongation axis in the Mn(1)and Mn(2)octahedron lies along the axial direction,and the Mn-Ncyanidebond distances range from 0.215 4(4)to 0.217 0(5)nm.The Mn-Ophenolatebond distances range from 0.260 3(4)to 0.271 9(5)nm,which are significantly shorter than those of the reported double phenolate-bridged Mn(Ⅱ)complexes[1,5,15,35].The Mn-N≡C bond angles are normal bent(141.0(4)°~142.9(4)°)for Mn(1)and Mn(2)ions. The Ncyanide-Mn-Ophenolatebond angles are nearly linear (169.8(4)°~172.5(4)°).The bridged Mn(1)-O(2)-Mn(1a) and Mn(2)-O(3)-Mn(2b)angles are also very similar within the range of 95.1(4)°~97.0(4)°for the two complexes.The adjacent metal-metal distances through the cyanide bridge distribute in a narrow range from 0.504 4(4)to 0.509 5(4)nm for Mn(1)…M and Mn(2)…M.
The Mn(3)ion also has an elongated octahedral coordination geometry in which the equatorial plane sites are occupied by N2O2atoms set from the tetradentate salen2-ligand and the two axial sites are coordinated by a nitrogen atom from the cyanide bridge and an oxygen atom from the coordinated water molecule.The Mn(3)-Ncyanidelengths are 0.223 7(5)nm for 1 and 0.222 6(4)nm for 2.The Mn(3)-O(1w)bond distances are 0.232 4(5)nm for 1 and 0.234 4(5)nm for 2,respectively,which are obviously shorter than the Mn-Ophenolatebond distances of Mn(1)and Mn(2). The Mn(3)-N≡C bond angles for bridging linkages are in a bent fashion with the angles of 144.8(4)°for 1 and 144.4(4)°for 2.The N(3)-Mn(3)-O(1w)bond angles are 171.78(15)°for 1 and 171.71(17)°for 2, which are similar with Mn(1)and Mn(2)ions.The intramolecular Mn(3)…M distances are 0.518 2(5)nm for 1 and 0.516 5(5)nm for 2,which are longer than those of the above Mn(1)…M and Mn(2)…M.
2.2 Magnetic properties
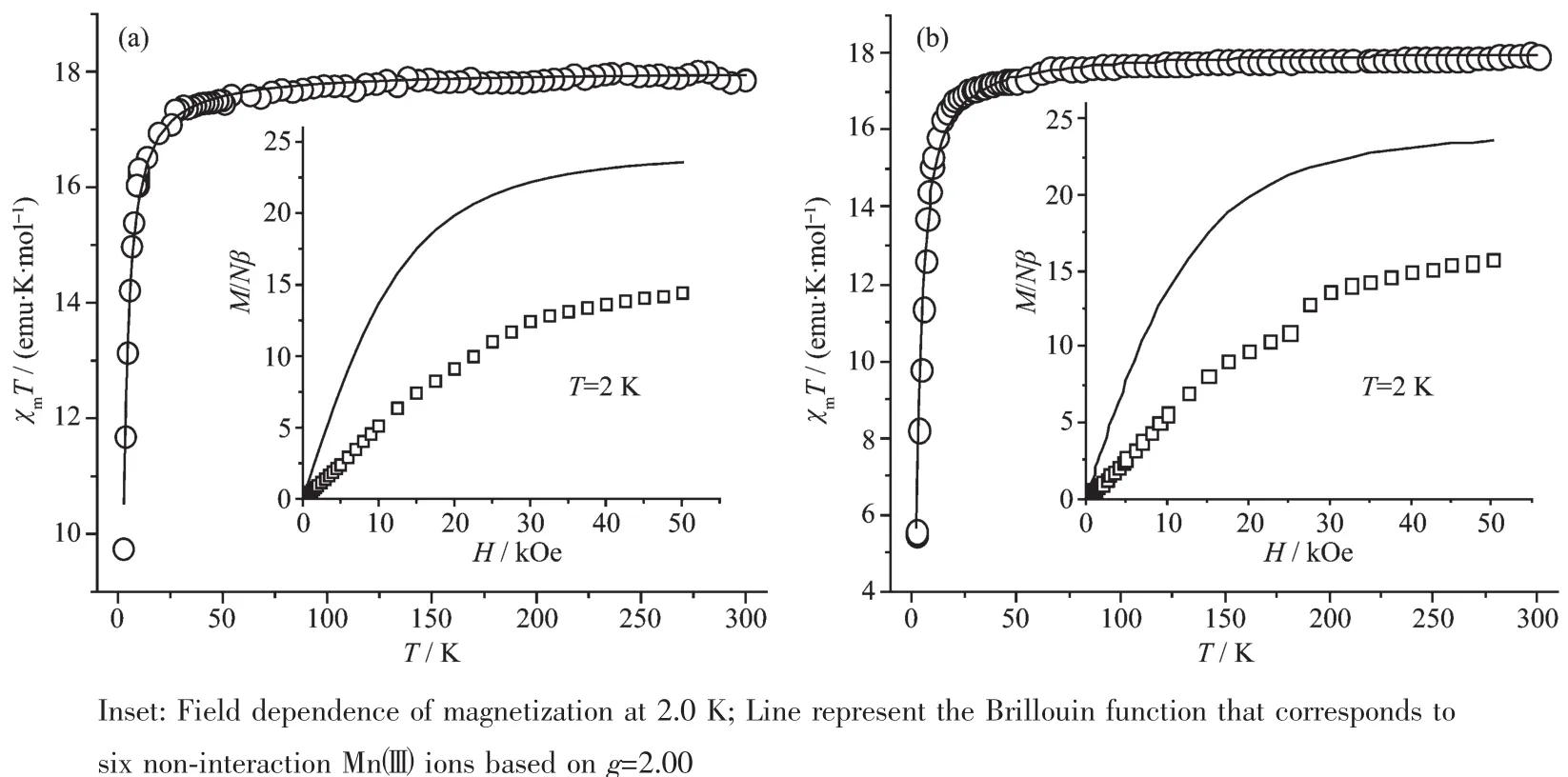
Fig.4Temperature dependences of χmT for the complexes 1(a)and 2(b)measured under an applied field of 1 000 Oe
Magnetic properties of the two complexes were measured in the temperature range of 2~300 K under an external magnetic field of 1 000 Oe,as shown in Fig.4.Obviously,there are very similar magnetic properties for the two complexes.The χmT values for complexes are in the range of 17.01~17.08 emu·K· mol-1at room temperature,which is nearly equal to the paramagnetic response of the six isolated highspin Mn(Ⅱ)cations based on S=2 and g=2.00.With the temperature decreasing,the χmT values remain nearly constant until the temperature decreases to 50 K and then sharply drop to their lowest values of 9.73 emu·K·mol-1for 1 and 5.48 emu·K·mol-1for 2 at 2 K,respectively,which indicate there are overall antiferromagnetic interactions for the complexes.On the other hand,the magnetic susceptibilities of two complexes conform well to Curie-Weiss law in the range of 10~300 K and give a negative Weiss constant θ=-1.38 K,Curie constant C=17.99 emu·K·mol-1for 1 and θ=-2.19 K,C=18.58 emu·K·mol-1for 2.Moreover,the field dependence of the magnetizations of complexes 1 and 2 is significantly lower than the corresponding Brillouin curve for the six Mn(Ⅱ)cautions with S=2 and g=2.00(Inset of Fig.4).On the basis of these above data,the overall antiferromagnetic interaction in the two complexes can be concluded.
According to their crystal structures of the two complexes,it can be seen that the main magnetic coupling contribution takes place in the adjacent double phenolate-bridgedMn(Ⅱ)dimersandthe isolated Mn(Ⅱ)ions.The magnetic exchanges for the two types of dimers[Mn(Ⅱ)1]2and[Mn(Ⅱ)2]2in the two complexes are nearly the same because their bond parameters are very similar.In order to quantitatively interpretthesedata,calculationsbasedonthe expression HamiltonianHˆ=-2JSˆMnSˆMn(a)for the magnetic susceptibility(χd)of the Mn(Ⅱ)dimer were performed, where J is the exchange coupling constant between Mn(Ⅱ)ions via phenolate bridges.And due to the symmetry of the structures of complexes,the final molar magnetic susceptibility(χm(Mn2Mn))can be calculatedintheformula2.Inaddition,the experimental curves of complexes 1 and 2 have been simulated as shown in Fig.3.The best fit gave the parameters of g=2.00,J=-0.340 cm-1for 1 and g= 2.03,J=-0.561 cm-1for 2.If considering the single ion zero-field splitting(D)of Mn(Ⅱ),the best fitting parameters are g=1.99,J=-0.192 cm-1and D=-0.342 cm-1for 1,and g=2.01,J=-0.243 cm-1and D=-0.384 cm-1for 2 based on the method of the literature[34].The negative J values support the overall antiferromagnetic coupling for the two complexes.
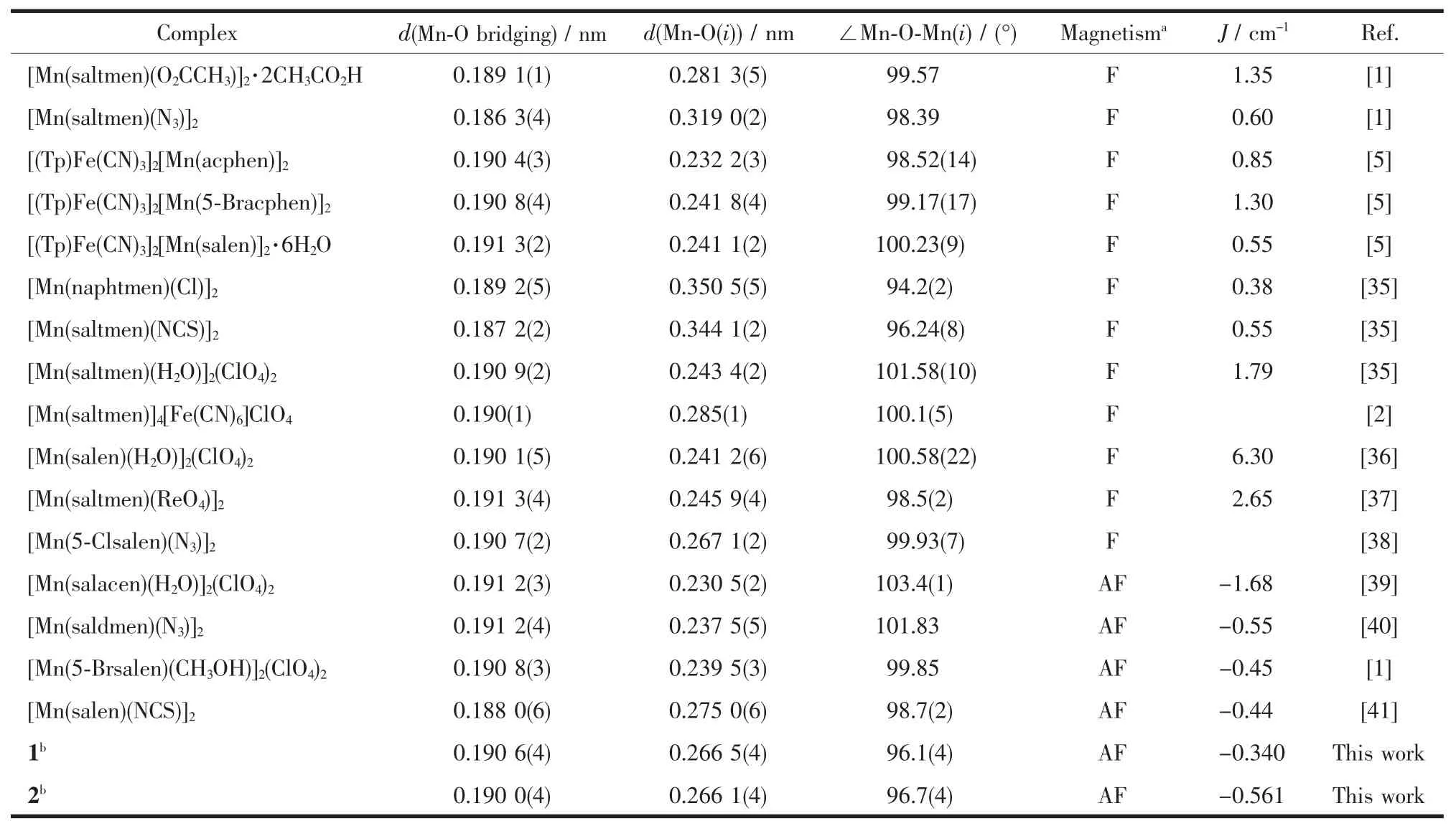
Table 3Specific structural parameters of selected dimeric Mn(Ⅱ)Schiff bases and their magnetic nature
Most of the complexes of Mn(Ⅱ)dimer bridged by phenolate oxygen atoms exhibit the ferromagnetic coupling with few exceptions.Nevertheless,the two complexesinthispaperpresenttheabnormal antiferromagnetic coupling.Table 3 summarized the pertinent bond distances and angles of the dimeric cores with the magnetic pathway through the phenolate linkages and the estimated magnetic parameters.The published paper once has reported the magnetostructural correlation of such magnetic systems and concludedonetheempiriccorrelationbetween magnetic coupling constants of two Mn(Ⅱ)ions through phenolate bridge and Mn-Ophenolatedistance,that is J= 4.572 4-11.868x(x is the Mn-Ophenolatedistance in the range of 0.24~0.37 nm)[35].According to this empiric correlation,themagneticcouplingshouldbe ferromagnetic with J value of 1.467 cm-1for 1 and 1.483 cm-1for 2.However,both of the complexes herein are all practically antiferromagnetic.In addition, several other examples also deviate from the above expression as shown in Table 3.From the limited examples in Table 3,the Mn-Ophenolatedistance is only one key factor for the final magnetic nature.The full establishment of magneto-structural correlation for this system needs further investigations in the future.
3 Conclusions
In summary,two new cyanide-and phenolatebridged M(Ⅱ)-Mn(Ⅱ)complexes based on[Mn(Ⅱ)(salen)]+and 4d/5d metal cyanide-containing[M(Ⅱ)(CN)6]4-(M= Ru and Os)building blocks have been successfully preparedandcharacterized.Thecomplexeshave similar single crystal structures in which the cyanidebridged heptanuclear[Mn(Ⅱ)6M(Ⅱ)]2+units are further linked by phenolate bridges from four sides to form 2D layer-like structures.Magnetic investigations reveal that the two complexesshow abnormallyoverall antiferromagneticcouplingfordoublephenolatebridged Mn(Ⅱ)systems.The magnetic susceptibilities of the two complexes have been fitted by the Mn2dimer model together with the contribution from the isolated Mn(Ⅱ)ion.
[1]Lü Z L,Yuan M,Pan F,Gao S,et al.Inorg.Chem.,2006,45:3538-3548
[2]Wang X Y,Avendao C,Dunar K R.Chem.Soc.Rev.,2011, 40:3213-3238
[3]Clérac R,Miyasaka H,Yamashita M,et al.J.Am.Chem. Soc.,2002,124:12837-12884
[4]Zhang R,Zhang T F,Ni Z H,et al.Inorg.Chim.Acta,2016, 453:494-500
[5]Kwak H Y,Ryu D W,Kim H C,et al.Dalton Trans.,2009, 11:1954-1961
[6]Kachi-Terajima C,Miyasaka H,Sugiura K,et al.Inorg.Chem., 2006,45:4381-4390
[7]Yoon J H,Lim J H,Kim H C,et al.Inorg.Chem.,2006,45: 9613-9615
[8]Pedersen K S,Schau-Magnussen M,Bendix J,et al.Chem. Eur.J.,2016,16:13458-13464
[9]Hoeke V,Stammler A,Bögge H,et al.Inorg.Chem.,2014,53: 257-268
[10]Xiang J,Jia LH,Wang H S,et al.Eur.J.Inorg.Chem., 2015,6:1065-1073
[11]Yoon J H,Yoo H S,Kim H C,et al.Inorg.Chem.,2009,48: 816-818
[12]Pedersen K S,Dreiser J,Nehrkorn J G,et al.Chem.Commun., 2011,47:6918-6920
[13]Ru J,Gao F,Yao M X,et al.Dalton Trans.,2014,43:18047-18055
[14]Ishikawa R,Nakano M,Breedlove B K,et al.Polyhedron, 2013,64:346-351
[15]Kopotkov V A,Yagubskii E B,Simonov S V,et al.New J. Chem.,2014,38:4167-4176
[16]Zhang R,Xu L,Ni Z H,et al.Inorg.Chem.Commun.,2016, 67:99-102
[17]Chen H,Miao B X,Zhang L F,et al.Inorg.Chim.Acta, 2013,404:34-39
[18]Liu W,Wang C F,Li Y Z,et al.Inorg.Chem.,2006,45: 10058-10065
[19]Ohba M,(Ⅱ)kawa H.Coord.Chem.Rev.,2000,198:313-328
[20]ZHANG Li-Fang(张丽芳),HAN Fang-Fang(韩方方),YANG Dai-Sheng(杨代胜),et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32(4):731-737
[21]Li G L,Zhang L F,Ni Z H,et al.Bull.Korean Chem.Soc., 2012,33:1675-1680
[22]Li G L,Cheng W Q,Zhang L F,et al.Transition Met.Chem., 2012,37:469-474
[23]Luminita M T,Lescouzec R,Cangussu D,et al.Inorg.Chem. Commun.,2005,8:382-385
[24]Yang D S,Zhang Z M,Zhang L F,et al.Transition Met. Chem.,2015,40:749-754
[25]Palii A V,Ostrovsky S M,Klokishner S I,et al.J.Am. Chem.Soc.,2004,126:16860-16867
[26]Guo J F,Wang X T,Wang B W,et al.Chem.Eur.J.,2010, 16:3524-3535
[27]Ni W W,Ni Z H,Cui A L,et al.Inorg.Chem.,2007,46:22-33
[28]Ene C D,Nastase S,Maxim C,et al.Inorg.Chim.Acta, 2010,363:4247-4256
[29]Miyasaka H,Nezu T,Sugimoto K,et al.Inorg.Chem.,2004, 43:5486-5488
[30]Miyasaka H,Clérac R,Mizushima K,et al.Inorg.Chem., 2003,42:8203-8213
[31]Ni Z H,Zheng L,Zhang L F,et al.Eur.J.Inorg.Chem., 2007:1240-1249
[32]Gentil L A,Navaza A,Olabe J A,et al.Inorg.Chim.Acta, 1991,179:89-96
[33]Sheldrick G M.Acta Crystallogr.Sect.C,2015,C71:3-8
[34]Yuan J,Chu Y X,Kou H Z.J.Coord.Chem.,2016,69:1218 -1225
[35]Miyasaka H,Clérac R,Ishii T,et al.J.Chem.Soc.,Dalton Trans.,2002:1528-1534
[36]Shyu H-L,Wei H-H,Wang Y.Inorg.Chim.Acta,1999,290: 8-13
[37]Miyasaka H,Clérac R,Wernsdorfer W,et al.Angew.Chem. Int.Ed.,2004,43:2801-2805
[38]Yoon J H,Lim J H,Choi S W,et al.Inorg.Chem.,2007,46: 1529-1531
[39]Matsumoto N,Zhang Z J,Okawa H,et al.Inorg.Chim. Acta,1989,160:153-157
[40]Saha S,Mal D,Koner S,et al.Polyhedron,2004,23:1811-1817
[41]Kennedy B J,Murray K S.Inorg.Chem.,1985,24:1552-1557
氰根和酚氧桥联M(Ⅱ)-Mn(Ⅱ)(M=Ru和Os)二维配合物的合成、结构与磁性
张丽芳 徐露季 玉洁 倪中海*
(中国矿业大学化工学院,徐州221116)
基于六氰根构筑单元[M(Ⅱ)(CN)6]4-与[Mn(Ⅱ)(salen)]+模块反应合成了2个新型酚氧和氰根混合桥联的M(Ⅱ)-Mn(Ⅱ)配合物{[Mn(Ⅱ)(salen)]4[Mn(Ⅱ)(salen)(H2O)]2[M(Ⅱ)(CN)6]}(ClO4)2·2H2O(M=Ru(1),Os(2),salen2-=双水杨酰胺乙基负离子)。单晶衍射结果表明:它们是结构类似的二维化合物,其中氰根桥联的七核[Mn(Ⅱ)6M(Ⅱ)]2+单元进一步通过双酚氧桥相互连接构成二维层状结构。磁性研究表明:2个化合物通过酚氧桥均呈现反常的反铁磁耦合,基于自旋哈密顿算符Hˆ=-2JMnMnSˆMn1SˆMn2拟合得到它们的磁耦合常数分别是J=-0.340 cm-1(1)和-0.561 cm-1(2)。
双金属配合物;氰根桥联;酚氧桥连;反铁磁耦合
O614.82+1;O614.82+4;O614.71+1
A
1001-4861(2017)06-1051-08
2016-12-06。收修改稿日期:2017-04-18。
10.11862/CJIC.2017.124
中央高校基本科研业务费专项资金(No.2015XKMS047)和江苏省高校优势学科建设工程项目资助。
*通信联系人。E-mail:nizhonghai@cumt.edu.cn;会员登记号:S06N0244M1703。
猜你喜欢
杂志排行
无机化学学报的其它文章
- CoAl2O4/蜂窝陶瓷催化剂的制备及其催化臭氧化性能
- Photocatalytic Hydrogen Production Based on Cobalt-Thiosemicarbazone Complex with the Xanthene Dye Moiety
- 两种金属-有机钙钛矿材料的负热膨胀性质
- Pyrazolate-Based Dipalladium(Ⅱ,Ⅱ)Complexes:Syntheses,Characterization and Catalytical Performance in Suzuki-Coupling Reaction
- 以滤纸为模板合成新型介孔生物活性玻璃微管材料
- Br-掺杂Bi2WO6的水热法合成及其可见光催化性能
