紫外线照射未成熟树突状细胞诱导小鼠同种抗原免疫耐受及其机制研究
2017-06-24郭轶先张蓝方孙雪静万岁桂夏长青
郭轶先 张蓝方 孙雪静 万岁桂 夏长青,2*
(1.首都医科大学宣武医院血液科, 北京 100053; 2.佛罗里达大学医学院病理学、免疫学和实验医学系,佛罗里达 32610)
· 基础研究 ·
紫外线照射未成熟树突状细胞诱导小鼠同种抗原免疫耐受及其机制研究
郭轶先1张蓝方1孙雪静1万岁桂1夏长青1,2*
(1.首都医科大学宣武医院血液科, 北京 100053; 2.佛罗里达大学医学院病理学、免疫学和实验医学系,佛罗里达 32610)
目的 探讨应用紫外线照射未成熟树突状细胞诱导C3H小鼠对Balb/c小鼠同种抗原免疫耐受及其免疫学机制。方法 ①用紫外线B(ultraviolet B, UVB)照射Balb/c(H-2d)小鼠骨髓来源的未成熟树突状细胞(immature dendritic cells,imDC),诱导其凋亡,随后将其输注到C3H(H-2k)小鼠体内,诱导C3H小鼠对Balb/c抗原免疫耐受。②检测诱免疫耐受小鼠体内调节T细胞以及细胞因子白细胞介素-10(interleukin-10, IL-10)和干扰素-γ(interferon-γ,IFN-γ)分泌以研究其耐受机制。结果 ①UVB-Balb/c imDC免疫的C3H小鼠对Balb/c抗原产生了免疫耐受,不能产生抗Balb/c抗体。②免疫耐受C3H小鼠不能排异注射到体内的Balb/c脾细胞。③UVB-Balb/c imDC免疫的C3H小鼠T细胞分泌IL-10增加,并且产生更多的FOX-P3+调节T细胞。结论 ①应用UVB-Balb/c imDC免疫C3H小鼠可以使C3H小鼠对Balb/c抗原产生完全彻底的免疫耐受。②免疫耐受C3H小鼠对Balb/c抗原产生免疫耐受的可能原因是由于其T细胞分泌IL-10增加,并且产生更多的FOX-P3+调节T细胞。
树突细胞;凋亡;调节T细胞;免疫耐受
免疫耐受重建和免疫耐受诱导是自身免疫病和异基因移植研究领域的重要研究课题和最终目标。此目标的实现将克服目前无选择性免疫抑制治疗带来的严重的健康问题。在这些维持自身免疫耐受的机制中,自身组织细胞在自我更新中不断发生的稳态凋亡发挥着非常重要的作用[1]。现有研究[2-3]表明,凋亡是机体维持免疫系统稳定的重要作用机制,凋亡细胞诱导耐受的独特之处是温和而高效。因此应用凋亡细胞诱导免疫耐受的治疗策略具有极好的临床前景。树突状细胞(dendritic cells,DC)是一类专职的抗原呈递细胞, 它本身并不起到直接杀伤病原的作用,但是在免疫应答及免疫耐受中发挥双向调节作用,稳态DC递呈抗原将会导致T细胞无应答,产生免疫耐受[4-6]。在本研究中,笔者拟通过凋亡树突状细胞诱导不同品系小鼠间同种免疫耐受,探求其免疫耐受机制。
1 材料与方法
1.1 实验动物
SPF级健康雌性Balb/c (H-2d)小鼠和雄性C3H(H-2k)小鼠购自北京维通利华实验动物技术有限公司。实验动物许可证号为SCXK-京-2014-0001。在首都医科大学实验动物科学部饲养繁殖。所有的小鼠均在SPF环境下IVC饲养系统中饲养。本研究通过首都医科大学宣武医院动物实验及实验动物伦理委员会审查批准。
1.2 树突状细胞培养及诱导
将冷冻在-80 ℃冰箱的Balb/c小鼠骨髓细胞复苏,将细胞接种到6孔板中,按10 ng/mL 加入重组小鼠粒细胞巨噬细胞集落刺激因子(recombinant murine granulocyte-macrophage colony stimulating factor,rmGM-CSF),按5 ng/mL加入重组小鼠白细胞介素(recombinant murine interleukin-4,rmIL-4),放37 ℃、饱和湿度、5%(体积分数)CO2培养箱中培养,倒置显微镜观察细胞形态及增生情况。细胞在体外培养4~5 d时增生显著,可见到大量具有典型树枝状突起的悬浮细胞,触突呈放射状,并有悬浮细胞呈集落生长,这些细胞为未成熟DC细胞(immature DC,imDC),将其中3孔加入脂多糖(lipopolysachrid,LPS)0.5 μg/mL诱导成熟DC细胞(mature DC,mDC)生成。加入 LPS继续培养24 h后,可见到LPS刺激的细胞体积有所增大,突起变长,这部分细胞为mDC细胞,此时将imDC及mDC收获。
1.3 树突细胞鉴定
1.3.1 倒置相差显微镜光镜及光镜分析
将上述培养收集的imDC及mDC 悬液甩片后镜下观察形态。
1.3.2 免疫表型分析
将上述培养收集的imDC及mDC 悬液离心沉淀,用 PBS 缓冲液调整细胞密度为 5×105/L,取100 μL标记CD11c、主要组织相容性复合体Ⅱ(major histocompatibility complex-Ⅱ, MHC-Ⅱ)、CD80和CD86荧光抗体,后行流式细胞术检测。
1.4 诱导C3H(H-2k)和Balb/c(H-2d)小鼠间免疫耐受
1)诱导Balb/c小鼠imDC凋亡:采用的紫外线B(ultraviolet, UVB)照射 (1 200 mJ/cm2)的方法照射使其由于DNA的交联而启动细胞凋亡的过程。然后立即将UVB-Balb/c imDC及Balb/c mDC经尾静脉输注到C3H小鼠体内。
2)C3H小鼠分组详见表1。
1.5 流式细胞术检测C3H小鼠免疫耐受情况
1.5.1 外周血抗Balb/c细胞抗体检测
取免疫结束后C3H小鼠内眦抗凝血,取血浆20 μL加入Balb/c脾细胞20 μL,混匀,室温下孵育30 min后用PBS彻底洗细胞3遍。标记抗小鼠Ig-FITC、CD4-Per-CP、CD8-PE,待流式细胞仪检测。
1.5.2 体内检测供者免疫耐受情况
将羧基荧光素琥珀酰亚胺酯(carboxyfluorescein succinimidyl ester,CFSE)标记的Balb/c小鼠脾细胞尾静脉输注到C3H小鼠体内。24 h后,将C3H小鼠处死,取脾细胞和腹股沟、腘窝淋巴结细胞,标记抗小鼠CD4-Per-CP、CD8-PE和B220-APC抗体,待流式细胞仪检测。
1.5.3 淋巴细胞增生实验
1)取之前经UVB-Balb/c imDC和Balb/c mDC免疫的免疫结束后的C3H小鼠脾细胞,CFSE染色后种植在96孔细胞培养板,分别用Balb/c mDC及C57 mDC在细胞比例为20∶1条件下刺激,对照组为空白组cell only和植物血凝素(phytohemagglutinin, PHA)刺激组。置37 ℃,5%(体积分数)CO2温箱培养4~5 d。培养4~5 d后,收集培养的细胞悬液,标记抗CD4单抗流式细胞术分析。2)实验分组详见表2。
1.5.4 细胞内因子检测
淋巴细胞增生实验步骤及分组同前,只是培养的细胞不经过CFSE染色。细胞收获前5 h加入含BolgiPlug白细胞活化混合物。收获细胞标记抗CD4抗体、干扰素-γ(interferon-γ,IFN-γ)和IL-10抗体,流式细胞仪检测。

表1 C3H小鼠分组Tab.1 Grouping of C3H mice
imDC: immature dendritic cells; mDC: mature dendritic cells.
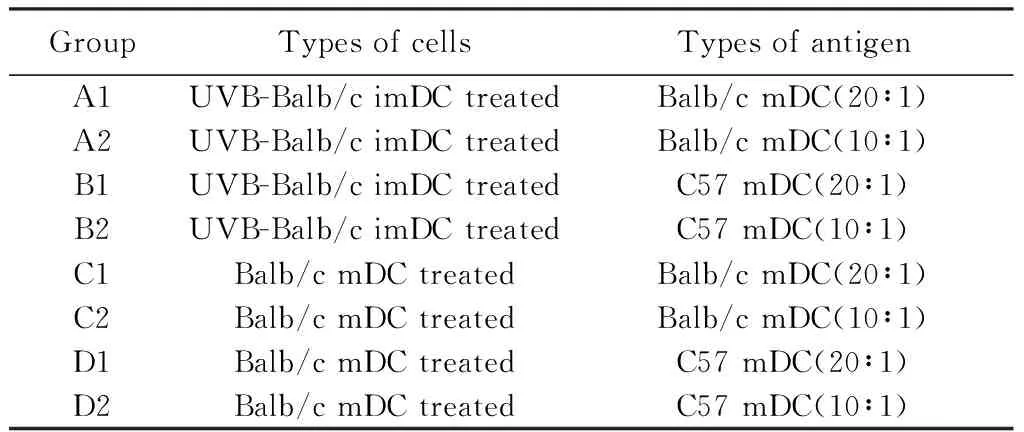
表2 C3H小鼠脾脏细胞分组Tab.2 Grouping of spleen cells of C3H mice
mDC: mature dendritic cells; imDC: immature dendritic cells.
1.5.5 调节T细胞检测
淋巴细胞增生实验步骤及分组同前,只是培养的细胞不经过CFSE染色。细胞收获标记CD4-Per-CP、FOX-P3抗体和CD25抗体,流式细胞仪检测。
1.6 统计学方法
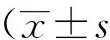
2 结果
2.1 小鼠DC体外诱导
2.1.1 细胞形态
小鼠骨髓细胞在体外经rmGM-CSF 和rmIL-4诱导24 h,倒置相差显微镜下可见呈簇状生长的细胞团。培养3 d,细胞簇较前增多,大多数细胞仍然贴壁,少数细胞呈半悬浮状态。培养5 d,大量细胞呈半悬浮生长,细胞体积较以前增大,呈圆形或梭型,细胞表面可见树突状突起(图1A、B)。将第5天的DC经LPS刺激24 h,细胞体积较前显著增大,细胞呈圆形、星形或梭形,细胞核明显,细胞表面突起较前增多、分支明显(图1C、1D)。
2.1.2 细胞免疫表型
流式细胞术分析表明,小鼠骨髓来源DC高表达CD11c,其中imDC低表达MHCⅡ、CD80和CD86,而mDC高表达MHCⅡ、CD80和CD86(图2)。
2.2 Balb/c(H-2d)和C3H(H-2K)小鼠间免疫耐受
2.2.1 输注了UVB-Balb/c imDC的C3H小鼠不能产生抗Balb/c抗体
输注Balb/c UVB-imDC的C3H小鼠不能产生抗Balb/c抗体,即彻底完全的免疫耐受。而输注未照射的Balb/c mDC的C3H小鼠体内可以检测出较高的抗Balb/c抗体。详见图3。
2.2.2 输注了UVB-Balb/c imDCs的C3H小鼠未能排斥CFSE染色的Balb/c脾细胞
输注UVB-Balb/c imDC的C3H小鼠脾脏和淋巴结可以检测到CFSE染色的Balb/c脾细胞,即对Balb/c脾细胞产生免疫耐受。而输注未照射的Balb/c imDC及mDC的C3H小鼠脾脏和淋巴结没有检测到CFSE染色的Balb/c脾细胞,即将Balb/c脾细胞排异掉了(图4)。

图1 小鼠BMDC倒置显微镜下及甩片后吉姆萨染色涂片光学显微镜下图像Fig.1 Images of BMDC under inverted microscope and Giemsa stained smears under optical microscope
A: immature DC under inverted microcope (20×); B: immature DC by Giemsa staining (60×); C: mature DC under inverted microcope (20×);D: mature DC by Giemsa staining (60×). Dendritic bulge was seen on the cell surface (A, B). Cell volume increased significantly compared with the former, cell surface protrusions increased more than before (C, D). DC: dendritic cells; BMDC:bone marrow derived dendritic cells.
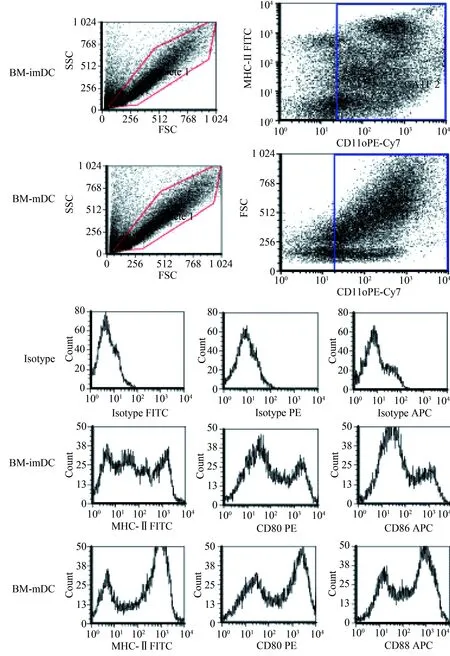
图2 流式细胞术分析小鼠骨髓来源DC免疫表型Fig.2 Flow cytometry analysis of DC immunophenotype of bone marrow derived from mice
CD11c was highly expressed in bone marrow-derived DCs, MHC Ⅱ, CD80 and CD86 were lowly expressed in imDC, while MHC Ⅱ, CD80 and CD86 were highly expressed in mDCs; BM-imDC: bone marrow immature dendritic cells; BM-mDC: bone marrow mature dendritic cells; MHC-Ⅱ:major histocompatibility complex-Ⅱ.

图3 Balb/C(H-2d) 和C3H(H-2k)小鼠间抗原特异性的免疫耐受诱导Fig.3 Antigen-specific immunotolerance induction in Balb/C (H-2d) and C3H (H-2k) mice
A:C3H; B:VVB-imDC; C:mDC; Antibody levels in C3H mice immunized by UVB- Balb/c imDCs(B), Balb/c mDCs(C), in non-immunized C3H mice(A) detected by flow cytometry. imDC: immature dendritic cells; mDC: mature dendritic cells.
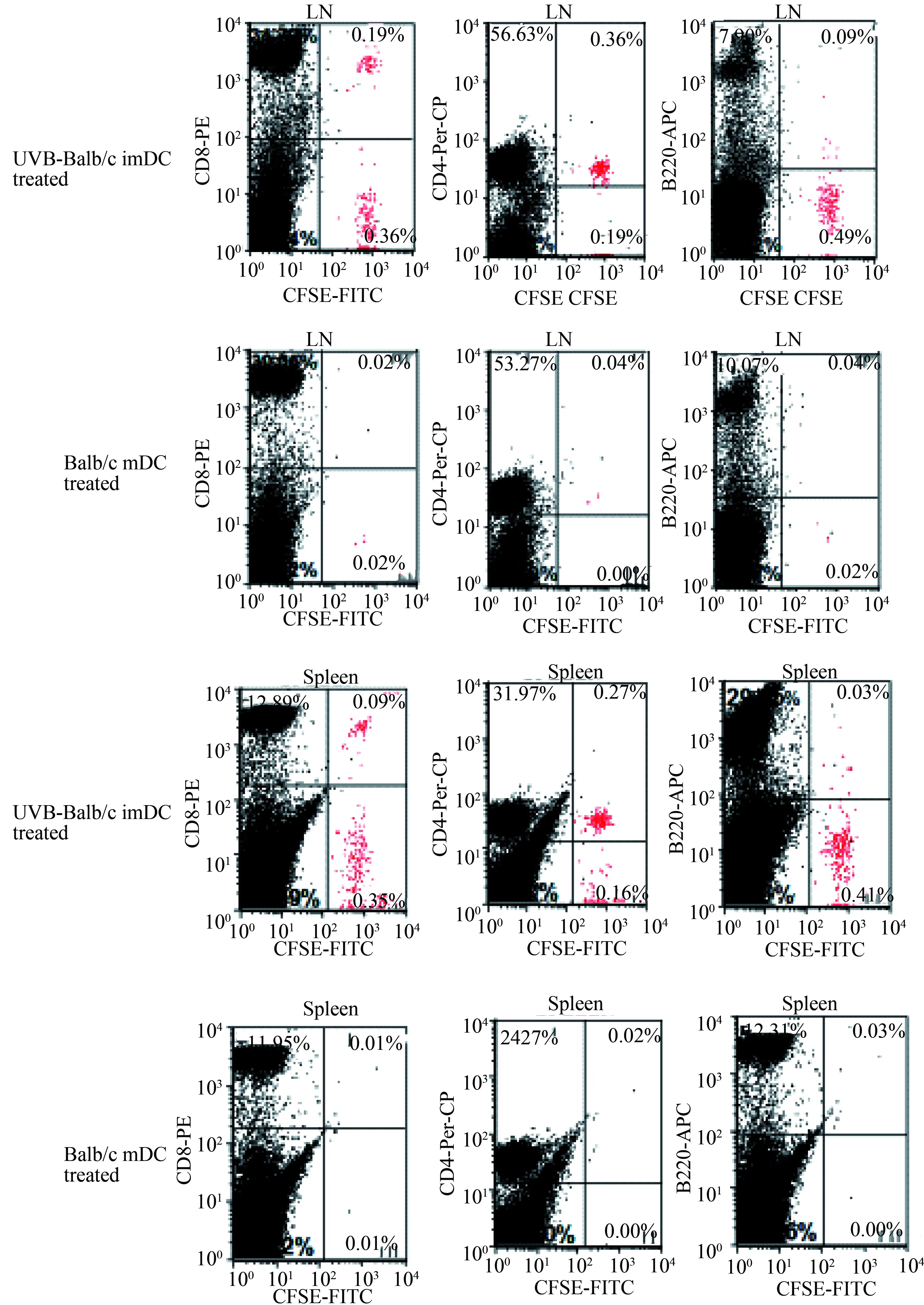
图4 流式细胞术检测静脉注射UVB-Balb/c imDC或mDC的C3H小鼠脾细胞及淋巴结中 CFSE染色的Balb/c小鼠脾细胞分布情况Fig.4 Distribution of spleen cells in CFSE-stained splenocytes of C3H mice injected with UVB-Balb/c imDCs or mDCs by intravenous injection of Balb/c mice by flow cytometry
The red cells were CFSE-stained Balb/c mouse spleen cells; imDC: immature dendritic cells; mDC: mature dendritic cells;CFSE:carboxyfluoresce in succinimidyl ester; LN:lymph node.
2.3 免疫耐受机制分析
流式细胞术分析结果提示UVB-Balb/c imDCs免疫的C3H小鼠脾脏中FOX-P3阳性的调节T细胞比例增高(图5)。
笔者将Balb/c UVB-imDC及mDC免疫的C3H小鼠脾细胞经CFSE染色后与Balb/c小鼠mDC细胞培养5 d后,标记抗体后用流式细胞仪检测发现各组淋巴细胞均有明显的增生(图6),随后对增生的细胞标记IFN-γ、IL-10、CD4,发现Balb/c UVB-imDC免疫的C3H小鼠T细胞较其他组T细胞分泌更多的IL-10(图7)。
3 讨论
自身免疫耐受的维持是机体预防自身免疫病的自我防御机制。当这一防御机制因某些原因被打破,便可诱发自身免疫病[7]。研究者[8]在对自身免疫的研究中发现,DC吞噬携带某种抗原的凋亡细胞后可以诱导机体对该抗原产生特异性免疫耐受。本实验结果显示,通过给C3H(H-2k)小鼠输注UVB-Balb/c(H-2d) imDC可以诱导C3H(H-2k)小鼠对Balb/c(H-2d)小鼠MHC抗原免疫耐受。C3H小鼠外周血中抗Balb/c抗体水平反映了C3H抗Balb/c MHC抗原的反应能力,较高的抗体水平提示较强的同种异体反应。接受UVB-Balb/c imDC输注的C3H小鼠不产生任何针对Balb/c的抗体。笔者还通过C3H小鼠静脉注射CFSE染色的Balb/c脾细胞,观察其是否对Balb/c抗原产生免疫耐受,这一结果和抗体检测结果一致,均证明了UVB-Balb/c(H-2d)imDC免疫的C3H(H-2k)小鼠对Balb/c(H-2d)小鼠MHC抗原产生了免疫耐受。并且,通过分析C3H小鼠脾脏和淋巴结中CFSE标记的Balb/c脾细胞,发现CD4+T细胞和CD8+T细胞较B细胞更易产生免疫耐受,因为在产生免疫耐受的C3H小鼠脾脏和淋巴结中B细胞数量较CD4+T和CD8+T细胞数量明显要少。这一结果提示UVB-Balb/c(H-2d)小鼠imDCs更倾向诱导MHC-Ⅰ抗原的免疫耐受,因为T细胞仅表达MHC-Ⅰ抗原,而B细胞不仅表达MHC-Ⅰ还表达MHC-Ⅱ。由于T细胞和B细胞是同时注射、归巢到脾脏和淋巴结的,所以这一结果并不能用B细胞较T细胞更多存在外周循环中来解释。
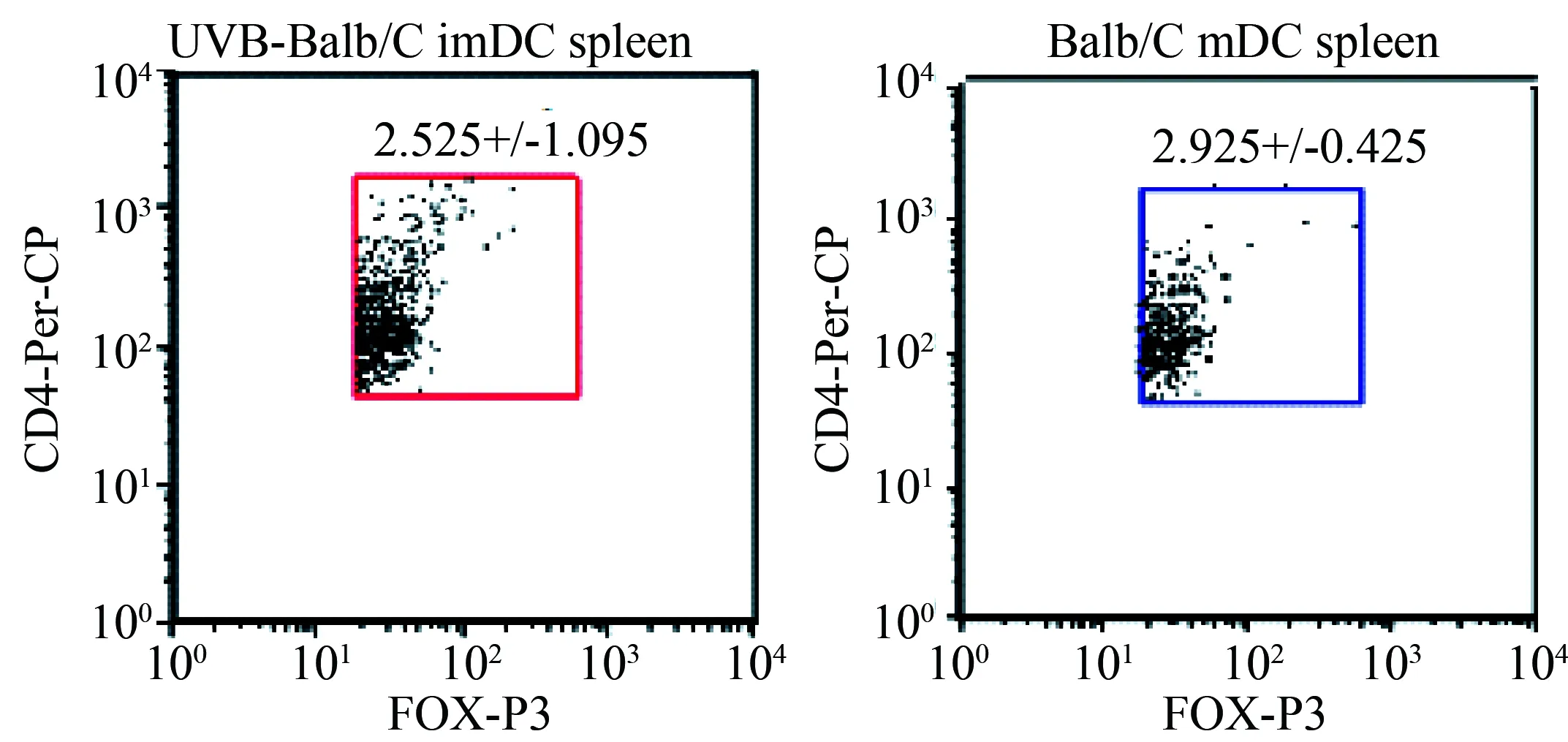
图5 流式细胞术检测静脉注射UVB-Balb/c imDC或 Balb/c mDC的C3H小鼠脾细胞中POX-P3阳性细胞比例Fig. 5 The ratio of FOX-P3 positive regulatory T cells in spleen cells of C3H mice treated by UVB-Balb/c imDC or Balb/c mDC
This observation was verified from three independent experiments;n=2;imDC: immature dendritic cells; mDC: mature dendritic cells.
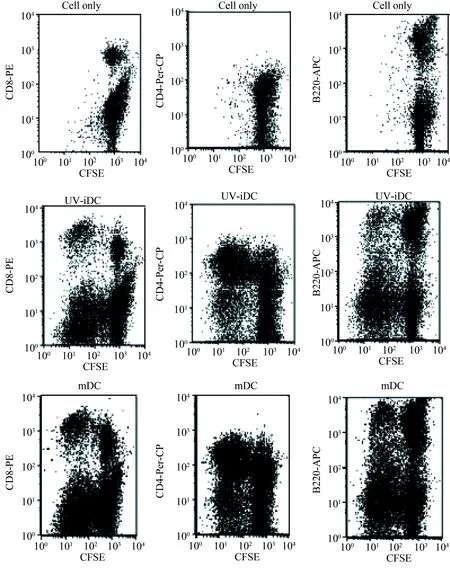
图6 Balb/c抗原诱导免疫后的C3H小鼠脾细胞Fig. 6 The immune responses of treated C3H mouse spleen cells induced by Balb/c antigen
C3H mouse spleen cells (1×106) treated by UVB-Balb/c imDC or Balb/c mDC intravenously were stained with
CFSE and culture with Balb/c mDCs. The proliferation was analyzed by flow cytometry after four days; imDC: immature dendritic cells; mDC: mature dendritic cells;CFSE:carboxyfluoresce in succinimidyl ester.

图7 流式细胞术分析免疫后C3H小鼠增生的CD4+T细胞分泌细胞因子的情况Fig.7 Flow cytometry analysis of cytokines secreted by CD4+ T cells in C3H mice after immunization
Cytokines secreted by proliferating CD4+T cells were analyzed by flow cytometry. This observation was verified from three independent experiments.n=2; CFSE: carboxyfluoresce in succinimidyl ester; IL-10:interleukin-10; IFN-γ:interferon-γ.
DC诱导免疫耐受的机制尚不完全清楚,已知的机制包括诱导T细胞无能、释放免疫抑制性细胞因子、介导T细胞的克隆清除、诱导和募集调节性T细胞等[9]。凋亡细胞所携带的抗原被DC吞噬后形成抗原肽-MHC-Ⅰ复合物,该复合物与 CD8+T细胞相互作用诱导其成为细胞毒T淋巴细胞(cytotoxic T lymphocyte,CTL)[10]。但是如果DC细胞没有同时激活CD4+T细胞,则CD8+T细胞表达肿瘤坏死因子相关凋亡诱导配体(tumor necrosis factor related apoptosis inducing ligand,TRAIL)死亡,从而诱导了免疫耐受[11- 12]。凋亡细胞免疫途径不同可以产生不同的免疫效果[13]。静脉注射凋亡细胞可以诱导机体对该凋亡细胞所携带的抗原产生免疫耐受[14],而皮下注射凋亡细胞则会产生抗原特异性的免疫应答[15]。脾脏CD8+DC细胞诱导免疫耐受而CD8-DC诱导免疫应答[13]。mDC由于表达MHC-Ⅱ和共刺激分子(CD80/CD86)增加而诱导免疫应答,而imDC则诱导免疫耐受[16]。笔者将UVB-Balb/c imDC、 Babl/c mDC免疫的C3H小鼠脾细胞CFSE染色后与Balb/c小鼠mDC细胞培养5 d后,发现UVB-Balb/c imDC免疫的C3H小鼠T细胞较其他组小鼠T细胞分泌更多的IL-10,并且FOX-P3+的调节T细胞比例也增高。研究结果提示FOX-P3+的调节T细胞在UVB-imDC诱导的免疫耐受中起到一定作用。
总之,凋亡树突状细胞具有很强的免疫耐受诱导作用,在免疫病治疗及移植免疫耐受中值得进一步研究。
[1] Skoberne M, Beignon A S, Larsson M, et al. Apoptotic cells at the crossroads of tolerance and immunity[J]. Curr Top Microbiol Immunol,2005,289:259-292.
[2] Sun E W, Shi Y F. Apoptosis: the quiet death silences the immune system[J]. Pharmacol Ther,2001,92(2-3):135-145.
[3] Hogquist K A, Baldwin T A, Jameson S C. Central tolerance: learning self-control in the thymus[J]. Nat Rev Immunol,2005,5(10):772-782.
[4] Gregori S. Dendritic cells in networks of immunological tolerance[J]. Tissue Antigens,2011,77(2):89-99.
[5] Lewis K L, Reizis B. Dendritic cells: arbiters of immunity and immunological tolerance[J]. Cold Spring Harb Perspect Biol,2012,4(8):a007401.
[6] Ganguly D, Haak S, Sisirak V, et al. The role of dendritic cells in autoimmunity[J]. Nat Rev Immunol,2013,13(8):566-577.
[7] Vives-Pi M, Rodriguez-Fernandez S, Pujol-Autonell I. How apoptotic beta-cells direct immune response to tolerance or to autoimmune diabetes: a review[J]. Apoptosis,2015,20(3):263-272.
[8] Griffith T S, Yu X, Herndon J M, et al. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance[J]. Immunity,1996,5(1):7-16.
[9] Chung C Y, Ysebaert D, Berneman Z N, et al. Dendritic cells: cellular mediators for immunological tolerance[J]. Clin Dev Immunol,2013,2013:972865.
[10]Schoenberger S P, Toes R E, van der Voort E I, et al. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions[J]. Nature,1998,393(6684):480-483.
[11]Janssen E M, Droin N M, Lemmens E E, et al. CD4+T-cell help controls CD8+T-cell memory via TRAIL-mediated activation-induced cell death[J]. Nature,2005,434(7029):88-93.
[12]Griffith T S, Kazama H, Vanoosten R L, et al. Apoptotic cells induce tolerance by generating helpless CD8+T cells that produce TRAIL[J]. J Immunol,2007,178(5):2679-2687.
[13]Dudziak D, Kamphorst A O, Heidkamp G F, et al. Differential antigen processing by dendritic cell subsets in vivo[J]. Science,2007,315(5808):107-111.
[14]Conlon P J, Miller S D, Claman H N. The induction of tolerance to DNFB contact sensitivity by using hapten-modified lymphoid cells. Ⅲ. Effects of hapten concentration on the ability of MLS-disparate cells to induce rapid unresponsiveness[J]. J Immunol,1980,125(2):807-813.
[15]Chaput N, De Botton S, Obeid M, et al. Molecular determinants of immunogenic cell death: surface exposure of calreticulin makes the difference[J]. J Mol Med (Berl),2007,85(10):1069-1076.
[16]Reis e Sousa C. Dendritic cells in a mature age[J]. Nat Rev Immunol,2006,6(6):476-483.
编辑 孙超渊
Alloantigen immune tolerance and its mechanism in mice immunized with the ultraviolet radiation immature dendritic cells
Guo Yixian1, Zhang Lanfang1, Sun Xuejing1, Wan Suigui1, Xia Changqing1,2*
(1.DepartmentofHematology,XuanwuHospital,CapitalMedicalUniversity,Beijing100053,China;2.DepartmentofPathology,ImmunologyandLaboratoryMedicine,UniversityofFloridaCollegeofMedicine,Florida32610,USA)
Objective To induce alloantigen immune tolerance between C3H mice and Balb/c mice by infusing the ultraviolet B(UVB) radiation immature dendritic cells (imDC) and and study the immunological mechanisms of this process.Methods ① The authors induced alloantigen tolerance in C3H mice (H-2k) by intravenous injecting ultraviolet B (UVB) irradiated Balb/c immature dendritic cells (UVB-Balb/c imDC) derived from the cultures of Balb/c bone marrow cells. ②Detection of immune tolerance induced regulatory T cells, interleukin-10 (IL-10) and interferon-γ (IFN-γ) in mice in order to study the tolerance mechanism. Results ①C3H mice immunized with UVB-Balb/c imDC can produce immune tolerance to Balb/c antigens and can not produce anti-Balb/c antibody. ②C3H mice immunized with UVB-Balb/c imDC can not clear the Balb/c spleen cells in vivo. ③The T cells of C3H mice immunized with UVB-Balb/c imDC can secrete more IL-10 and produce more FOX-P3+regulatory T cells than the control group in vitro. Conclusion ①Immunization of C3H mice with UVB-Balb/c imDC allows C3H mice to fully tolerate Balb/c antigen. ②The possible cause of immune tolerance to Balb/c antigens in C3H mice is due to the increased secretion of IL-10 from T cells and the production of more FOX-P3+regulatory T cells.
dendritic cells; apoptosis; regulatory T cells; immune tolerance
国家自然科学基金(81172854,81240015)。This study was supported by National Natural Science Foundation of China (81172854, 81240015).
时间:2017-06-09 18∶30 网络出版地址:http://kns.cnki.net/kcms/detail/11.3662.r.20170609.1830.060.html
10.3969/j.issn.1006-7795.2017.03.020]
R3
2016-01-15)
*Corresponding author, E-mail:bjxwgyx@163.com
