盐旱复合胁迫对小麦幼苗生长和水分吸收的影响
2017-06-27翁亚伟田中伟靳雪莹李梦雅余钟毓戴廷波
翁亚伟, 张 磊, 张 姗, 田中伟, 靳雪莹, 李梦雅, 余钟毓, 姜 东, 戴廷波
南京农业大学农学院/农业部作物生理生态与生产管理重点实验室,江苏省现代作物生产协同创新中心, 南京 210095
盐旱复合胁迫对小麦幼苗生长和水分吸收的影响
翁亚伟, 张 磊, 张 姗, 田中伟, 靳雪莹, 李梦雅, 余钟毓, 姜 东, 戴廷波*
南京农业大学农学院/农业部作物生理生态与生产管理重点实验室,江苏省现代作物生产协同创新中心, 南京 210095
为明确盐害、干旱及盐旱复合胁迫对小麦幼苗生长和水分吸收的影响,从而为盐害和干旱胁迫下栽培调控提供理论依据。以2个抗旱性不同的小麦品种(扬麦16和耐旱型洛旱7号)为材料,采用水培试验,以NaCl和PEG模拟盐旱复合胁迫,研究了盐旱复合胁迫下小麦幼苗生长、根系形态、光合特性及水分吸收特性的变化。结果表明,盐、旱及复合胁迫下小麦幼苗的生物量、叶面积、总根长与根系表面积、叶绿素荧光和净光合速率均显著下降,但是复合胁迫处理的降幅却显著低于单一胁迫。盐旱复合胁迫下根系水导速率和根系伤流液强度显著大于单一胁迫,从而提高了小麦幼苗叶片水势和相对含水量。盐胁迫下小麦幼苗Na+/K+显著大于复合胁迫,但复合胁迫下ABA含量却显著小于单一的盐害和干旱胁迫。因此,盐旱复合胁迫可以通过增强根系水分吸收及降低根叶中ABA含量以维持较高光合能力,这是盐旱复合胁迫提高小麦适应性的重要原因。洛旱7号和扬麦16对盐及盐旱复合胁迫的响应基本一致,但在干旱胁迫下洛旱7号表现出明显的耐性。
小麦;盐旱复合胁迫;光合作用;根系水导速率;水分吸收
盐害与干旱一直是制约农业生产的严重自然灾害。近些年,盐害和干旱发生频率和强度愈来愈高,尤其是重大盐害和干旱直接威胁到国家的长期粮食安全和社会稳定[1- 4]。有研究指出干旱时,土壤水分蒸发增强导致地表盐分不断积累,同时由于植物根系吸水使得地表土壤的水分减少、盐分浓度增加[5-6],因此会形成盐害与干旱的复合胁迫。小麦是我国重要粮食作物,小麦幼苗生长发育的好坏会直接影响产量和品质,并且小麦苗期处于干旱少雨的秋季与土壤返盐的春季[7],因此明确盐旱复合胁迫对小麦幼苗生长的影响及生理机制具有一定实际意义。盐害与干旱都会造成渗透胁迫,增加植物根系吸水阻力,降低植物光合速率,限制植物生长;但目前对盐旱复合胁迫研究,主要集中在探讨渗透调节物质与抗逆境酶在复合胁迫中的作用,而对复合胁迫下植物对水分吸收和运输以及光合能力的研究较少[7- 10]。由于作物种类和处理方式的不同,因此得出的一些不同的结论[8- 12]。 有研究指出:盐与旱复合胁迫会加重对植物损伤[8,10];但也有研究指出:植物面临盐与旱双重胁迫时却可以表现出一定的适应性[6- 7,11-13]。而且目前对其表现出适应性的原因解释较少。因此本文在前人研究基础上,采用水培试验,以NaCl和PEG模拟盐旱复合胁迫,选用2个抗旱性不同的小麦品种(扬麦16和耐旱型洛旱7号)为材料,分析复合胁迫下小麦幼苗根系吸水能力、叶片光合作用以及小麦幼苗生长的关系,明确小麦幼苗表现出适应性的原因,以期为揭示小麦幼苗对盐旱复合胁迫的适应机制提供科学依据。
1 材料与方法
1.1 实验材料与处理
于2014—2015年在南京农业大学牌楼试验基地遮雨棚中进行水培试验。供试材料为抗旱性不同的小麦品种洛旱7号(耐旱型品种)和扬麦16。选取饱满且大小一致的种子,用15%过氧化氢浸种消毒10min后冲洗3—4次,移至铺有两层灭菌滤纸的周转箱中,于20℃光照培养箱中黑暗条件下催芽,待种子露白后移至石英砂中培养。当小麦出现第二片真叶时,选择健壮且长势一致的幼苗,定植于装1/2 Hoagland营养液的培养箱(38cm×32cm×27cm) 中,每箱30株,待4叶1心时进行胁迫处理。设干旱(D,12.7%PEG营养液,-0.25MPa)、盐(S,50mmol/L NaCl营养液,-0.25MPa)和盐旱复合胁迫(SD,含12.7% PEG+50mmol/LNaCl营养液,-0.45MPa)3个处理,以完全营养液为对照(CK),每处理重复3次,每重复5箱。溶液水势采用WP4露点水势仪测定,营养液pH5.5—6.0,用气泵维持营养液溶氧浓度6—8mg/L,生长期平均温度20℃/8℃(昼/夜)、光强300μmol m-2s-1,光照周期15h/9h(昼/夜)。
1.2 测定项目与方法
处理后7d,测定顶展叶片的水分状况、相对电导率和光合参数,测定根系面积,并取样品鲜样于液氮中速冻保存,用于各生理指标进行测定。
1.2.1 植株干重和根冠比
取样后110℃杀青10min,75℃烘干至恒重称重。根冠比=根系干重/地上部。
1.2.2 叶片水势和叶片相对含水量
采用WP4露点水势仪测定顶展叶片的水势。采用烘干法,测定叶片相对含水量,叶片相对含水量=(鲜生物量-干生物量)/(饱和鲜生物量-干生物量)×100%。
1.2.3 金属离子
Na+、K+含量的测定,参照王宝山等的方法[14]。
1.2.4 根系形态
总根长、根系表面积和根系体积采用WinRHIZO全自动根系扫描分析仪测定。
1.2.5 激素(ABA)
激素测定按照酶联免疫吸附测定法(ELISA)测定,参照中国农业大学提供的试剂盒。
1.2.6 光合生理参数和叶绿素荧光参数
光合生理参数的测定,采用 Li- 6400便携式光合作用测定仪(美国)测定净光合速率(Pn),气孔导度(Gs),胞间CO2浓度(Ci)和蒸腾速率(Tr)。仪器采用开放式气路,CO2浓度为385μmolL-1,选择红蓝光源叶室,光合有效辐射为1000μmol m-2s-1。每处理取五片生长状态一致的顶展叶测定。
叶绿素荧光参数采用PAM- 2000便携式调制荧光仪(德国)测定。将叶片暗适应15 min,测PSⅡ最大光化学效率(Fv/Fm)。在3000μmol m-2s-1强闪光下,测PSⅡ实际光化学效率(Fv′/Fm′)。
1.2.7 叶绿素含量
称0.1g顶展叶,剪碎放入50 mL提取液(V乙醇∶V丙酮=1∶1),在25℃黑暗条件下提取24 h,分光光度计测定吸光值,并计算叶绿素和类胡萝卜素含量[15]。
1.2.8 根系导水速率和木质部伤流液
根系导水速率采用压力室(3005型,美国SEC公司)测定。取小麦幼苗,剪除地上部并留茬1cm,清除切口液体 (以防污染),将根系放入压力室中的蒸馏水中,施压(每次增加0.2 MPa,增至1.0MPa),每个压强下出流稳定后用脱脂棉吸取汁液,时间1min,万分之一天平上称量吸水前后脱脂棉的质量,其质量差为1min内通过测试根系的水流通量m(mg/min)。最后将测量过的根系称重M(g),最后计算根系水导速率Lpr(mg g-1min-1MPa-1)。 然后向压力室中的蒸馏水中添加HgCl2(50μmol/L),再次测量根系水导速率[16]。
根系伤流液收集,参照李合生的方法略加改进。称取约0.5g脱脂棉,剪除小麦幼苗地上部并留茬1cm,清除切口液体 (以防污染),然后迅速用脱脂棉包裹住切面,并用保鲜膜包好脱脂棉,收集12 h (19:00—7:00) 后,称量法测定伤流量[15]。
1.3 数据处理
采用Excel软件进行数据分析,采用Duncan新复极差法进行差异显著性检验(α=0.05),采用Sigmaplot 12.5作图。
2 结果与分析
2.1 盐旱复合胁迫对小麦幼苗生长的影响
表1表明,2个小麦品种在盐、干旱和盐旱复合胁迫下干物重均显著降低,且盐胁迫下降低幅度最大,而盐旱复合胁迫下减少最小。盐胁迫下根冠比显著降低,但干旱和盐旱复合胁迫下根冠比显著增加且干旱对其影响大于盐旱复合胁迫;叶片面积在3种胁迫下均显著减小,但复合胁迫对其影响最小,表明复合胁迫下小麦幼苗可保持较强的叶片生长和物质积累。品种间比较,洛旱7号在干旱胁迫下表现出明显的抗性,但在盐胁迫及复合胁迫下与扬麦16无显著差异。
不同小写字母表示处理间差异显著(P<0. 05)
2.2 盐旱复合胁迫对小麦幼苗根系形态的影响
表2表明,2个小麦品种在盐、干旱和盐旱复合胁迫下总根长、表面积以及根系体积显著下降,其中盐害对小麦幼苗的总根长、表面积以及根系体积影响最大而复合胁迫对其影响最小.这与干物质重积累结果一致.对洛旱7号和扬麦16的次生根和初生根研究表明:在单一的盐害和干旱胁迫下次生根变化大于初生根;而在复合胁迫下初生根变化大于初生根.

表2 盐旱复合胁迫对小麦幼苗的根长、根系表面积和根系体积的影响
2.3 盐旱复合胁迫对小麦幼苗叶片水势和叶片含水量的影响
图1指出,2个小麦品种在胁迫下叶片水势和相对含水量均显著降低.复合胁迫下扬麦16叶片相对含水量显著高于干旱,但是显著低于盐害胁迫;而其叶片水势在盐害和干旱胁迫下无显著差异且显著低于复合胁迫.复合胁迫下其叶片水势和相对含水量与单一胁迫相比却均有显著提高.在复合胁迫下洛旱7号叶片相对含水量显著低于盐害胁迫,但与干旱胁迫差异不显著;而在盐害下其叶片水势显著小于干旱胁迫且显著小于复合胁迫.这是因为洛旱7号作为耐旱品种,所以其在干旱胁迫下有较高水势和含水量.说明扬麦16和洛旱7号在复合胁迫下有着较高的保水能力,洛旱7号在干旱胁迫下表现出抗性.

图1 盐旱复合胁迫对小麦幼苗叶片水势和叶片相对含水量的影响Fig.1 Effects of salt combined with drought stress on leaf water potential and relative water content of wheat seedlings
2.4 盐旱复合胁迫对小麦幼苗叶绿素含量的影响
图2指出,在逆境下2个品种的Chl(a+b)含量显著下降,而Chla/Chlb和Caro含量显著升高,盐旱复合胁迫下Chl(a+b)含量、Chla/Chlb和Caro含量显著大于单一胁迫.盐害胁迫下Chl(a+b)和Caro含量显大于干旱,而Chla/Chlb显著小于干旱胁迫.洛旱7号在干旱胁迫下叶绿素含量显著大于盐害,而扬麦16在两种胁迫下差异不显著,说明洛旱7号在干旱胁迫下表现出明显的耐性.

图2 盐旱复合胁迫对小麦幼苗叶绿素含量的影响Fig.2 Effects of salt combined with drought stress on chlorophyll and carotenoid content in leaves of wheat seedlings
2.5 盐旱复合胁迫对小麦幼苗光合特性的影响
由图3可知净光合速率在胁迫下会显著下降,且盐害对其影响最大,复合胁迫对其影响最小,在干旱下洛旱7号光合速率显著高于扬麦16。洛旱7号在盐害复合胁迫下Gs和Tr显著高于盐害和干旱单一胁迫;因此复合胁迫小麦幼苗保持较高的Gs和Tr,以维持较高的蒸腾拉力。盐害下小麦幼苗Gs大于干旱,而Ci低于干旱,说明盐害主要是通过非气孔因素造成光合速率下降。
图4指出:2个品种幼苗的Fv/Fm表现趋势一致,在逆境下其显著下降,盐害对其影响最大,而盐旱复合胁迫对其影响最小。逆境下小麦幼苗的Fv′/Fm′会显著下降,且其表现趋势与Fv/Fm表现趋势一致。两个品种幼苗在干旱胁迫下其Fv/Fm和Fv′/Fm′都显著高于盐害胁迫而显著低于盐旱复合胁迫,说明盐害会造成小麦幼苗光合机构损伤,从而使得其光合能力下降,这与光合参数得出:盐害主要是通过非气孔因素造成光合速率下降的结论相一致。复合胁迫下小麦幼苗的光合机构受到的损伤较小,因而其光合能力下降较小。
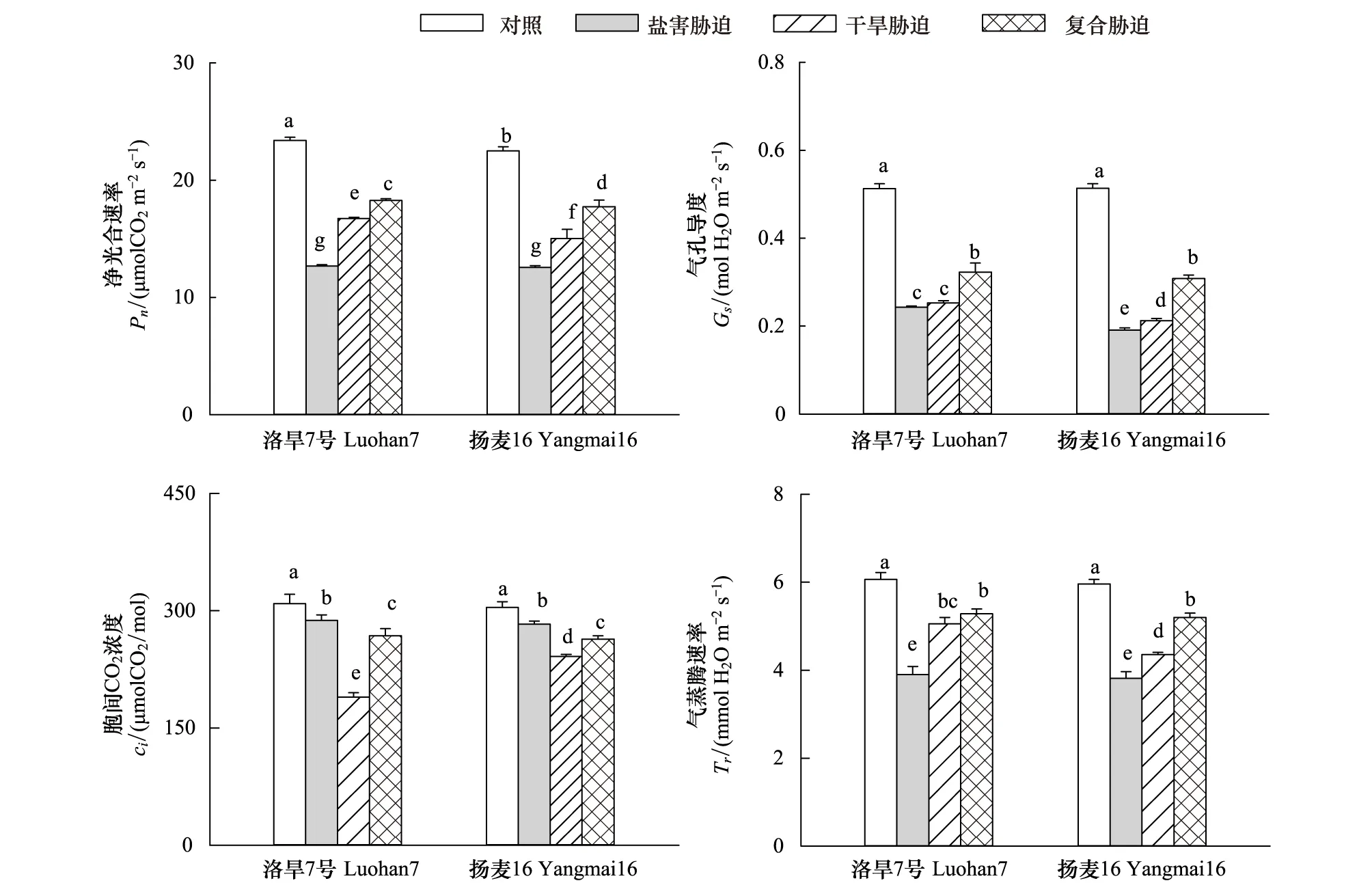
图3 盐旱复合胁迫对小麦幼苗叶片光合参数的影响Fig.3 Effects of salt combined with drought stress on photosynthetic parameters in leaves of wheat seedlings

图4 盐旱复合胁迫对小麦幼苗叶绿素荧光参数的影响Fig.4 Effects of salt combined with drought stress on chlorophyll fluorescence parameters in leaves of wheat seedlings
2.6 盐旱复合胁迫对小麦幼苗根系水导速率和伤流强度的影响
图5表示未添加HgCl2和添加HgCl2后小麦幼苗的根系水导速率。水通道蛋白通道处的氨基酸残基会与Hg2+发生反应,造成物理性阻塞,因此HgCl2是水通道蛋白专一抑制剂[16- 17]。未添加HgCl2时复合胁迫下的小麦幼苗根系水导速率显著大于单一胁迫,说明复合胁迫小麦幼苗水分运输能力较强;添加HgCl2后复合胁迫下根系水导速率显著小于单一胁迫,说明复合胁迫下小麦幼苗会表达更多水通道蛋白以抵御逆境。图5指出在复合胁迫下,小麦幼苗的伤流强度显著高于干旱,与盐害差异不显著。说明盐害和复合胁迫下小麦根系可以保持较高水分吸收能力。

图5 盐旱复合胁迫对小麦幼苗根系水导速率和根系伤流液强度的影响Fig.5 Effects of salt combined with drought stress on root hydraulic conductance and root bleeding intensity of wheat seedlings
2.7 盐旱复合胁迫对小麦幼苗ABA含量和Na+/K+的影响
图6指出在复合胁迫下,两个品种小麦幼苗根叶中ABA含量显著低于单一胁迫。说明单一胁迫下根系会产生更多的ABA传至地上部引起气孔关闭。表明复合胁迫下,小麦幼苗可以通过相对的降低ABA含量以促进气孔开放增加蒸腾拉力,同时促进根系和地上部生长发育,用于抵御逆境。图6指出与盐害相比复合胁迫下小麦幼苗Na+/K+和ABA含量显著下降,说明,复合胁迫下小麦幼苗会通过降低Na+/K+缓解离子毒害。

图6 盐旱复合胁迫对小麦幼苗含量和Na+/K+的影响Fig.6 Effects of salt combined with drought stress on on ABA content and Na+/K+ in leaves and roots of wheat seedlings
3 讨论
研究指出,植物面临盐与旱双重胁迫时表现出一定的适应性,盐旱复合胁迫植物[10- 12]。盐害和干旱植物的,光合能力因此本研究主要通过对盐旱复合下小麦幼苗吸水能力、光合能力及其关系研究以阐述植物面临盐与旱双重胁迫时表现出适应性的原因。
渗透胁迫会导致根系伤流强度下降,引起气孔关闭并导致蒸腾拉力降低,使得植物体吸水困难[18-19]。本试验中,与单一盐、旱胁迫相比复合胁迫下小麦幼苗的根系伤流强度和根系水导速率的增加,使得小麦幼苗叶片水势和相对含水量升高,保证了小麦幼苗相对高效光合作用。相对高效光合作用使得小麦幼苗的气孔导度和蒸腾速率增加,提高了植株的蒸腾拉力,进一步增强小麦幼苗的吸水动力。
渗透胁迫会导致植物产生大量ABA使其生长发育受抑制生理活性降低,并引起光合速率下降[18-20]。盐害下由于Na+的过多积累,在引起渗透胁迫同时亦会造成植物的Na+/K+失衡甚至是离子毒害[21],导致光合器官损伤,引起光合速率的下降[22-23]。本研究中:复合胁迫下小麦幼苗体内的Na+/K+显著低于单一的盐害胁迫,复合胁迫下小麦幼苗的体内的ABA含量显著高于单一胁迫,而其叶面积,叶绿素含量,Fv/Fm和Fv′/Fm′显著大于单一胁迫,因此复合胁迫下小麦幼苗可以保持较高光合能力。
本研究表明,与干旱相比小麦幼苗在复合胁迫下可以保持较高水分吸收能力以提高植株含水量,与盐害相比在复合胁迫下小麦幼苗会通过降低Na+/K+缓解离子毒害,从而保证相对高效的光合速率,因此表现出一定的适应性,与前人研究结果相一致[6- 7,11]。
4 结论
与单一盐、旱胁迫相比,盐旱复合胁迫提高了小麦幼苗根系吸水能力和叶片含水量,降低了Na+/K+和ABA含量,从而缓解了对叶片光合能力的抑制,保证了小麦的生长,使之表现出一定的适应性。因此,维持较高的水分吸收和降低Na+/K+是小麦在盐旱复合胁迫下表现出适应性重要原因。
[1] Yang S L, Chen K, Wang S S, Gong M. Osmoregulation as a key factor in drought hardening-induced drought tolerance inJatrophacurcas. Biologia Plantarum, 2015, 59(3): 529-536.
[2] Weng B S, Zhang P, Li S N. Drought risk assessment in China with different spatial scales. Arabian Journal of Geosciences, 2015, 8(12): 10193-10202.
[3] Narjesi V, Mardi M, Hervan E M, Azadi A, Naghavi M R, Ebrahimi M, Zali A A. Analysis of Quantitative Trait Loci (QTL) for grain yield and agronomic traits in wheat (TriticumaestivumL.) under normal and salt-stress conditions. Plant Molecular Biology Reporter, 2015, 33(6): 2030-2040.
[4] 王宁, 杨杰, 黄群, 苏桂兰, 周红, 许庆华, 董合林, 严根土. 盐胁迫下棉花K+和Na+离子转运的耐盐性生理机制. 棉花学报, 2015, 27(3): 208-215.
[5] 毛海涛,樊哲超,何华祥,邵东国,王晓菊. 干旱、半干旱区平原水库对坝后盐渍化的影响. 干旱区研究, 2016, 33(1): 74-79.
[6] 解卫海, 马淑杰, 祁琳, 张振华, 柏新富. Na+吸收对干旱导致的棉花叶片光合系统损伤的缓解作用. 生态学报, 2015, 35(19): 6549-6556.
[7] 陈成升, 谢志霞, 刘小京. 旱盐互作对冬小麦幼苗生长及其抗逆生理特性的影响. 应用生态学报, 2009, 20(4): 811-816.
[8] Sun C X, Gao X X, Fu J Q, Zhou J H, Wu X F. Metabolic response of maize (ZeamaysL.) plants to combined drought and salt stress. Plant and Soil, 2015, 388(1-2): 99-117.
[9] Zhang X K, Lu G Y, Long W H, Zou X L, Li F, Nishio T. Recent progress in drought and salt tolerance studies inBrassicacrops. Breeding Science, 2014, 64(1): 60-73.
[10] Slama I, Ghnaya T, Messedi D, Hessini K, Labidi N, Savoure A, Abdelly C. Effect of sodium chloride on the response of the halophyte speciesSesuviumportulacastrumgrown in mannitol-induced water stress. Journal of Plant Research, 2007, 120(2): 291-299.
[11] 刘建新, 王金成, 王瑞娟, 贾海燕. 旱盐交叉胁迫对燕麦幼苗叶片生理特性的影响. 干旱地区农业研究, 2014, 32(3): 24-28.
[12] Hussain M I, Lyra D A, Farooq M, Nikoloudakis N, Khalid N. Salt and drought stresses in safflower: a review. Agronomy for Sustainable Development, 2016, 36: 4.
[13] Meggio F, Prinsi B, Negri A S, Di Lorenzo G S, Lucchini G, Pitacco A, Failla O, Scienza A, Cocucci M, Espen L. Biochemical and physiological responses of two grapevine rootstock genotypes to drought and salt treatments. Australian Journal of Grape and Wine Research, 2014, 20(2): 310-323.
[14] 王宝山, 赵可夫. 小麦叶片中Na、K提取方法的比较. 植物生理学通讯, 1995, 31(1): 50-52.
[15] 李合生. 植物生理生化实验原理和技术. 北京: 高等教育出版社, 2006.
[16] Adiredjo A L, Navaud O, Grieu P, Lamaze T. Hydraulic conductivity and contribution of aquaporins to water uptake in roots of four sunflower genotypes. Botanical Studies, 2014, 55: 75.
[17] Zarebanadkouki M, Ahmed M A, Carminati A. Hydraulic conductivity of the root-soil interface of lupin in sandy soil after drying and rewetting. Plant and Soil, 2015, 398(1-2): 267-280.
[18] Ma Y, Qin F. ABA Regulation of Plant Responses to Drought and Salt Stresses//Zhang D, ed: Abscisic Acid: Metabolism, Transport and Signaling. Netherlands: Springer, 2014: 315-336.
[19] 马富举, 李丹丹, 蔡剑, 姜东, 曹卫星, 戴廷波. 干旱胁迫对小麦幼苗根系生长和叶片光合作用的影响. 应用生态学报, 2012, 23(3): 724-730.
[20] Habibi G, Ajory N. The effect of drought on photosynthetic plasticity inMarrubiumvulgareplants growing at low and high altitudes. Journal of Plant Research, 2015, 128(6): 987-99.
[21] Wang H, Zhang M S, Guo R, Shi D C, Liu B, Lin X Y, Yang C W. Effects of salt stress on ion balance and nitrogen metabolism of old and young leaves in rice (OryzasativaL.). BMC Plant Biology, 2012, 12: 194.
[22] Valifard M, Mohsenzadeh S, Kholdebarin B. Sodium chloride induced changes in photosynthetic performance and biochemical components ofSalviamacrosiphon. Indian Journal of Plant Physiology, 2015, 20(1): 79-85.
[23] Medeiros C D, Neto J R C F, Oliveira M T, Rivas R, Pandolfi V, Kido É A, Baldani J I, Santos M G. Photosynthesis, antioxidant activities and transcriptional responses in two sugarcane (SaccharumofficinarumL.) cultivars under salt stress. Acta Physiologiae Plantarum, 2014, 36(2): 447-459.
Effects of salt with drought stress on growth and water uptake of wheat seedlings
WENG Yawei, ZHANG Lei, ZHANG Shan, TIAN Zhongwei, JIN Xueying, LI Mengya,YU Zhongyu, JIANG Dong, DAI Tingbo*
AgronomyCollegeofNanjingAgriculturalUniversity/KeyLaboratoryofCropPhysiologyEcologyandProductionManagementofMinistryofAgriculture/JiangsuCollaborativeInnovationCenterforModernCropProduction,Nanjing210095,China
Salt and drought stress are two major limiting factors to wheat (TriticumaestivumL.) productivity. In north or northwest China, salt and drought stress often occur simultaneously owing to less rainfall and higher evaporation in winter and spring, which results in higher wheat yield loss. Recently, several studies have indicated that certain crop species exhibit lower growth inhabitation under the combined stress of salt and drought compared with salt and drought stress separately, but less information about adaptation mechanisms of these plants is available. Drought-tolerant and susceptible cultivars may possess variable morphological and metabolic adaptation processes in response to salt and drought stress that may contribute differently to their adaptation capability towards stress conditions. This study aims to investigate the combined effects of salt with drought stress(SD) on wheat seedling growth and water absorption characteristics, therefore providing a theoretical basis for wheat cultivation and management under salt and drought stress conditions. For this purpose two wheat cultivars, Yangmai16 (drought-susceptible) and Luohan7 (drought-tolerant), were used in a hydroponic experiment to investigate the effects of SD on root morphology, photosynthesis, and water absorption characteristics at the seedling stage of wheat. Sodium chloride(NaCl) and polyethylene glycol 6000(PEG) were applied to solution to simulate salt and drought stress, respectively. Leaf gas exchange, chlorophyll fluorescence parameters, leaf water potential and root hydraulic conductivity was determined, and hormone concentrations were estimated according to the enzyme-linked immunosorbentassaymethod. The results showed that both salt and drought stress significantly affected the plant growth and physiological activities for both wheat cultivars. However, the combined effects of SD on plant growth and dry matter production reduction were lower than their sole effects. The root length, root surface area and root volume in SD-treated plants were higher than those in single stress treated plants although these were significantly lower when compared with the control. This indicates that SD has less negative effects on root growth than the single stress does. Similarly, chlorophyll content, chlorophyll fluorescence parameters (Fv/FmorFv′/Fm′), net photosynthetic rate, and stomatal conductance under SD treatment were all significantly higher than the single salt or drought stress, showing that SD caused less damage to the photosynthetic apparatus than their single application. Root hydraulic conductivity and xylem sap intensity under SD were observed to be significantly higher than those for the single stress, which resulted in higher leaf water potential and relative water content under SD than under single stress. The Na+/K+ratio in leaves and roots under SD treatment were significantly lower than that for the single salt stress, and the abscisic acid (ABA) content in SD-treated plants was lower than that in single stress-treated plants, although those were significantly higher than those for the control, indicating that SD could reduce root ABA formation as compared to single stress. Compared with single salt and drought stress, SD not only improved the root water uptake capacity and leaf water status, but also decreased the Na+/K+and ABA content, hence alleviating inhibition of leaf photosynthetic capacity. Overall, maintaining a higher water absorption capacity and photosynthesis were the major contributors for wheat seedlings to adapt SD. Luohan7 and Yangmai16 responded similarly to salt and SD, whereas Luohan7 showed more obvious tolerance to drought stress than Yangmai16.
wheat; salt combined with drought; photosynthesis; root hydraulic conductivity; water uptake
10.5846/stxb201601040020
国家自然科学基金资助项目(31471443,31501262); 江苏省自然科学基金资助项目(BK20140705)
2016- 01- 04; 网络出版日期:2016- 08- 30
翁亚伟, 张磊, 张姗, 田中伟, 靳雪莹, 李梦雅, 余钟毓, 姜东, 戴廷波.盐旱复合胁迫对小麦幼苗生长和水分吸收的影响.生态学报,2017,37(7):2244- 2252.
Weng Y W, Zhang L, Zhang S, Tian Z W, Jin X Y, Li M Y,Yu Z Y, Jiang D, Dai T B.Effects of salt with drought stress on growth and water uptake of wheat seedlings.Acta Ecologica Sinica,2017,37(7):2244- 2252.
*通讯作者Corresponding author.E-mail: tingbod@njau.edu.cn
