Role of [18F] fludeoxyglucose positron emission tomography in the selection of liver transplantation candidates in patients with hepatocellular carcinoma
2017-06-19
Hangzhou, China
Role of [18F] fludeoxyglucose positron emission tomography in the selection of liver transplantation candidates in patients with hepatocellular carcinoma
Yu-Fu Ye, Wei Wang, Ting Wang, Jun Yu, Lei Geng, Song-Feng Yu, Sheng Yan and Shu-Sen Zheng
Hangzhou, China
BACKGROUND: The Milan criteria are widely accepted among many centers. However, patients with hepatocellular carcinoma beyond the Milan criteria might still benefit from liver transplantation (LT) when tumor itself is not aggressive. [18F] fluorodeoxyglucose positron emission tomography/computed tomography imaging could provide useful information of tumor behaviors, which is helpful to predict the prognosis for many tumors.
METHOD: In order to determine its role in candidate selection for LT, we therefore retrospectively analyzed 103 recipients with preoperative positron emission tomography (PET) findings.
RESULTS: Positive PET findings (PET+) were significantly associated with tumor nodule numbers (P=0.013), tumor grade (P=0.025), macro- (P=0.002) and micro-vascular invasion (P=0.002), as well as the Milan criteria (P=0.018). PET+patients had significantly increased risk of tumor recurrence post-LT compared to PET negative (PET¯) patients (P=0.007). The 1-, 3-, and 5-year overall survival rate of PET¯ patients were 96.0%, 87.2% and 76.2%, compared to 74.7%, 55.4% and 49.9% in PET+patients, respectively (P<0.05). The 1-, 3-, and 5-year recurrence-free survival rate of PET¯ patients were 91.8%,81.9% and 76.0%, compared to 70.1%, 39.3% and 21.9% in PET+patients, respectively (P<0.05). Recipients within the Milan criteria showed comparable 1-, 3-, and 5-year survival rates in comparison with those beyond the Milan criteria with a PET¯ findings (1-, 3-, and 5-year overall survival rates, 97.5%, 83.3%, and 83.3% vs 90.0%, 80.0%, and 66.7%,P= 0.123; 1-, 3-, and 5-year recurrence-free survival rates, 95.1%, 73.1%, and 73.1% vs 90.0%, 78.8%, and 65.6%,P=0.148).
CONCLUSIONS: Certain patients with hepatocellular carcinoma and negative PET findings, who have exceeded the Milan criteria, are also eligible candidates for LT. Preoperative PET/CT imaging is an important marker, which should be incorporated in extended candidate selection criteria for LT.
(Hepatobiliary Pancreat Dis Int 2017;16:257-263)
liver transplantation; hepatocellular carcinoma; positron emission tomography/computed tomography; prognostic factors
Introduction
Hepatocellular carcinoma (HCC) has been increasingly prevalent throughout the world, ranking the fifth in cancer incidence and the third in cancer mortality.[1]Liver transplantation (LT) is a curative treatment for selected HCC patients, and the prognosis of these patients improved significantly when proper criteria were used. The 4-year tumor-free survival rate is 83% in HCC patients within the Milan criteria after LT.[2]However, high drop-off rate in the waiting list remains a debate that organ allocation according to the Milan criteria was too strict. Salvage LT was selected as a possible way to buffer this situation.[3]Indeed, several expanded criteria, such as the University of California San Francisco (UCSF) criteria, had reported similar survival outcomes compared with the Milan criteria.[4]
The Milan criteria focuses merely on morphological information, which is the key dispute that some scholars criticizes its limitation. Basically, the imaging evaluation itself is more likely to over- or under-estimate the real tumor size.[5]Therefore, morphological information from the Milan criteria, for instance, tumor size or tumor number, is not sufficient to predict prognosis after LT.[6]Recently, several tumor behavior markers, such as microvascular invasion (MVI), were reported to have a remarkable impact on the prognosis of HCC patients after LT.[7]Of note, MVI has a close relationship with early tumor recurrence or poor overall survival after LT.[8]Hence, the identification of tumor behavior markers is also essential for the prognosis. Moreover, the prognostic evaluation requires preoperative biopsy, but seems to be a problem due to the risk of bleeding and potential tumor spread. Besides, this procedure is not routinely performed. In addition, China has a large amount of HCC cases, and many of them need LT.[9]To make the organ allocation more effective, we need more sophisticated tools to predict the prognosis. It is, therefore, of great importance to identify novel methods to evaluate tumor behavior before LT.
[18F] fluorodeoxyglucose positron emission tomography/computed tomography ([18F] FDG PET/CT) has been identified as a useful tool in evaluating and staging malignancy in esophageal carcinoma, lymphoma and colorectal carcinoma.[10-12]This method was also considered effective to identify metastases, and to monitor tumor recurrence.[6]The mechanism of [18F] FDG is the enhanced intake of glucose by the tumor cells. The amount of [18F] FDG intake is associated with the tumor behavior.[13]Preoperative [18F] FDG PET/CT predicts tumor differentiation, MVI and overall survival after liver resection or transplantation in HCC patients.[6,8,14]However, whether preoperative PET imaging plays a role in LT candidate selection is still controversial. We therefore conducted this study to further determine the clinical value of preoperative [18F] FDG PET/CT in prognostic prediction, and verify its role in candidate selection for LT among HCC patients. Our study also provides an evidence of using [18F] FDG PET/CT as anin vivobiomarker to identify patients at risk for HCC recurrence after LT.
Methods
Patients selection
A database of 214 registered HCC patients, who underwent orthotopic liver transplantation (OLT) at our center between January 2006 and June 2013, were analyzed. The diagnosis of HCC was made by at least two abdominal imaging modalities showing consistent results, including ultrasound and CT or magnetic resonance imaging (MRI). Among them, 103 patients who had received [18F] FDG PET/CT scan 2 months before LT were enrolled. The immunosuppressive therapy consisted of a calcineurin inhibitor-based induction regimen, which was augmented by mycophenolate mofetil or azathioprine. All patients received hepatitis B immune globulin (HBIg) therapy and an oral anti-viral agent from the time of transplant. The study protocol was approved by the Institutional Review Board of the First Affiliated Hospital, Zhejiang University School of Medicine. All participants provided their written informed consent to participate in this study.
[18F] FDG PET/CT and image analysis
Whole-body [18F] FDG PET scan was performed before LT, using a Biograph Sensation 16 PET/CT scanner (Siemens, Erlangen, Germany). Patients were fasted for at least 6 hours before the injection of [18F] FDG, and the blood glucose level was less than 8 mmol/L. Based on the study from Kornberg et al, a static emission scan was performed from the neck to the knee level in a 2-dimensional mode after 1 to 1.5 hours of [18F] FDG (360 MBq) injection.[8]When PET scans were done, subsequent CT scan or MRI was performed to locate the tumor. Two nuclear medicine specialists evaluated the coronal, sagittal, and axial PET images independently. The tumor uptake of [18F] FDG was assessed semi-quantitatively using the tumor/background (T/B) ratio. Regions of interest (ROIs) were drawn over the normal liver and the tumor nodules. The maximum standardized uptake values (SUVmax), the mean SUV (SUVmean), and the ratio of tumor SUV-max to normal liver SUVmax were calculated. A receiver operating characteristic (ROC) analysis was performed to define the optimal cut-off for the [18F] FDG intake to predict the prognosis. In the present study, we defined PET positive as a T/B ratio >1.35.
Histopathological study
All explants were examined by a pathologist. The histological grade was assessed according to the modified Edmondson criteria and the final classification was based on the area with the poorest differentiation.[15]MVI was defined as the presence of tumor emboli within any vein such as the central vein, the portal vein, large capsular veins, the lobar or segmental branches of the portal vein or hepatic veins.
Statistical analysis
All analyses were performed using statistical softwareSPSS (version 11.0; SPSS Inc., Chicago, IL, USA). Comparison of categorical and continuous variables was performed using Chi-square test and Mann-WhitneyUtest, respectively. Overall survival and recurrence-free survival were analyzed by using the Kaplan-Meier method. Patient survival rates comparison in different groups were performed using log-rank test. Risk factors identified by univariate analysis were enrolled into multivariate analysis to determine the independent effect, using Cox proportional hazard model (stepwise: conditional). APvalue less than 0.05 was considered statistically significant.
Results
Clinical characteristics of enrolled patients
There were 93 males and 10 females with a mean age of 49.5±8.1 years. Eighty-five patients (82.5%) underwent LT with deceased donors, 18 (17.5%) with living donors. Of 103 patients, 41 (39.8%) were within the Milan criteria, 62 (60.2%) were beyond the Milan criteria. All enrolled patients had HBV-related chronic liver diseases, but only 42 (40.8%) had detectable HBV DNA (>1×103copies) before LT. Complications of liver cirrhosis, such as esophageal/gastric varices, splenomegaly, thrombocytopenia or ascites, were recorded in 90 patients. In the present study, 68 patients (66.0%) had received the preoperative adjuvant therapy, including transcatheter arterial chemoembolization (TACE) in 51 patients, radiofrequency ablation (RFA) in 8 patients or a combination regimen of TACE and RFA in 9 patients. All enrolled patients were routinely followed up postoperatively, and mean duration of the follow-up was 25.7 months. During the follow-up, 53 patients (51.5%) experienced HCC recurrence. The clinical data of all enrolled patients were summarized in Table 1.

Table 1. Patient and tumor characteristics (n, %)
[18F] FDG PET status correlated with prognostic factors for HCC
Among all, 78 patients had positive PET findings (PET+, 75.7%), while 25 patients (PET¯, 24.3%) had no increased [18F] FDG uptake on preoperative PET scans. The PET findings between PET+and PET¯ patients were significantly associated with tumor nodule numbers (P=0.013), tumor grade (P=0.025), macro- (P=0.002) and micro-vascular invasion (P=0.002), as well as theMilan criteria (P=0.018). However, PET findings were not significantly associated with preoperative AFP level (P=0.317), overall tumor diameter (P=0.222) and pre-LT HBV DNA load (P=0.577) (Table 2).
[18F] FDG PET positivity correlated with the risk of HCC recurrence after LT
The mean post-LT follow-up duration ranged from 6.1 to 85 months (median: 25.7). The overall 1-, 3-, and 5-year survival rates and recurrence-free survival rates were 80.1%, 65.2%, 48.4% and 77.5%, 64.4%, 37.5%, respectively. Among 78 patients with PET+findings, 46 had HCC recurrence post-LT (59.0%), which was significantly higher than that in PET¯ group (28.0%). HCC patients with PET+findings had increased risk of tumor recurrence post-LT compared to PET¯ patients (P=0.007) (Table 3). The 1-, 3-, and 5-year recurrence-free survival rates of PET¯ patients were 91.8%, 81.9% and 76.0%, compared to 70.1%, 39.3% and 21.9% in PET+patients (P=0.001). Likewise, the 1-, 3-, and 5-year overall survival rates of PET¯ patients was 96.0%, 87.2% and 76.2%, compared to 74.7%, 55.4% and 49.9% in PET+patients (P=0.001) (Fig. 1). Univariate analysis showed that patients with AFP level ≤400 ng/mL, HBV DNA load ≤1× 103copies (negative), well/moderately tumor differentiation, tumor nodules number ≤3, a total tumor diameter≤8 cm, negative PET findings, within the Milan criteria,no macro- and micro-vascular invasion had a better prognosis. Subsequent multivariate analysis revealed that a PET¯ status, an AFP level ≤400 ng/mL, within the Milan criteria, no macro- and micro-vascular invasion were independent factors of better overall and recurrence-free survival (P〈0.05, Table 4). There was no survival difference between patients in terms of gender (P=0.219), age (P=0.901), or adjuvant therapy (P=0.489).
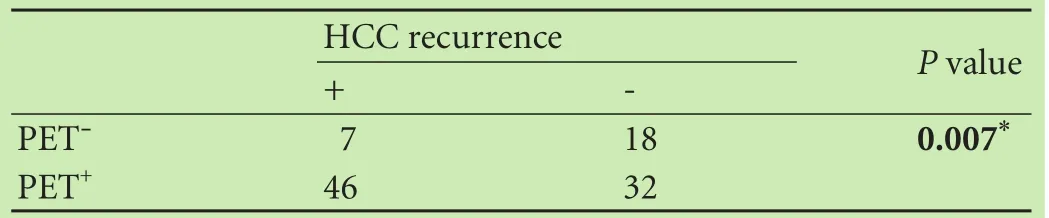
Table 3. Association between tumor recurrence and [18F] FDG PET/CT findings
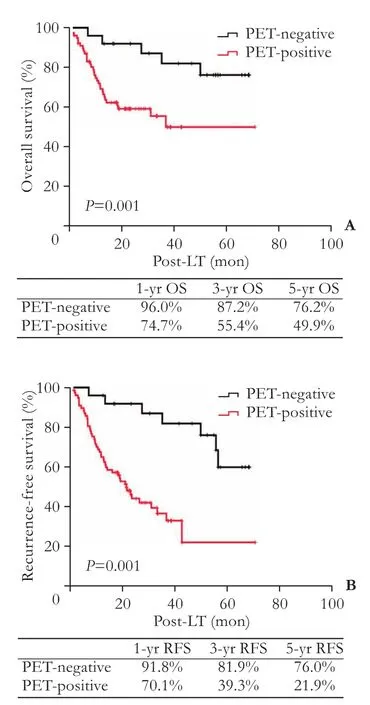
Fig. 1. Overall and recurrence-free survival rates of patients with hepatocellular carcinoma after LT according to the [18F] FDG PET/CT findings.
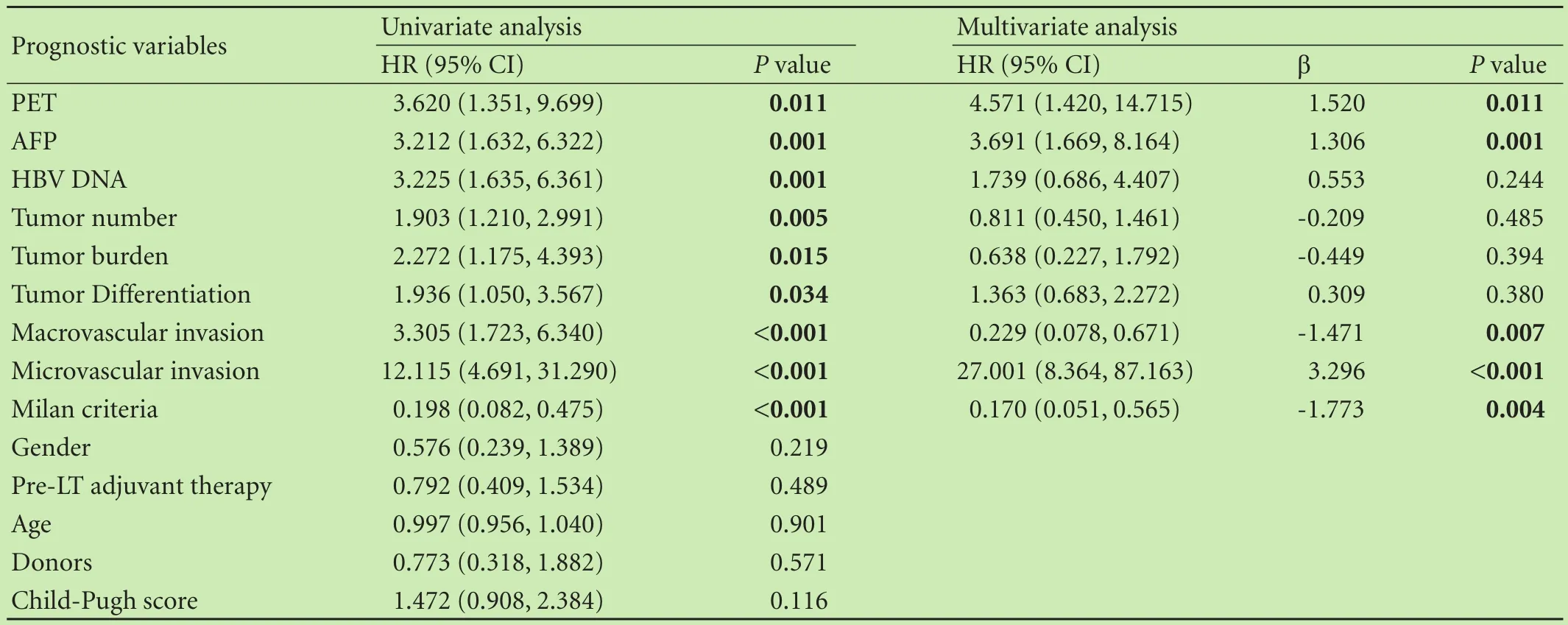
Table 4. Univariate and multivariate analyses of factors related to overall survival, using the Cox proportional hazards model
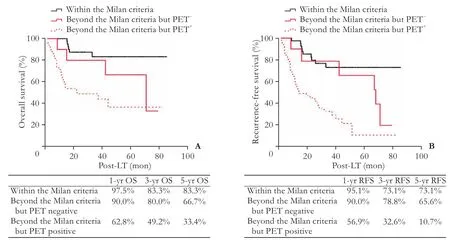
Fig. 2. Overall and recurrence-free survival rates between the recipients within the Milan criteria and those beyond the Milan criteria with a PET-status.
[18F] FDG PET scanning is helpful for LT candidate selection
The 5-year overall survival rate was significantly higher for recipients within the Milan criteria than those beyond the Milan criteria (83.3% vs 32.7%,P〈0.001). Likewise, recipients within the Milan criteria got a better 5-year recurrence-free survival rate compared to those beyond the Milan criteria (73.1% vs 19.0%,P〈0.001). For recipients within the Milan criteria, the incidence of HCC recurrence was higher in PET+patients compared to that in PET¯ patients (33.3% vs 7.1%,P=0.032). For recipients beyond the Milan criteria, the incidence of HCC recurrence was also higher in PET+patients compared to that in PET¯ patients (72.5% vs 54.5%,P=0.005). Subsequent Kaplan-Meier curve showed that PET¯ recipients beyond the Milan criteria had a better 5-year overall survival rate (66.7% vs 33.4%,P=0.044) and recurrence-free rate (65.6% vs 10.7%,P=0.002) than those with PET+. Additionally, PET¯ recipients within the Milan criteria also had a better 5-year recurrencefree survival rate (91.7% vs 64.5%,P〈0.05) compared to those with the PET+. However, 5-year overall survival rates between PET+and PET¯ recipients within the Milan criteria were similar (80.8% vs 91.7%,P=0.233). In particular, recipients within the Milan criteria showed comparable 1-, 3-, and 5-year overall survival rates in comparison with those beyond the Milan criteria with a PET¯ status (1-, 3-, and 5-year overall survival rates, 97.5%, 83.3%, and 83.3% vs 90.0%, 80.0%, and 66.7%,P=0.123; 1-, 3-, and 5-year recurrence-free survival rates, 95.1%, 73.1%, and 73.1% vs 90.0%, 78.8%, and 65.6%,P=0.148, Fig. 2), the 1-, 3-, and 5-year overall survival rats of patients beyond the Milan criteria were significantly higher in PET¯ patients compared with PET+patients.
Discussion
There was a concern that candidates will not benefit from LT if tumors have an aggressive course, even though they meet the Milan criteria, and that HCC patients beyond the Milan criteria might still benefit from LT when the tumor itself is not aggressive. Apart from morphological parameters, other factors, such as AFP, histological grade and vascular invasion, may accurately reflect tumor biological aggressiveness and risk of tumor recurrence following LT, which are also crucial factors affecting candidate selection. Accumulating evidences have demonstrated that MVI has an important impact on tumor recurrence and long-term survival after liver resection or LT.[16,17]However, the information of MVI from HCC patients cannot be acquired preoperatively by using noninvasive methods.
[18F] FDG PET/CT has become a standard procedure for the detection of extrahepatic HCC metastases and pre-operative evaluation of candidates for LT in our and other transplant centers. [18F] FDG competes with glucose to enter the cells, and it is involved in someintracellular enzymatic pathways.[18,19]Due to different enzymatic activities, [18F] FDG metabolism within well differentiated HCC cells equals to the level in normal tissues, whereas poorly differentiated HCC cells take more [18F] FDG and show hot spots on the scans.[19,20]In the present study, an increase of18F-FDG uptakes was signif icantly associated with tumor numbers, high tumor grade, micro- and macro-vascular invasion. Yang et al showed that PET/CT findings were correlated with tumor grade. Of HCC candidates within the Milan criteria, 4 of 6 had HCC recurrence and these patients had PET+findings.[21]Our data also showed that the ratio of poorly differentiated tumors was much higher in PET+patients compared with that in PET¯ patients. Therefore, [18F] FDG PET/CT reflects the tumor behavior. Our study demonstrated that preoperative [18F] FDG PET/CT scanning was useful in the prediction of HCC recurrence among LT candidates. Specifically, we also confirmed that [18F] FDG PET/CT was an independent predictor of tumor recurrence, and [18F] FDG PET/CT positivity was correlated with bad tumor behaviors.
Living donor liver transplantation (LDLT) has a potential advantage over deceased donor liver transplantation (DDLT), because DDLT presents with longer waiting time and higher drop-out rate on the waiting list.[3,22-24]Therefore, LDLT becomes a reliable solution to deal with the reality of organ shortage. However, higher recurrence of tumor in LDLT still remains a debate among scientists. Some arguments are, the patients with biologically more aggressive tumors drop out due to tumor progression when waited for DDLT, which may be the reason that the candidates with DDLT had lower tumor recurrence compared with those with LDLT.[25]Lee et al[22]investigated the predictive value of [18F] FDG PET/CT in 191 patients with LDLT and found that PET positivity was an independent prognostic factor for early tumor recurrence. The present study combined LDLT and DDLT and did not find the correlation between the donor type and recurrence-free survival (P=0.569).
In 2009, Kornberg et al reported the role of [18F] FDG PET/CT in identifying eligible candidates for LT.[8]The authors indicated that PET negative patients could be selected for LT cautiously, because patients with tumors beyond the Milan criteria and negative PET scans achieved comparable recurrence-free survival compared to those with tumors within the Milan criteria.[8]In 2012, the same group investigated the predictive role of [18F] FDG PET/CT in a larger cohort and found that there was no significant difference in terms of 5-year recurrencefree survival between patients within the Milan criteria (86.2%) and those with PET¯ findings and beyond the Milan criteria (81%).[19]In view of this, should candidates beyond the Milan criteria but with PET¯ findings be considerable for LT? The present study validated Kornberg’ s theory and found that the 1-, 3-, and 5-survival rates were comparable between the patients whose HCC were within the Milan criteria and those whose HCC were beyond the Milan criteria but PET was negative. The present study emphasized the importance of combining tumor morphology with biological information in selecting eligible HCC candidates for LT.
This study was limited to the patients with HBV-related HCC. Therefore, the data are not applicable to patients with HCV-related HCC. Another limitation was the retrospective nature and a single transplant center, and selection bias might have an impact on the survival data. Further multi-center, prospective cohort study is needed to verify the findings.
In conclusion, [18F] FDG PET/CT not only excludes potential tumor metastasis during preoperative assessment for LT, but also is a predictor of tumor recurrence after LT. Certain candidates who have negative PET findings should be considered for LT although they exceed the Milan criteria. [18F] FDG PET/CT imaging, same as AFP, vascular invasion and the Milan criteria, is an independent indicator of patient prognosis after LT.
Acknowledgments:The authors would like to thank Lin Zhang for her excellent work on the daily maintenance and management of transplant database.
Contributors:ZSS proposed the study. YYF and WW performed the research and wrote the first draft. WT collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. YYF and WW contributed equally to this article. ZSS is the guarantor.
Funding:This study was supported by grants from the National Natural Science Foundation of China (91542205, 81400673 and 81470893), Specialized Research Fund for the Doctoral Program of Higher Education (20130101120125), and Zhejiang Provincial Natural Science Foundation (LY14H03003).
Ethical approval:The study protocol was approved by the Institutional Review Board of the First Affiliated Hospital, Zhejiang University School of Medicine. All participants provided their written informed consent to participate in this study.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365: 1118-1127.
2 Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-699.
3 Hu Z, Wang W, Li Z, Ye S, Zheng SS. Recipient outcomes ofsalvage liver transplantation versus primary liver transplantation: a systematic review and meta-analysis. Liver Transpl 2012;18:1316-1323.
4 Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-1403.
5 Taouli B, Krinsky GA. Diagnostic imaging of hepatocellular carcinoma in patients with cirrhosis before liver transplantation. Liver Transpl 2006;12:S1-7.
6 Asman Y, Evenson AR, Even-Sapir E, Shibolet O. [18F]fludeoxyglucose positron emission tomography and computed tomography as a prognostic tool before liver transplantation, resection, and loco-ablative therapies for hepatocellular carcinoma. Liver Transpl 2015;21:572-580.
7 Figueras J, Ibañez L, Ramos E, Jaurrieta E, Ortiz-de-Urbina J, Pardo F, et al. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: results of a multicenter study. Liver Transpl 2001;7:877-883.
8 Kornberg A, Freesmeyer M, Bärthel E, Jandt K, Katenkamp K, Steenbeck J, et al.18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant 2009;9:592-600.
9 Wang W, Ye Y, Wang T, Zhang F, Geng L, Yu J, et al. Prognostic prediction of male recipients selected for liver transplantation: With special attention to neutrophil to lymphocyte ratio. Hepatol Res 2016;46:899-907.
10 Guo H, Zhu H, Xi Y, Zhang B, Li L, Huang Y, et al. Diagnostic and prognostic value of18F-FDG PET/CT for patients with suspected recurrence from squamous cell carcinoma of the esophagus. J Nucl Med 2007;48:1251-1258.
11 Carrillo-Cruz E, Marín-Oyaga VA, de la Cruz Vicente F, Borrego-Dorado I, Ruiz Mercado M, Acevedo Báñez I, et al. Role of18F-FDG-PET/CT in the management of marginal zone B cell lymphoma. Hematol Oncol 2015;33:151-158.
12 Delbeke D, Martin WH. FDG PET and PET/CT for colorectal cancer. Methods Mol Biol 2011;727:77-103.
13 Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, et al. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol 2000;32: 792-797.
14 Lin CY, Chen JH, Liang JA, Lin CC, Jeng LB, Kao CH.18F-FDG PET or PET/CT for detecting extrahepatic metastases or recurrent hepatocellular carcinoma: a systematic review and metaanalysis. Eur J Radiol 2012;81:2417-2422.
15 Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48 900 necropsies. Cancer 1954;7:462-503.
16 Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 2011;254:108-113.
17 Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11-22.
18 Fass L. Imaging and cancer: a review. Mol Oncol 2008;2:115-152.
19 Kornberg A, Küpper B, Tannapfel A, Büchler P, Krause B, Witt U, et al. Patients with non-[18F]fludeoxyglucose-avid advanced hepatocellular carcinoma on clinical staging may achieve longterm recurrence-free survival after liver transplantation. Liver Transpl 2012;18:53-61.
20 Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging 1999;2:159-171.
21 Yang SH, Suh KS, Lee HW, Cho EH, Cho JY, Cho YB, et al. The role of (18)F-FDG-PET imaging for the selection of liver transplantation candidates among hepatocellular carcinoma patients. Liver Transpl 2006;12:1655-1660.
22 Lee SD, Kim SH, Kim YK, Kim C, Kim SK, Han SS, et al. (18)FFDG-PET/CT predicts early tumor recurrence in living donor liver transplantation for hepatocellular carcinoma. Transpl Int 2013;26:50-60.
23 Sarasin FP, Majno PE, Llovet JM, Bruix J, Mentha G, Hadengue A. Living donor liver transplantation for early hepatocellular carcinoma: A life-expectancy and cost-effectiveness perspective. Hepatology 2001;33:1073-1079.
24 Cheng SJ, Pratt DS, Freeman RB Jr, Kaplan MM, Wong JB. Living-donor versus cadaveric liver transplantation for nonresectable small hepatocellular carcinoma and compensated cirrhosis: a decision analysis. Transplantation 2001;72:861-868.
25 Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol 2008;15:1001-1007.
October 4, 2016
Accepted after revision January 25, 2017
Author Af filiations: Division of Hepatobiliary and Pancreatic Surgery, First Af filiated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Ye YF, Wang W, Wang T, Yu J, Geng L, Yu SF, Yan S and Zheng SS); Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health; Collaborative Innovation Center for Diagnosis Treatment of Infectious Diseases, Hangzhou 310003, China (Ye YF and Zheng SS)
Prof. Shu-Sen Zheng, MD, PhD, FACS, Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, First Affiliated Hospital, Zhejiang University School of Medicine; Collaborative Innovation Center for Diagnosis Treatment of Infectious Diseases, Hangzhou 310003, China (Tel/Fax: +86-571-87236466; Email: shusenzheng@ zju.edu.cn)
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(17)60011-0
Published online May 3, 2017.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Patients with early recurrence of hepatocellular carcinoma have poor prognosis
- Combined cavo-atrial thrombectomy and hepatectomy in hepatocellular carcinoma
- Traditional surgical planning of liver surgery is modified by 3D interactive quantitative surgical planning approach: a single-center experience with 305 patients
- Hepatobiliary & Pancreatic Diseases International
- Crosstalk of liver immune cells and cell death mechanisms in different murine models of liver injury and its clinical relevance
- Circulating autoantibodies to endogenous erythropoietin are associated with chronic hepatitis C virus infection-related anemia
