含二硫键的蛋白质/多肽的MALDI-TOF MS源内裂解研究
2017-06-21姚文斌汪亿晗连文慧杨洪梅陈长宝刘淑莹
姚文斌,汪亿晗,苏 蕊,连文慧,杨洪梅,2,陈长宝,刘淑莹
(1.长春中医药大学,吉林省人参科学研究院,吉林 长春 130117;2.中国科学院长春应用化学研究所,吉林 长春 130022)
含二硫键的蛋白质/多肽的MALDI-TOF MS源内裂解研究
姚文斌1,汪亿晗1,苏 蕊1,连文慧1,杨洪梅1,2,陈长宝1,刘淑莹1
(1.长春中医药大学,吉林省人参科学研究院,吉林 长春 130117;2.中国科学院长春应用化学研究所,吉林 长春 130022)
应用基质辅助激光解吸电离飞行时间质谱(MALDI-TOF MS)法研究含二硫键的人胰岛素与甘精胰岛素酶解液的源内裂解(ISD)。比较了不同基质种类及不同结晶状态对含二硫键的人胰岛素与甘精胰岛素酶解液的源内裂解的影响。结果表明,含二硫键的蛋白质的ISD发生受激光点照射位置的影响,在不同基质与结晶形态的条件下,含二硫键的蛋白质的ISD碎片信息不同。通过分析比较,含二硫键的蛋白质的ISD较容易控制,并且其基质的种类及结晶状态作用很关键。需要获得大量碎片时,使激光照射在样品和阿魏酸(FA)基质形成的大结晶处;不希望出现碎片时,可使用2,4,6-三羟基苯乙酮(THAP)为基质,或使激光照射在样品和其他基质形成的细小均匀结晶处。
含二硫键的蛋白质/多肽;基质辅助激光解吸电离飞行时间质谱(MALDI-TOF MS);源内裂解(ISD);基质;结晶
基质辅助激光解吸电离(MALDI)技术是由Karas和Tanaka等[1-2]发明的,由于基质辅助激光解吸电离飞行时间质谱(MALDI-TOF MS)具有质量范围宽、耐盐和灵敏度高等优点[3-5],利用该技术分析蛋白质的应用日益广泛。二硫键是一种常见的蛋白质翻译后修饰,对稳定蛋白质的空间结构[6-9]、维持正确的折叠构象[10-12]、保持及调节生物活性[13-15]等都有着重要作用。确定二硫键在蛋白质中的位置对于鉴定蛋白质一级结构有重要的意义,是研究含有二硫键的活性多肽/蛋白质化学结构的重要方面。目前,MALDI MS已经成为分析二硫键的强有力工具[16-17]。
虽然MALDI通常被认为是一种软离子化过程,但是在离子化过程中也会出现严重的碎裂,例如,会发生二硫键连接的多肽的快速裂解[18-20]现象。这种快速裂解受激光强度和所用基质的影响[20-22],在以芥子酸为基质的质谱图中,这种快速碎裂方式并不常见。通过研究二硫键连接的多肽的快速裂解现象发现,激光强度起着重要的作用,其他参数对碎裂程度的影响则很小。Zhao等[22]报道的二硫键源内裂解具有如下规律:如果多肽结构中是成对的链间二硫键,主要发生自由基重排;只要结构中有奇数个半胱氨酸就可以获得还原型的源内裂解碎片。
本研究拟分析结晶状态和基质种类对含二硫键的蛋白质源内裂解(ISD)的影响,以促进或避免ISD的发生,希望为使用MALDI-TOF MS分析含二硫键的蛋白质/多肽提供支持信息。
1 实验部分
1.1 仪器和试剂
Voyager DE-STR MALDI-TOF MS仪:美国AB公司产品,配有N2激光器(337 nm);α-氰基-4-羟基肉桂酸(CHCA)、芥子酸(SA)、阿魏酸(FA)、2,5-二羟基苯甲酸(DHB)、2,4,6-三羟基苯乙酮(THAP)、3-氨基喹啉(3-AQ)等基质:均为美国Sigma公司产品;胰蛋白酶: Promega(北京)生物技术有限公司产品;人胰岛素和甘精胰岛素:通化东宝制药有限公司产品;三氟乙酸(TFA):色谱级,美国Tedia公司产品;实验用水:由美国Millipore公司Milli-Q超纯水机制备。
1.2 蛋白质酶解
取50 μL 1 g/L的人胰岛素和甘精胰岛素,分别溶解在pH 7.8的50 mmol/L NH4HCO3溶液中,于37 ℃用胰蛋白酶进行水解;酶/底物的质量比为1∶50,酶解8 h;最后将酶解液放入-20 ℃冰箱中终止酶解反应。
1.3 MALDI-MS分析
样品直接在MALDI靶上制备:取0.5 μL0.2 g/L的被分析物与同体积10 g/L的基质混合,基质溶解在含0.1%TFA的水-乙腈(1∶1,V/V)溶液中。将50 mmol/L NH4HCO3-NH3·H2O缓冲溶液(pH 9.0)添加到混合溶液中,调节溶液至pH 8。
采用正离子模式采集,测试每一个样品时均优化了加速电压、光栅电压和延迟时间等参数。激光频率为3 Hz,扫描50次。利用外标法进行校正,校正液包括CHCA(190.050 4 u)和血管紧张素Ⅰ(1 296.685 3 u)。
2 结果与讨论
采用MALDI-TOF MS分析含二硫键的蛋白质时,注意到含二硫键的蛋白质ISD的发生受激光点照射位置的影响。以人胰岛素和甘精胰岛素为例,评价了结晶状态对ISD碎裂的影响。酶切位点示于图1。激光照射人胰岛素与FA基质形成非常细小的均匀结晶位置的谱图没有产生碎片,示于图2a。从图2a可见,仅有2个离子m/z859.46和m/z4 866.23的酶解肽段。把激光照射在大结晶处获得图2b,比较图2b与图2a可看出,图2b中含有质量差为32 u的3个特征离子(m/z2 454.24、2 486.21、2 518.18),是通过对称和非对称断裂m/z4 866.23的多肽的二硫键而产生,可能是热力学的原因,是断裂偶数个化学键后的自由基重排反应,而非还原性的ISD,这与前期的文献[22]报道一致。

图1 人胰岛素(a)和甘精胰岛素(b)的结构式和相对分子质量Fig.1 Structures and molecular weights of human insulin (a) and insulin glargine (b)

注:左图为以FA为基质的经胰蛋白酶酶解后人胰岛素的位点照片图2 均匀结晶位置(a)和大结晶处(b)所得的MALDI质谱图Fig.2 MALDI mass spectrum recorded in a region where crystals are very finely distributed (a) and recorded on a large crystal (b)
分析甘精胰岛素的质谱图观察到了相似的现象,结果示于图3。从图3可见,除了m/z4 809.20离子外,所有质谱峰与图2中结果相同。结果证实,含二硫键的蛋白质ISD的发生依赖于其结晶状态,这与采用MALDI ISD研究全甲基化糖的结果[23]一致。在其报道中,因为糖对阳离子的亲和性较高,所以碎裂可以由样品点无定形区域的高浓度钠所避免。另一方面,由于基质晶体处没有钠离子,糖苷键被质子化而断裂。这种机理不能解释当前的实验现象,因为多肽是被质子化的,对于此现象的合理解释是:结晶越小,样品吸收的激光强度越小,MALDI过程越温和;反之,结晶越大,样品吸收的激光强度越大,MALDI过程越剧烈[24]。
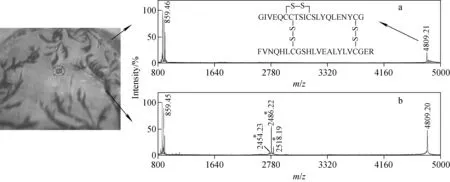
注:左图为以FA为基质的经胰蛋白酶酶解后甘精胰岛素的位点照片;※号标记为通过对称和非对称断裂二硫键而产生的碎片离子图3 均匀结晶位置(a)和大结晶处(b)所得到的MALDI质谱图Fig.3 MALDI mass spectrum recorded in a region where crystals are very finely distributed (a) and recorded on a large crystal (b)
为更加透彻地理解这种现象,考察了CHCA、SA、DHB、AQ和THAP基质。在这些基质中,与FA相似,CHCA和SA也可以使含二硫键的蛋白质断裂,同样具有结晶状态依赖性。与FA不同的是,ISD并不是在所有的大结晶上都能观察到,表明CHCA(SA)与FA形成结晶过程的差异性。使用DHB和AQ为基质时,在大结晶处可以观察到较小的碎片,结果示于图4,小结晶处观察不到碎片。使用THAP为基质时,观察不到碎片,可能由于它的结构中,三个羟基相对于每一个羟基而言都处于中间位置,无法发生氧化还原反应[23]。

注:*号标记为通过对称断裂二硫键而产生的碎片离子图4 以DHB(a)与AQ(b)为基质,甘精胰岛素经胰蛋白酶酶解后所得的MALDI质谱图Fig.4 MALDI mass spectra of insulin glargine tryptic digests using DHB (a) and AQ (b) as the matrices
3 结论
本实验应用MALDI-TOF MS研究了结晶状态和基质对人胰岛素与甘精胰岛素两个含二硫键的蛋白质的ISD影响。通过分析比较,含二硫键的蛋白质的ISD较容易控制,其基质的种类及结晶状态起着关键的作用。在基质考察中,FA、CHCA和SA均可以使含二硫键的蛋白质断裂,同样具有结晶状态依赖性。与FA不同的是,使用CHCA和SA为基质时,并不能在所有的大结晶上都观察到ISD,表明CHCA(SA)与FA形成结晶过程具有差异性。使用DHB和AQ为基质时,可以观察到较小的碎片。使用THAP为基质时,观察不到碎片。因此,当需要大量碎片时,可以使用FA为基质,不希望出现碎片时可以使用THAP为基质。这为使用MALDI-TOF MS分析含二硫键的蛋白质/多肽提供理论指导。
[1] KARAS M, HILLENKAMP F. Laser desorption ionization of proteins with molecular masses exceeding 10 000 daltons[J]. Analytical Chemistry, 1988, 60(20): 2 299-2 301.
[2] TANAKA M, WAKI H, IDO Y, et al. Protein and polymer analysis up tom/z100,000 by laser ionization time-of-flight mass spectrometry[J]. Rapid Communications in Mass Spectrometry, 1988, 2(2): 151-153.
[3] GATLIN-BUNAI C L, CAZARES L H, COOKE W E, et al. Optimization of MALDI-TOF MS detection for enhanced sensitivity of affinity-captured proteins spanning a 100 kDa mass range[J]. Journal of Proteome Research, 2007, 6(11): 4 517-4 524.
[4] OSULA O, SWATKOSKI S, COTTER R J. Identification of protein SUMOylation sites by mass spectrometry using combined microwave-assisted aspartic acid cleavage and tryptic digestion[J]. Journal of Mass Spectrometry, 2012, 47(5): 644-654.
[5] WANG Z, YU X, CUI M, et al. Investigation of calmodulin-peptide interactions using matrix-assisted laser desorption/ionization mass spectrometry[J]. Journal of the American Society for Mass Spectrometry, 2009, 20(4): 576-583.
[6] LEHLE K, KOHNERT U, STERN A, et al. Effect of disulfide bonds on the structure, function, and stability of the trypsin/tPA inhibitor from Erythrina caffra: site-directed mutagenesis, expression, and physiochemical characterization[J]. Nature Biotechnology, 1996, 14(4): 476-480.
[7] MATSUMURA M, SIGNOR G, MATTHEWS B W. Substantial increase of protein stability by multiple disulphide bonds[J]. Nature, 1989, 342(6 247): 291-293.
[8] CREIGHTON T E. Disulphide bonds and protein stability[J]. Bioessays, 1988, 8(2): 57-63.
[9] PACE C N, GRIMSLEY G R, THOMSON J A, et al. Conformational stability and activity of ribonuclease T1 with zero, one, and two intact disulfide bonds[J]. Journal of Biological Chemistry, 1988, 263(24): 11 820-11 825.
[10]WEDEMEYER W J, WELKER E, NARAYAN M, et al. Disulfide bonds and protein folding[J]. Biochemistry, 2000, 39(15): 4 207-4 216.
[11]PITT J J, da SILVA E, GORMAN J J. Determination of the disulfide bond arrangement of Newcastle disease virus hemagglutinin neuraminidase. Correlation with a beta-sheet propeller structural fold predicted for paramyxoviridae attachment proteins[J]. Journal of Biological Chemistry, 2000, 275(9): 6 469-6 478.
[12]HARRISON P M, STERNBERG M J. Analysis and classification of disulphide connectivity in proteins. The entropic effect of cross-linkage[J]. Journal of Molecular Biology, 1994, 244(4): 448-463.
[13]REINDERS J, SICKMANN A. Modificomics: posttranslational modifications beyond protein phosphorylation and glycosylation[J]. Biomolecular Engineering, 2007, 24(2): 169-177.
[14]ABKEVICH V I, SHAKHNOVICH E I. What can disulfide bonds tell us about protein energetics, function and folding: simulations and bioninformatics analysis[J]. Journal of Molecular Biology, 2000, 300(4): 975-985.
[15]NELSON J W, CREIGHTON T E. Reactivity and ionization of the active site cysteine residues of DsbA, a protein required for disulfide bond formation in vivo[J]. Biochemistry, 1994, 33(19): 5 974-5 983.
[16]张平. HPLC MALDI-TOF质谱法检测IFN-α2b二硫键结构[J]. 海峡药学,2016,28(5):65-69.
ZHANG Ping. Determination of disulfide bond in IFN-α2b by HPLC MALDI-TOF MS[J]. Strait Pharmaceutical Journal, 2016, 28(5): 65-69(in Chinese).
[17]王勇,李水明,何曼文. 基质辅助激光解吸电离一串联飞行时间质谱鉴定鲨鱼硒结合蛋白[J]. 质谱学报,2013,34(5):257-262.
WANG Yong, LI Shuiming, HE Manwen. Identification of selenium binding protein of shark by MALDI-TOF/TOF mass spectrometry[J]. Journal of Chinese Mass Spectrometry Society, 2013, 34(5): 257-262(in Chinese).
[18]YANG H, YU Y, SONG F, et al. Structural characterization of neutral oligosaccharides by laser-enhanced in-source decay of MALDI-FTICR MS[J]. Journal of the American Society for Mass Spectrometry, 2011, 22(5): 845-855.
[19]QIU X, CUI M, LI H, et al. Prompt disulfide fragmentations of disulfide-containing proteins in a matrix-assisted laser desorption/ionization source[J]. Rapid Communications in Mass Spectrometry, 2007, 21(21): 3 520-3 525.
[20]BROWN R S, CARR B L, LENNON J J. Factors that influence the observed fast fragmentation of peptides in matrix-assisted laser desorption[J]. Journal of the American Society for Mass Spectrometry, 1996, 7(3): 225-232.
[21]PATTERSON S D, KATTA V. Prompt fragmentation of disulfide-linked peptides during matrix-assisted laser desorption ionization mass spectrometry[J]. Analytical Chemistry, 1994, 66(21): 3 727-3 732.
[22]ZHAO L, ALMARAZ R T, XIANG F, et al. Gas-phase scrambling of disulfide bonds during matrix-assisted laser desorption/ionization mass spectrometry analysis[J]. Journal of the American Society for Mass Spectrometry, 2009, 20(9): 1 603-1 616.
[23]SMARGIASSO N, de PAUW E. Optimization of matrix conditions for the control of MALDI in-source decay of permethylated glycans[J]. Analytical Chemistry, 2010, 82(22): 9 248-9 253.
[24]TRIMPIN S, RDER H J, MÜLLEN K. Investigations of theoretical principles for MALDI-MS derived from solvent-free sample preparation part Ⅰ. preorganization[J]. Internationl Journal of Mass Spectrometry, 2006, 253(1/2): 13-21.
In-Source Decay of Disulfide Bond-Containing Proteins/Peptides by MALDI-TOF MS
YAO Wen-bin1, WANG Yi-han1, SU Rui1, LIAN Wen-hui1, YANG Hong-mei1,2, CHEN Chang-bao1, LIU Shu-ying1
(1.JilinGinsengAcademy,ChangchunUniversityofChineseMedicine,Changchun130117,China;2.ChangchunInstituteofAppliedChemistryChineseAcademyofSciences,Changchun130022,China)
The disulfide bond is one of the most common post-translational modifications in proteins, of which determination is essential to the comprehensive understanding of protein structures. Disulfide bond analysis has gone through great improvement due to the development of matrix-assisted laser desorption/ionization mass spectrometry (MALDI MS), especially in terms of speed and sensitivity. In general, the characterization of disulfide-containing peptides is achieved by the reduction of disulfide bonds followed by alkylation. The identification of disulfide/cysteine-containing peptides in digests of proteins is essential to structure elucidation of a protein. In order to present a deep understanding of some phenomena occurring in MALDI MS of disulfide/cysteine-containing proteins, the current work systematically investigated effects of co-crystal size and matrix on MALDI-In Source Decay (ISD) fragmentation of disulfide-containing proteins. Imaging experiments were performed to evaluate the influence of laser shot location on the fragmentation of human insulin and insulin glargine, which were selected as model compounds. The spectrum that was recorded on very finely distributed crystals of FA spot does not exhibit fragments. While a characteristic ‘triplet’ ions with a mass separation of 32 u generated by both symmetric and nonsymmetric cleavages of the disulfide bonds was observed on large crystals. Probably for thermodynamic reasons, the tryptic peptide was subjected to the cleavage of even number of chemical bonds gave rise to radical recombination without reductive ISD. Among several matrices tested including ferulic acid (FA),α-cyano-4-hydroxycinnamic acid (CHCA), sinapinic acid (SA), 2,5-dihydroxybenzoic acid (DHB), 3-aminoquinolin (AQ), and 2,4,6-trihydroxy acetophenone (THAP), FA was shown to be a versatile matrix allowing one to induce or prevent ISD according to the location of laser shots. CHCA and SA were found to promote ISD of disulfide-containing proteins, in a location dependent manner. However, unlike in CHCA (or SA), ISD was not systematically observed on all crystals for FA, suggesting differences between the crystallization processes of CHCA (or SA) and FA. Minor fragments were observed when using DHB and AQ as matrices. As for THAP, no fragmentation was observed probably because its three OH-groups in meta-position relative to each other resulted in the nonoccurrence of redox reaction. The studies provide insights into the experimental conditions required for determination of disulfide-containing protein by MALDI MS and are helpful for mass spectrum interpretation, opening the way to more rational studies of disulfide/cysteine-containing proteins by MALDI mass spectrometry.
disulfide-containing protein/peptide; MALDI-TOF MS; in-source decay; matrix; crystal
2016-05-06;
2016-09-21
吉林省科技厅项目(20170623026TC,20160101220JC,20160204027YY);吉林省卫生技术创新项目(2016J098)资助
姚文斌(1991—),男(汉族),山西人,硕士研究生,中药化学专业。E-mail: 2390697021@qq.com
陈长宝(1967—),男(汉族),吉林人,研究员,从事中药及生物制药研究。E-mail: ccb2021@126.com
刘淑莹(1943—),女(汉族),黑龙江人,研究员,从事中药化学和有机质谱学研究。E-mail: liusy01@ccucm.edu.cn
时间:2017-04-13;网络出版地址:http:∥www.cnki.net/kcms/detail/11.2979.TH.20170413.0929.012.html
O657.63
A
1004-2997(2017)03-0302-06
10.7538/zpxb.2016.0075
