3,4-二羟基丁酸及其内酯的合成研究进展
2017-06-05董润安
高 玉, 董润安
3,4-二羟基丁酸及其内酯的合成研究进展
高 玉, 董润安
(北京理工大学生命学院,北京,100081)
3,4-二羟基丁酸(3,4-dihydroxybutyric acid, 3,4-DHBA)及其内酯3-羟基-γ-丁内酯(3-hydroxy-γbutyrolactone, 3HBL)均是重要的手性C4平台化合物,它们是许多天然产物合成的重要原料,也是重要的医药中间体。3,4-二羟基丁酸及其内酯目前的合成方法主要为化学合成,但因其较低的产率﹑严苛的反应条件﹑无法避免的副产物生成和环境污染等缺点使其难以实现大规模生产。本综述对近年来3,4-二羟基丁酸及其内酯的相关合成研究进行总结,详细论述了相关化学合成法,同时总结了现有的生物合成3,4-二羟基丁酸及其内酯的研究进展。
3,4-二羟基丁酸;3-羟基-γ-丁内酯;化学合成;生物合成
1 3,4-二羟基丁酸及其内酯简介
3,4-二羟基丁酸(3,4-dihydroxybutyric acid, 3,4-DHBA)是一种易溶于水﹑乙醇﹑乙醚的手性化合物,通过对其分子结构中的羧基和羟基进行修饰可形成许多有价值的衍生物[1],如可合成抗生素[2],β-和α-氨基酸和多肽[3,4]等,或是作为手性合成的基础材料。3,4-DHBA可通过简单酯化形成环状的内酯物质3-羟基-γ-丁内酯(3-hydroxy-γ-butyrolactone, 3HBL),该内酯也是一种非常重要的手性C4化合物,它可用于合成各种药物﹑聚合物和溶剂。例如制备降血脂药物阿托伐他汀[5]﹑神经介质L-肉毒碱[6]﹑HIV蛋白酶抑制剂氨普那韦[7]﹑饱感剂(2S,4S)-2-羟基-4-羟甲基-4-丁内酯[8]﹑皮肤病药物羟基二十碳四烯酸 (12-HETE)[9]和抗癌药aplysistatin[10]等,其中阿托伐他汀是世界范围内销售量最大的处方药,其年销售额超过100亿美元。将(S)-3-羟基-γ-丁内酯还原可得(S)-3-羟基四氢呋喃,后者是治疗艾滋病药物的一种重要中间体;将(S)-3-羟基-γ-丁内酯转化为(S)-5-羟甲基-1,3-唑啉-2-酮,可得最新一代的抗菌药物[11]。此外,由3-羟基-γ-丁内酯经简单转换得到的(s)-N-甲基-3-羟基吡咯和(R)-N-甲基-3-甲氮基吡咯也都具有重要的生理活性[11]。由于(S)-3-羟基-γ-丁内酯广泛的用途,其被美国能源部定义为最具价值的化合物之一[12]。

图1.3,4-二羟基丁酸及其内酯的应用Fig 1.Application of 3,4-dihydroxybutyric acid and 3-hydroxy-γbutyrolactone
3,4-dihydroxybutyric acid:3,4-二羟基丁酸;thienamycin:噻嗯霉素;a-amino acids :a-氨基酸;β-amino acids:β-氨基酸;peptides:多肽类;3-hydroxyγ-butyrolactone:3-羟基-γ-丁内酯;aplysistatin:抗癌药物单环金合欢烷类;12-HETE:羟基二十碳四烯酸;Amprenavir:氨普那韦;Lipitor:阿托伐他汀;L-carnitine:L-肉毒碱;(S)/(R) -N-methyl-3-hydroxypyrrole:(s)/(R)-N-甲基-3-羟基吡咯;(2S, 4S)-2-hydroxy-4-hydroxymethyl-4-butyrolactone:(2S,4S)-2-羟基-4-羟甲基-4-丁内酯;(S)-3-hydroxytetrahydrofuran:(S)-3-羟基四氢呋喃.
2 3,4-二羟基丁酸及其内酯的合成研究现状
2.1 化学法合成3,4-二羟基丁酸及其内酯
有关3,4-二羟基丁酸的化学合成报道主要以专利为主,如Chem[13]首先通过降解4位含取代基的葡萄糖化合物如4-O-甲基-D-葡萄糖﹑麦芽糖﹑支链淀粉和纤维素等,取代4位碳上的基团形成2-羰基化合物即4-脱氧-2,3-邻酮己糖,之后利用该二羰基化合物与碱反应即可形成3,4-DHBA,但产量较低(图2)。Chem[14]继续改进该方法使二羰基化合物与过氧化氢反应以生成3,4-DHBA,但会伴有副产物乙醇酸生成,而且该反应中由于互变异构作用会有少量3,4-DHBA的同分异构体存在;同时由于过度氧化,目标物3,4-DHBA会被降解形成甲酸和乙醇酸使得该方法不具备工业价值。利用类似的方法以二羰基化合物作为中间代谢物制备3,4-DHBA的技术方法还有很多,但据报道产率都很低约为30%。而且在这些合成方法中,除3,4-二羟基丁酸外还会生成多种副产物,包括乙醇酸﹑异糖酸﹑甲酸﹑酮和二酮等。另外,若以二糖如麦芽糖或乳糖作为底物的情况下,二糖中仅一个糖单元会形成3,4-二羟基丁酸,而另一个糖单元官能团作为离去基团不参与3,4-DHBA的合成而与目标产物共存形成1:1混合物,因此要从反应混合物中分离和纯化3,4-二羟基丁酸是非常困难的。同时由于3,4-二羟基丁酸的产率非常低,这些方法并不适合于工业使用。
之后Cho 等人[15]利用特定酶将市售的支链淀粉制备成具有结构特异性的寡糖以制备光学纯的(S)-3,4-二羟基丁酸及其相关衍生物,但反应过程较复杂,且反应时间长。Hollingsworth等人[16]利用葡萄糖和碱金属氢氧化物作为反应物,在双氧水存在的情况下经70℃加热反应24小时获得3,4-DHBA,因该过程产率低,反应时间长,并不存在实际生产的可能性。或是将R-3-氯-1,2-丙二醇经氰化和水解直接形成3,4-DHBA,缺点是该反应过程中当反应温度高于所需温度时会生成大量副产物,如3,4-二羟基丁腈,3,4-二羟基丁酸的氨基化合物等[17]。
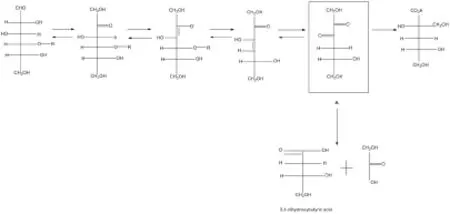
图2.通过形成羰基基团化学合成3,4-二羟基丁酸[14]Fig 2.Reacting the formed dicarbonyl compound to chemical synthesis 3,4-dihydroxybutyric acidA: 4-deoxy-2,3-hexodiulose, 4-脱氧-2,3-邻酮己糖;3,4-dihydroxybutyric acid:3,4-二羟基丁酸
而3-羟基-γ-丁内酯由于其广泛的用途,近年来国内外有关它的合成报道较多,主要包括以下8种:(1)以L-苹果酸为原料,制成二甲酯后用LiBH4 选择性还原制得3HBL,收率可达到90%[18];(2)以(S)-4-氯-3-羟基丁腈为原料,酸性条件水解后在强碱条件下环化生成3HBL,收率可达88.8%[19];(3)以(2R,3R)- 2,3-二羟基-γ-丁内酯为原料,将其溶于二氯甲烷后经冷却到0℃,加入有机缚酸剂,经过滤除去固体,之后经蒸除溶剂﹑加碱﹑加有机溶剂﹑脱氢﹑减压蒸馏最终制得3HBL[20];(4)以4-苄氧基- 3-羟基丁酸酯为原料,不对称加氢后在酸性条件下成环生成目标产物,收率可达88.8%[21];(5)以(S)-碱为原料,在碱性的极性溶剂中100~190℃下反应0.5~5h,即可得到3HBL,收率为82%[22];(6)以(S)- 4-卤代-羟基丁酸酯为原料,在含水溶剂中回流即可得到3HBL,收率为75.1%[23];(7)以D-异抗坏血酸为原料,经六步反应最后在盐酸条件下成环生成3HBL,收率为40%[24];(8)以异己糖源为原料,也可使用乳糖﹑麦芽糖或一些低聚物糖类[25,26],在一定条件下经氧化形成3HBL。以上方法中,路线1中的还原合成法需在固定床反应器内以金属钌作为催化剂对L-苹果酸高压加氢来合成3HBL,工序复杂危险,并需要昂贵的催化剂和纯化过程,目前已经工业化,年产量约120吨[27,28];路线8采用价格便宜﹑原料易得的乳糖作为起始原料合成了(S)-3 -羟基-γ-丁内酯,收率达44.5%[29],该路线的不同之处在于原料简单易得,具有一定的工业化前景,但该路线容易出现糖源过度氧化问题,且副产物二羰基化合物也难以分离。
综上所述,可见3,4-二羟基丁酸及其内酯的化学合成法虽多,但这些方法都存在一些难以避免的缺点,例如催化剂价格昂贵[15,18]﹑反应条件严苛[16,19]﹑过程不易控制[14,15]﹑产量较低[13,14]或是不可避免的副产物的形成[13,17]﹑及后续处理繁杂等问题[14]。因此,亟需开发一种反应条件温和﹑原材料安全易得且副产物较少的3,4-二羟基丁酸及其内酯的合成方法。
2.2 生物法合成3,4-二羟基丁酸及其内酯
合成生物学是二十一世纪发展最快的学科之一,利用其进行微生物代谢改造,可以合成许多非天然﹑高附加值的化合物。生物合成法通过操作微生物的遗传密码,调整细胞代谢网络,重新配置代谢流,在温和条件下有效利用可再生生物质资源[30],广泛受到研究人员和政府的重视。
2013年,麻省理工大学化学工程系Martin 等人[31]以葡萄糖和一些可提供酰基辅酶A的前体物质如丁酸盐﹑异丁酸盐﹑丙酸盐和乙醇酸等作为原料,构建了以3-羟基酸作为平台化合物合成3,4-二羟基丁酸和其他多种平台化合物的方法。我们已知羟基酸是一类通用的手性化合物,可用于合成多种极具价值的化合物,据已有文献报道,3-羟基丁酸(3HB)[32]和3-羟基戊酸酯(3HV)[33]可通过生物合成途径成功合成。在该合成通路中,3HB和3HV的合成起始于两个乙酰CoA分子﹑或乙酰CoA和丙酰CoA的缩合,该过程由硫解酶PHaA 或BktB催化;随后生成的β-酮酰基辅酶A分别被3-羟基丁酰CoA还原酶PHaB或Hbd还原成(R)或(S)型的对映异构体醇类,继续经磷酸转乙酰酶Ptb 和丁酸盐激酶 Buk[32]或硫解酶ⅡTesB[34,35]水解即可产生各种游离酸。需要注意的是在该过程中TesB可以水解3HB-CoA的所有异构体,而Ptb-Buk酶系只特异性针对(R)型异构体[36]。Martin等人从上述生物合成3HB和3HV的通路中受到启发,希望通过改造该通路以合成更多更具价值的3-羟基酸衍生物,如3,4-DHBA。如图3所示,他们利用来自埃氏巨球型菌(Megasphaera elsdenii)中具有广泛底物特异性的乙酰CoA转移酶(Pct)[37,38]或来自于鼠伤寒沙门菌(Salmonella typhimurium LT2)的乙酰或丙酰CoA合成酶(PrpE)[39]对前体物质进行反应使其可提供酰基辅酶A,之后利用3-羟基酸途径来缩合乙酰CoA和乙醇酰CoA。根据生物体内不同通路的酶组合对最终产物效率高低的影响,Pct﹑BktB﹑PHaB 和 TesB被认为是最高效的酶组合,利用该通路可直接合成五种通用物质,包括3,4-DHBA﹑3羟基丁酸(3HB)﹑3-羟基戊酸(3HV)﹑3-羟基己酸(3HH)和3-羟基-4-甲基戊酸(3H4MV),其中合成了555±52 mg/L的3,4-DHBA,而3,4-DHBA经过酸处理后可产生221±15 mg/L的3HBL。
2014年,该课题组Dhamankar等人继续优化该通路,通过利用乙酰CoA转移酶Pct将CoA转移到乙醇酸上,之后经缩合和立体定向还原形成4-羟基-3-酮丁基CoA,该中间代谢物可经硫解酶TesB作用直接生成3,4-DHBA,最终同时合成了0.7 g/ L的3,4-DHBA和0.3 g/L的3HBL[40]。该通路中的乙醇酸可由重组Escherichia coli菌株在代谢过程中合成,不需要外源添加,提高了菌株的合成效率。
随后Cheong等人[41]又发现在细菌体内利用非脱羧的克莱森反应,以ω-功能化引物和α-功能化的延伸基团为底物经多步反应可合成多种极具功能的小分子化合物,其中若以乙醇酰基为引物,乙酰辅酶A作为延伸基团可经Pct,BktB和 PhaB1等相关酶催化反应产生0.35 g/L的3-羟基-γ-丁内酯,相较于以葡萄糖作为底物的生物合成通路,3HBL的产量稍有提高。
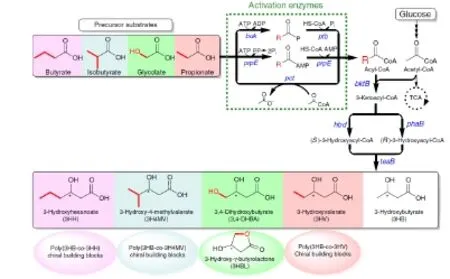
图3.3-羟基羧酸生物合成途径[31]Fig 3.Schematic representation of the 3-hydroxyacid pathway Butyrate:丁酸盐;Isobutyrate:异丁酸盐;Glycolate:乙醇酸;Propionate:丙酸盐;Glucose:葡萄糖;3-Ketoacyl-CoA:3-酮酰基辅酶A;(S)-3-Hydroxyacyl-CoA:(S)-3-羟丁酰辅酶A;(R)-3-Hydroxyacyl-CoA:(R)-3-羟丁酰辅酶A;3-Hydroxybutyrate(3HB):3-羟基丁酸;3-Hydroxyvalerate(3HV):3-羟基戊酸;3-Hydroxy-4-methylvalerate(3H4MV):3-羟基-4甲基戊酸;3-Hydroxyhexanoate(3HH):3-羟基己酸Ptb: 磷酸转乙酰酶;Buk: 丁酸盐激酶;PrpE: 乙酰COA或丙酰CoA合成酶;Pct: 乙酰CoA转移酶;BktB: 硫解酶;Hbd: 3-羟基丁酰CoA还原酶;PhaB: 3-羟基丁酰CoA还原酶;TesB:硫解酶
在合成生物学研究中,木质纤维素作为自然界产量最丰富的可再生能源在代替石油资源方面被广泛利用。其中木糖作为木质纤维素原料中第二丰富的糖类被广泛用于合成各种生物制品,如1,4-丁二醇[42]﹑中康酸酯[43]﹑D-1,2,4-丁三醇[44]﹑乙醇[45]﹑乙二醇[46]等。因此,相关研究者转而开始研究是否可以利用更为丰富的木糖来生物合成3,4-DHBA及其内酯。北京化工大学化学工程系Yajun Yan团队[47]利用木糖为底物通过人为设计构建了一条可经五步反应直接生成3,4-DHBA的合成通路。在该通路中木糖经木糖脱氢酶(XDH)﹑D-硅藻糖基酶(XL)和D-木糖苷脱水酶(XD)的逐步催化转化为2-酮-3-脱氧-D-戊酮糖酸,该物质接着被酮酸脱羧酶(KDC)脱羧形成3,4-二羟基丁醛,之后在合适的醛脱氢酶(ALDH)作用下转化为3,4-二羟基丁酸(如图4所示)。通过优化该通路相关基因和敲除竞争途径最终产生了1.27 g/L的3,4-DHBA,这是目前国际上关于生物合成3,4-DHBA的最高产量。
3.展望

图4.利用大肠杆菌体内构建的以木糖为底物生物合成3,4-DHBA的新通路[47]Fig.4.A novel biosynthetic pathway for the production of 3,4-DHBA from D-xylose in E.coli.D-xylose: D-木糖; D-xylonolactone: D-己酸内酯; D-xylonate: D-木糖苷;2-keto-3-deoxy-D-xylonate:2-酮-3-脱氧-D-戊酮糖酸; 3,4-dihydroxybutanal:3,4-二羟基丁醛; 3,4-dihydroxybutyric acid:3,4-二羟基丁酸.xylA: D-木糖异构酶; yagE or yjhH: 醛缩酶; XDH: 木糖脱氢酶; XL: D-硅藻糖基酶; XD: D-木糖苷脱水酶;KDC: 酮酸脱羧酶; ALDH: 醛脱氢酶.
由于3,4-二羟基丁酸及其内酯的应用越来越广泛,全球范围内对其的需求持续增长,因此,亟需找到一种既经济安全又高产的3,4-二羟基丁酸及其内酯的生产方法。通过微生物生物合成来生产3,4-二羟基丁酸及其内酯是较好的选择,但上述无论是利用代谢工程改造的大肠杆菌以葡萄糖作为底物异源生物合成3,4-DHBA和3HBL的研究,或是通过克莱森酯缩合反应产生3HBL的反应,都存在反应步骤多﹑产率低﹑副产物多等难以实现大规模生物化生产的瓶颈。而最新的以木糖为底物经五步催化反应生物合成3,4-DHBA的通路在克服了上述通路现有的瓶颈后,构建了以更为丰富的木糖为原料生物合成3,4-DHBA的通路,且该过程副产物少,产量较高,为实现3,4-二羟基丁酸及其内酯的大规模生产奠定了基础。
[1] Chen G Q, Wu Q.Microbial production and applications of chiral hydroxyalkanoates[J].Applied Microbiology and Biotechnology, 2005, 67(5): 592-599.
[2] Chiba T, Nakai T.A synthetic approach to (+)-thienamycin from methyl (R)-3-hydroxybutanoate.A new entry to (3R, 4R)-3-((R)-1-hydroxyethyl)-4-acetoxy-2-
azetidinone[J].Chemistry Letters, 1985, 5: 651-654.
[3] Park S H, Lee S H, Lee S Y.Preparation of optically active β-amino acids from microbial polyester polyhydroxyalkanoates[J].Journal of Chemical Research, 2001, 11: 498-499.
[4] Seebach D, Albert M, Arvidsson P I.From the biopolymer PHB to biological investigations of unnatural β-and γ-peptides[J].CHIMIA International Journal for Chemistry, 2001, 55(4): 345-353.[5] Brower P L,Butler D E,Deering C, et al.The synthesis of (4R-cis)-1,1-dimethylethyl 6-cyanomethyl-2,2-dimethyl-1,3-dioxane-4-acetate, a key intermediate for the preparation of CI-981, a highly potent, tissue selective inhibitor of HMGCoA reductase[J].Tetrahedron Letters, 1992, 33(17):2279-2282.
[6] Larcheveque M.Enantiomerically pure β,γ-epoxyeters from β-hydroxylactones: Synthesis of β-hydroxyesters and (-)-GABOB[J].Tetrahedron, 1990, 46(12): 4277-4282.
[7] Kim E E, Baker C T, Dwyer M D, et al.Crystal structure of HIV-1 protease in complex with VX-478, a potent and orally bioavailable inhibitor of the enzyme[J].Journal of the American Chemical Society, 1995, 117(3): 1181-1182.
[8] Uchikawa O, Okukado N, Sakata T, et al.Synthesis of (S) and (R)-3-hydroxy- 4-butanolide and (2S,4S)-, (2R,4S)-,(2S,4R)-and(2R,4R)-2-hydroxy-4- (hydroxymethyl)-4-butanolide and their satiety and hungermodulating activities[J].Bulletin of the Chemical Society of Japan, 1988, 61(6): 2025-2029.
[9] Corey E J, Niwa H, Knolle J.Total sythesis of (S)-12-hydroxy-5,8,14-cis,-10-trans-eicosatetraenoic acid (Samuelesson’s HETE)[J].Journal of the American Chemical Society, 1978, 100(6): 1942-1944.
[10] Shieh H M, Prestwich G D.Chiral, biomimetic total synthesis of (-)-aplysistatin[J].Tetrahedron Letters, 1982, 23(45):4643-4646.[11] Inoue K, Matsumoto M.Method for preparing optically active 3,4-dihyoxybuthric acid derivatives:US,US4994597[P].1991.
[12] Werpy T, Peterson G.Top Value Added Chemicals from Biomass, Vol 1: Results of Screening for Potential Candidates from Sugars and Synthesis Gas[M].US: The United States Department of Energy,2004:1-76.
[13] Machell G, Richards G N.Mechchanism of saccharinic acid formation part I .competing reactions in the alkaline degradation of 4-O-methyl-D-glucose,maltose,amylase,and cellulose[J].Journal of the Chemical Society, 1960, 384:1924-1931.
[14] Machell G, Richards G N.Mechchanism of saccharinic acid formation part II .the apdicarbonyl intermediate in formation of D-glucoisosaccharinic acid[J].Journal of the Chemical Society, 1960, 385:1932-1937.
[15] Cho Y H, Chun J, Park Y M, et al.Process for preparing optically pure (S)-3,4-dihydroxybutyric acid derivatives:US,US 6221639B1[P].2001.
[16] Hollingsworth R I.Process for the preparation of 3,4-dihydroxybutanoic acid and salts thereof: US, US 5292939A[P].1994.
[17] Inoue K, Matsumoto M, Takahashi S.Method for preparing optically active 3,4-dihydroxybutyric acid derivatives: US, US4994597[P].1991.
[18] Saito S, Hasegawa T, Inaba M.Combination of boranedimethyl sulfide complex with catalytic sodium tetrahydroborate as a selective reducing agent for a-hydroxy esters.Versatile chiral building block from (S)-(-)-malic acid[J].Chemistry Letters, 1984, (8): 1389-1392.
[19] Nakagawa A, Idogaki H, Kato K, et al.Improvement on production of (R)-4-chloro-3-hydroxybutyrate and (S)-3-hydroxyγ-butyrolactone with recombinant Escherichia coli cells[J].Journal of bioscience and bioengineering, 2006, 101(2): 97-103.
[20] Suzuki T, Idogaki H, Kasai N.Dual production of highly pure methyl (R)-4-chloro-3-hydroxybutyrate and (S)-3-hydroxygamma-butyrolactone with Enterobacter sp[J].Enzyme and Microbial Technology, 1999, 24:13-20.
[21] Kumar P, Deshmukh A N, Upadhyay R K, et al.A simple and practical approach toenantiomerically pure (S)-3-hydroxy-γbutyrolactone: synthesis of (R)-4-cyano-3-hydroxybutyric acid ethylester[J].Tetrahedron Asymmetry, 2005, 16(16): 2717-2721.
[22] Kumar S, Kurur N D, Chawla H M, et al.[J].Synthetic Communications, 2001, 31(5):775.
[23]Yong L I, Wang J S, Wang Q, et al.[J].Chinese Chemical Letters, 2004, 15(4):400~403.
[24] Tanaka A, Yamashita K.A novel synthesis of (R)- and (S)-4-hydroxytetrahydrofuran-2-ones[J].Synthesis, 1987, (6):570-576.
[25] Jacks T E, Butler D E.Process for the synthesis of protected esters of (S)-3, 4-dihydroxybutyric acid: US.US 5998633[P].1999.[26] 千钟弻, 赵翼行.制备光学纯(S)-3-羟基-γ-丁内酯的连续方法: CN1166782 [P].2001.
[27] Agranat I, Caner H, Caldwell J.Putting chirality to work: the strategy of chiral switches[J].Nature Reviews Drug Discovery, 2002, 1(10): 753-768.
[28] Kwak B S, Chung K N, Kim T Y, et al.Continuous process for the production of optically pure (S)-β-hydroxy-γ-butyrolactone: US.US 10528246[P].2002.
[29] 章小波, 蒋永祥, 汪劲松.(S)-3-羟基-γ-丁内酯的合成[J].精细化工中间体, 2004, 35(3):25-26.
[30] Xu P, Bhan N, Koffas M A G.Engineering plant metabolism into microbes: from systems biology to synthetic biology[J].Current Opinion in Biotechnology, 2013, 24:291-299.
[31] Martin C H, Dhamankar H, Tseng H C, et al.A platform pathway for production of 3-hydroxy acids provides a biosynthetic route to 3-hydroxy-gamma-butyrolactone[J].Nature Communications, 2013, 4:17-27.
[32] Gao H J, Wu Q, Chen G Q.Enhanced production of D-(-)-3-hydroxybutyric acid by recombinant Escherichia coli[J].FEMS Microbiology Letters, 2002, 213:59-65.
[33] Tseng H C, Harwell C L, Martin C H, et al.Biosynthesis of chiral 3-hydroxyvalerate from single propionate-unrelated carbon sources in metabolically engineered E.coli[J].Microbial Cell Factories, 2010, 9:96-104.
[34] Liu S J, Steinbuchel A.Exploitation of butyrate kinase and pHospHo transbutyrylase from Clostridium acetobutylicum for the in vitro biosynthesis of poly (hydroxyalkanoic acid)[J].Applied Microbiology and Biotechnology, 2000, 53:545-552.
[35] Naggert J, Narasimhan M L, DeVeaux L, et al.Cloning, sequencing, and characterization of Escherichia coli Thioesterase-II[J].Journal of Biological Chemistry, 1991, 266:11044-11050.
[36] Liu Q, Ouyang S P, Chung A, et al.Microbial production of R-3-hydroxybutyric acid by recombinant E.coli harboring genes of phbA,phbB, and tesB[J].Applied Microbiology and Biotechnology, 2007, 76:811-818.
[37] Schweiger G, Buckel W.On the dehydration of (R)-lactate in the fermentation of alanine to propionate by clostridiumpropionicum[J].FEBS Letters, 1984, (171):79-84.
[38] Taguchi S, Yamada M, Matsumoto K, et al.A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme[J].Proceedings of the National Academy of Sciences, 2008, 17323-17327.
[39] Liu X W, Wang H H, Chen J Y, et al.Biosynthesis of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by recombinant Escherichia coli harboring propionyl-CoA synthase gene (prpE) or propionate permease gene(prpP)[J].Biochemical Engineering Journal, 2009, 43: 72-77.
[40] Dhamankar H, Tarasova Y, Martin C H, et al.Engineering E.coli for the biosynthesis of 3-hydroxy-γ-butyrolactone (3HBL) and 3, 4-dihydroxybutyric acid (3, 4-DHBA) as value-added chemicals from glucose as a sole carbon source[J].Metabolic engineering, 2014, 25: 72-81.
[41] Cheong S, Clomburg J M, Gonzalez R.Energy and carbon efficient synthesis of unctionalized small molecules in bacteria using non-decarboxylative Claisen condensation reactions[J].Nature Biotechnology, 2016, 34(5):556.
[42] Tai Y, Xiong M, Jambunathan P, et al.Engineering nonphosphorylative metabolism to generate lignocellulose-derived products[J].Nature Chemical Biology, 2016, 12:247-253.
[43] Bai W, Tai Y S, Wang J, et al.Engineering nonphosphorylative metabolism to synthesize mesaconate from lignocellulosic sugars in Escherichia coli[J].Metabolic Engineering, 2016,38:285-292.
[44] Zhang N N, Wang J B, Zhang Y, et al.Metabolic pathway optimization for biosynthesis of 1,2,4-butanetriol from xylose by engineered Escherichia coli[J].Enzyme and Microbial Technology, 2016, 93:51-58.
[45] Sakihama Y, Hasunuma T, Kondo A.Improved ethanol production from xylose in the presence of acetic acid by theoverexpression of the HAA1 gene in Saccharomyces cerevisiae[J].Journal of Bioscience and Bioengineering, 2015, 119:297-302.
[46] Pereira B, Li Z J, Mey D, et al.Efficient utilization of pentoses for bioproduction of the renewable two-carbon compounds ethylene glycol and glycolate[J].Metabolic Engineering, 2016,34:80-87.
[47] Wang J, Shen X L, Jain R, et al.Establishing a novel biosynthetic pathway for the production of 3,4-dihydroxybutyric acid from xylose in Escherichia coli[J].Metabolic Engineering, 2017, 41: 39-45.
Study on Synthesis of 3,4 - Dihydroxybutyric Acid and Its Lactone Form
Gao Yu, Dong Runan
(School of Life Science, Beijing Institute of Technology, Beijing, 100081)
3,4-dihydroxybutyric acid (3,4-DHBA) and its lactone form 3-hydroxy-γ-butyrolactone are all versatile chiral C4 compounds which could be used as a raw materials to synthesis many natural products, or pharmaceutical intermediates.Currently production of 3,4-DHBA and its lactone form mainly employs chemical synthesis, which has many drawbacks, such as harsh reaction conditions, low yield, more byproducts, severe environment contamination and so on.This review summarizes the progress of the chemical synthesis for the 3,4-dihydroxybutyric acid and its lactone form, and discusses the existing research about biosynthesis in detail.
3,4-dihydroxybutyric acid; 3-hydroxy-γ-butyrolactone; Chemical synthesis ; Biosynthesis
Q81 [Document Code] A
10.11967/ 2017150203
Q81
A DOI:10.11967/ 2017150203
高玉(1991-),女,内蒙古乌海市,硕士研究生,主要研究方向:微生物合成与代谢,Email:gaoyu0324@163.com.
董润安(1964-),男,博士研究生,副教授,硕士生导师,主要研究方向:细胞生物学,Email: dongra@bit.edu.cn.
高玉:联系地址:北京市海淀区中关村南大街5号北京理工大学;联系电话:18401621435;Email:gaoyu0324@163.com.本课题无基金项目。
