白细胞介素-33和血管内皮生长因子C在胃癌中的表达及临床意义*
2017-06-05夏兵祥袁振华郑苏文张业伟
夏兵祥,李 凡,徐 健,袁振华,郑苏文,张业伟
(南京医科大学附属肿瘤医院/江苏省肿瘤医院/江苏省肿瘤防治研究所普外科,南京 210009)
白细胞介素-33和血管内皮生长因子C在胃癌中的表达及临床意义*
夏兵祥,李 凡,徐 健,袁振华,郑苏文,张业伟△
(南京医科大学附属肿瘤医院/江苏省肿瘤医院/江苏省肿瘤防治研究所普外科,南京 210009)
目的 探讨白细胞介素-33(IL-33)和血管内皮生长因子C(VEGF-C)在胃癌患者组织标本及血清中表达,以及二者与胃癌淋巴结转移的关系。方法 分别应用免疫组织化学SP法和酶联免疫双抗夹心法(ELISA)检测98例胃癌患者和36名健康体检者胃黏膜组织标本及血清中IL-33和VEGF-C水平。结果 胃癌组织中IL-33和VEGF-C的阳性表达率分别为67.35%和74.49%,显著高于正常胃组织(47.22%和61.11%),比较差异有统计学意义(P<0.01)。IL-33和VEGF-C表达在不同的肿瘤分化程度、组织浸润、淋巴结转移、远处转移及临床分期间比较差异有统计学意义(P<0.05)。胃癌淋巴结转移患者的IL-33和VEGF-C的表达阳性率高于未发生淋巴结转移患者(P<0.05)。胃癌患者血清IL-33和VEGF-C水平为(50.24±13.08)pg/mL和(210.73±58.35)pg/mL,高于健康体检者(P<0.05);淋巴结转移患者血清IL-33和VEGF-C水平高于未发生淋巴结转移患者,比较差异有统计学意义(P<0.05)。结论 胃癌患者血清中高水平IL-33可能诱导VEGF-C分泌,促进胃癌淋巴结转移,可作为评估胃癌预后的重要指标。
胃肿瘤;血管内皮生长因子C;白细胞介素33;淋巴结转移
胃癌是消化道最常见的恶性肿瘤,病死率居恶性肿瘤的第2位[1]。胃癌患者死亡的主要原因是肿瘤复发和转移,其中最常见的转移途径为淋巴结转移。临床上有超过80%的进展期胃癌患者出现淋巴结转移,研究证明其是胃癌患者预后不良的独立危险因素[2]。近年来有关肿瘤侵袭转移的机制研究发现,血管内皮生长因子C(VEGF-C)与肿瘤的淋巴结转移密切相关[3-4],同时白细胞介素-33(IL-33)可刺激并诱导白细胞和肥大细胞VEGF mRNA表达与VEGF蛋白分泌,促进胃癌细胞侵袭和转移[5]。然而,关于IL-33、VEGF-C与肿瘤淋巴结转移的研究少有报道。因此,本研究通过检测胃癌患者组织标本和血清中IL-33和VEGF-C表达情况,分析IL-33和VEGF-C与胃癌淋巴结转移的关系,探讨其在胃癌淋巴结转移中的作用,以及二者表达水平的相关性及临床意义。
1 资料与方法
1.1 一般资料 收集2012年1月至2014年12月在本院接受胃癌根治手术的98例胃癌患者(胃癌组)手术切除标本,术前未接受任何放、化疗等。其中男50例,女48例;年龄42.0~86.0岁,中位年龄60.8岁;肿瘤直径1.0~10.0 cm,中位直径4.6 cm,所有标本经病理学证实为胃癌。根据WHO(2010年)胃癌组织学分类标准[6],管状腺癌(高、中分化)35例,低分化腺癌40例,黏液腺癌14例,印戒细胞癌9例;根据国际抗癌联盟(UICC)胃癌TNM分期标准[6],Ⅰ期20例,Ⅱ期27例,Ⅲ期38例,Ⅳ期13例;早期胃癌16例,进展期胃癌82例;有淋巴结转移76例;有远处转移16例。另选取同期36名在本院门诊体检的健康人,胃镜下取正常胃黏膜组织为对照组,均知情同意,自愿参与。本研究经过南京医科大学伦理委员会批准,批准编号(2011)132号。
纳入标准:经病理诊断为胃癌,年龄、性别不限,入院行D2淋巴结清扫的胃切除术患者。排除标准:合并严重心脑血管疾病;合并多种肿瘤病史;外科手术前接受放疗、化疗、靶向药物治疗等其他治疗手段;有研究者认为影响预后、评估及难以完成临床观察的其他严重疾病或残疾。
1.2 方法 胃癌组患者于术前3 d采集清晨空腹静脉血2 mL,对照组采集1次空腹静脉血2 mL,采血后静置离心(1 000 r/min,10 min),分离血清,置20 ℃保存待测。所有组织标本均经10%甲醛固定24 h,行常规石蜡包埋,连续切取4 μm厚的切片,采用免疫组织化学SP法染色,具体步骤按说明书进行。每批染色过程中均设磷酸盐缓冲液(PBS)代替一抗的阴性对照组。以细胞质呈清晰的棕黄色颗粒且其染色强度高于背景非特异染色者为阳性细胞。由两位经验丰富的病理科医生在相同条件下观察,正常胃组织计数细胞(500)中的阳性细胞数,以百分率表示。无阳性反应细胞或阳性反应细胞数小于10%者为阴性,阳性反应细胞数大于或等于10%者为阳性。酶联免疫吸附试验(ELISA)检测血清IL-33和VEGF-C浓度,免疫组织化学染色SP法检测IL-33和VEGF-C在胃癌中的表达情况。人IL-33和人VEGF-C ELISA试剂盒均购自上海润裕生物科技有限公司,兔抗人IL-33和VEGF-C多克隆抗体及S-P试剂盒均购自Dako公司,操作步骤严格按照说明书进行。
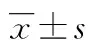
2 结 果
2.1 IL-33和VEGF-C在胃癌组织及正常胃组织中的表达 光镜下正常胃黏膜上皮细胞质中IL-33和VEGF-C染色弱或呈阴性。胃癌组织细胞内IL-33和VEGF-C呈弥漫性或簇状分布的棕黄色颗粒,瘤间质中有时可见IL-33和VEGF-C呈簇状的浅棕色染色,瘤旁正常胃黏膜中少见IL-33和VEGF-C染色。低分化腺癌中IL-33和VEGF-C染色程度较高、中分化腺癌略深(图1)。IL-33和VEGF-C在正常胃组织和进展期胃癌及淋巴结阳性组胃癌间的表达有统计学意义(P<0.05);早期胃癌及淋巴结阴性组胃癌与正常胃组织间表达比较差异无统计学意义(P>0.05);早期胃癌和进展期胃癌,淋巴结无转移组和淋巴结转移组胃癌中IL-33和VEGF-C表达比较差异有统计学意义(P<0.05),见表1~3。

A~E:SP法(×100);F:SP法(×400)。
图1 IL-33与VEGF-C在胃癌组织及正常胃组织中的表达
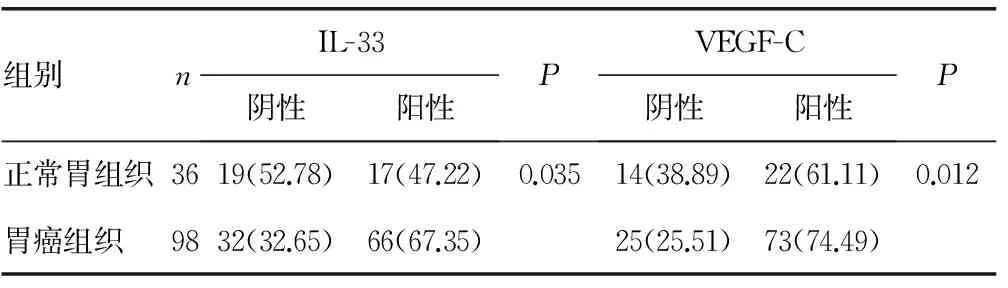
表1 IL-33和VEGF-C在胃癌组织及正常胃组织中的表达[n(%)]
2.2 IL-33和VEGF-C表达与胃癌临床病理指标之间的关系 IL-33和VEGF-C表达与胃癌患者性别、年龄、肿瘤位置和大小无相关性(P>0.05),但与肿瘤分化程度、组织浸润、淋巴结转移、远处转移及UICC分期有相关性(P<0.05)。肿瘤分化程度越低、组织浸润越严重、UICC分期越晚,IL-33和VEGF-C水平越高(P<0.05);有淋巴结转移的胃癌IL-33和VEGF-C水平显著高于无淋巴结转移者(P<0.05);有远处转移的胃癌中IL-33和VEGF-C水平明显高于无远处转移者(P<0.05),见表4。

表2 IL-33和VEGF-C表达与胃癌分期的关系[n(%)]

表3 IL-33和VEGF-C表达与胃癌淋巴结转移的关系[n(%)]

表4 IL-33和VEGF-C表达与胃癌临床病理指标之间的关系[n(%)]
2.3 胃癌组织标本中IL-33和VEGF-C表达情况的相关性 98例胃癌组织标本中IL-33和VEGF-C共阳性为54例,66例IL-33阳性患者中VEGF-C阳性率为81.82%,32例IL-33阴性患者中VEGF-C阳性率为59.38%,二者表达水平呈正相关(tau-b等级相关系数Kendall′s tau-b=0.241,P=0.025)。
2.4 胃癌组与对照组间血清IL-33和VEGF-C浓度的比较 98例胃癌患者血清IL-33和VEGF-C水平分别为(50.24±13.08)pg/mL和(210.73±58.35) pg/mL,与对照组血清IL-33和VEGF-C水平[(26.38±9.10)pg/mL和(82.26±35.79)pg/mL]相比,差异有统计学意义(P<0.01)。
2.5 胃癌患者血清IL-33和VEGF-C水平与胃癌淋巴结转移的关系 本研究98例胃癌患者中,76例患者经组织病理学证实有淋巴结转移,22例未发生淋巴结转移。淋巴结转阴性患者血清IL-33水平和VEGF-C水平分别为(38.37±7.45)pg/mL和(157.97±34.66)pg/mL,较健康人[IL-33:(26.38±9.10)pg/mL,VEGF-C:(82.26±35.79)pg/mL]有升高趋势,但比较差异无统计学意义(P=0.427、0.734)。淋巴结转阳性患者血清IL-33和VEGF-C水平分别为(50.24±13.08)pg/mL和(226.00±54.91)pg/mL,较健康人和淋巴结阴性患者明显升高,比较差异有统计学意义(P<0.05)。
3 讨 论
胃癌作为消化道最常见的恶性肿瘤,已严重威胁到人类健康。但胃癌的发生与转移是一个涉及多步骤、多因素的复杂过程,有关其复发转移机制尚不明确,持续存在的慢性炎症刺激在胃癌的发生、发展及促进肿瘤复发、转移过程中发挥重要作用。IL-33具有抑制和(或)促进炎症反应的双重生理效应[7-8],在炎症、肿瘤等多种疾病中发挥重要的调控作用[9-10]。VEGF-C可诱导肿瘤中心部位新生淋巴管生成[11-12],并在早期胃癌中均有高表达,与肿瘤浸润和淋巴结转移显著相关[13-15]。
近年来研究结果表明,IL-33可通过致癌抑制因子2-细胞外调解蛋白激酶1/2(ST2-ERK1/2)和c-Jun氨基末端激酶(JNK)信号通路促进胃癌细胞侵袭和转移[16],体内IL-33及其受体ST2表达水平的变化对胃癌等恶性肿瘤患者的病情进展、诊断及预后具有重要的参考价值[17-20]。本研究发现胃癌患者血清及组织标本中IL-33表达水平明显高于健康人,而且其升高程度与胃癌的恶性程度呈正相关,这进一步证实IL-33与胃癌的侵袭和转移密切相关。
VEGF-C是血管内皮生长因子家族最强的促血管生成因子之一,其主要通过刺激新生血管生成,促进血管内皮细胞增生和迁移,增加微血管通透性及促进细胞外基质的降解等途径促进肿瘤血管生成、浸润及转移[21-22]。诸多研究发现,VEGF-C在胃癌组织中高表达,其表达程度与淋巴结转移、淋巴管浸润、静脉浸润及肿瘤浸润生长方式、患者预后呈显著相关性[23-26]。本研究结果与其一致,VEGF-C在胃癌组织及血清标本中表达水平明显高于健康人,并且胃癌患者血清VEGF-C浓度与淋巴结转移、病理分期及分化程度等密切相关,表明VEGF-C可作为反映胃癌侵袭转移能力的重要指标。
血管生成是许多生理和病理过程的基本事件之一,其中病理性血管生成是肿瘤形成、增殖与转移的关键环节[27-28]。血管生成是一个涉及微血管结构和功能上一系列变化的复杂过程,与多种生长因子相关,如细胞因子、促血管生成因子、黏附分子等[29]。 IL-33能诱导内皮细胞增生、迁移和组织分化,从而促进新生血管生成[30],同时还与上皮细胞浸润密切相关,参与肿瘤的发生和发展[31]。
综上所述,胃癌组织及血清中IL-33和VEGF-C表达水平升高,二者之间存在正相关,并与胃癌的恶性进展、淋巴结转移等生物学行为有关,提示IL-33参与肿瘤新生血管的形成及肿瘤的浸润和转移,可能成为胃癌靶向治疗的潜在靶点之一。
[1]Torre LA,Bray F,Siegel RL,et al.Global cancer statistics,2012[J].CA Cancer J Clin,2015,65(2):87-108.
[2]Degiuli M,Borasi A,Forchino F,et al.Lymph-nodal ratio in gastric cancer staging system[J].Minerva Chir,2011,66(3):177-182.
[3]Yang LP,Fu LC,Guo H,et al.Expression of vascular endothelial growth factor C correlates with lymphatic vessel density and prognosis in human gastroesophageal junction carcinoma[J].Onkologie,2012,35(3):88-93.
[4]Dai Y,Jiang J,Wang Y,et al.The correlation and clinical implication of VEGF-C expression in microvascular density and lymph node metastasis of gastric carcinoma[J].Am J Transl Res,2016,8(12):5741-5747.
[5]Yamashita K,Hosoda K,Ema A,et al.Lymph node ratio as a novel and simple prognostic factor in advanced gastric cancer[J].Eur J Surg Oncol,2016,42(9):1253-1260.
[6]Li ZS,Li Q.The latest 2010 WHO classification of tumors of digestive system[J].Zhonghua Bing Li Xue Za Zhi,2011,40(5):351-354.
[7]Sattler S,Smits HH,Xu D,et al.The evolutionary role of the IL-33/ST2 system in host immune defence[J].Archi Immunol Ther Exp(Warsz),2013,61(2):107-117.
[8]Yang Q,Li G,Zhu Y,et al.IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+T cells[J].Eur J Immunol,2011,41(11):3351-3360.
[9]Gangemi S,Allegra A,Profita M,et al.Decreased plasma levels of IL-33 could contribute to the altered function of Th2 lymphocytes in patients with polycythemia vera and essential thrombocythemia[J].Cancer Invest,2013,31(3):212-213.
[10]Pichery M,Mirey E,Mercier P,et al.Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues,lymphoid organs,brain,embryos,and inflamed tissues:in situ analysis using a novel IL-33-LacZ gene trap reporter strain[J].J Immunol,2012,188(7):3488-3495.
[11]Lieto E,Ferraraccio F,Orditura M,et al.Expression of vascular endothelial growth factor(VEGF) and epidermal growth factor receptor(EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients[J].Ann Surg Oncol,2008,15(1):69-79.
[12]Yonemura Y,Endo Y,Tabata K,et al.Role of VEGF-C and VEGF-D in lymphangiogenesis in gastric cancer[J].Intern J Clin Oncology,2005,10(5):318-327.
[13]Gretschel S,Astrosini C,Vieth M,et al.Markers of tumour angiogenesis and tumour cells in bone marrow in gastric cancer patients[J].Eur J Surg Oncol,2008,34(6):642-647.
[14]Miyamoto N,Yamamoto H,Taniguchi H,et al.Differential expression of angiogenesis-related genes in human gastric cancers with and those without high-frequency microsatellite instability[J].Cancer Lett,2007,254(1):42-53.
[15]Onogawa S,Kitadai Y,Amioka T,et al.Expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in early gastric carcinoma:correlation with clinicopathological parameters[J].Cancer Lett,2005,226(1):85-90.
[16]Yu XX,Hu Z,Shen X,et al.IL-33 Promotes gastric cancer cell invasion and migration via ST2-ERK1/2 pathway[J].Dig Dis Sci,2015,60(5):1265-1272.
[17]Bergis D,Kassis V,Ranglack A,et al.High serum levels of the interleukin-33 receptor soluble ST2 as a negative prognostic factor in hepatocellular carcinoma[J].Transl Oncol,2013,6(3):311-318.
[18]Hu LA,Fu Y,Zhang DN,et al.Serum IL-33 as a diagnostic and prognostic marker in non-small cell lung cancer[J].Asian Pac J Cancer Prev,2013,14(4):2563-2566.
[19]Ye XL,Zhao YR,Weng GB,et al.IL-33-induced JNK pathway activation confers gastric cancer chemotherapy resistance[J].Oncol Rep,2015,33(6):2746-2752.
[20]Tong X,Barbour M,Hou K,et al.Interleukin-33 predicts poor prognosis and promotes ovarian cancer cell growth and metastasis through regulating ERK and JNK signaling pathways[J].Mol Oncol,2016,10(1):113-125.
[21]Bose D,Meric-Bernstam F,Hofstetter W,et al.Vascular endothelial growth factor targeted therapy in the perioperative setting:implications for patient care[J].Lancet Oncol,2010,11(4):373-382.
[22]Lal G,Hashimi S,Smith BJ,et al.Extracellular matrix 1 (ECM1) expression is a novel prognostic marker for poor long-term survival in breast cancer:a hospital-based cohort study in Iowa[J].Ann Surg Oncol,2009,16(8):2280-2287.
[23]Sun L,Duan J,Jiang Y,et al.Metastasis-associated in colon cancer-1 upregulates vascular endothelial growth factor-C/D to promote lymphangiogenesis in human gastric cancer[J].Cancer Lett,2015,357(1):242-253.
[24]Zhang J,Zhu Z,Sun Z,et al.Survivin gene expression increases gastric cancer cell lymphatic metastasis by upregulating vascular endothelial growth factor-C expression levels[J].Mol Med Rep,2014,9(2):600-606.
[25]Wang L,Li HG,Wen JM,et al.Expression of CD44v3,erythropoietin and VEGF-C in gastric adenocarcinomas:correlations with clinicopathological features[J].Tumori,2014,100(3):321-327.
[26]Ikeda K,Oki E,Saeki H,et al.Intratumoral lymphangiogenesis and prognostic significance of VEGFC expression in gastric cancer[J].Anticancer Res,2014,34(8):3911-3915.
[27]Griffioen AW,Molema G.Angiogenesis:potentials for pharmacologic intervention in the treatment of cancer,cardiovascular diseases,and chronic inflammation[J].Pharmacol Rev,2000,52(2):237-268.
[28]Gasparini G,Toi M,Biganzoli E,et al.Thrombospondin-1 and -2 in node-negative breast cancer:correlation with angiogenic factors,p53,cathepsin D,hormone receptors and prognosis[J].Oncology,2001,60(1):72-80.
[29]Deban L,Correale C,Vetrano S,et al.Multiple pathogenic roles of microvasculature in inflammatory bowel disease:a Jack of all trades[J].Am J Pathol,2008,172(6):1457-1466.
[30]Choi YS,Choi HJ,Min JK,et al.Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production[J].Blood,2009,114(14):3117-3126.
[31]Sundlisaeter E,Edelmann RJ,Hol J,et al.The alarmin IL-33 is a notch target in quiescent endothelial cells[J].Am J Pathol,2012,181(3):1099-1111.
Expression of interleukin-33 and vascular endothelial growth factor-C in gastric cancer and its clinical significance*
XiaBingxiang,LiFan,XuJian,YuanZhenhua,ZhengSuwen,ZhangYewei△
(DepartmentofGeneralSurgery,TumorHospitalAffiliatedtoNanjingMedicalUniversity/JiangsuProvincialCancerHospital/JiangsuProvincialCancerResearchInstitute,Nanjing,Jiangsu210009,China)
Objective To investigate the expression of interleukin-33 (IL-33) and vascular endothelial growth factor C (VEGF-C) in gastric cancer tissues and serum,and to explore the relationship between these two indicators and gastric cancer lymph node metastasis.Methods The levels of IL-33 and VEGF-C in the tissues of gastric mucosa and serum were detected by immunohistochemical SP method and enzyme-linked immunosorbent assay (ELISA) in 98 patients with gastric cancer and 36 healthy subjects.Results The expression rates of IL-33 and VEGF-C in gastric cancer were 67.35% and 74.49%,which were significantly higher than the rates in normal gastric tissue (47.22% and 61.11%).The difference was statistically significant (P<0.01).The expression of IL-33 and VEGF-C was correlated with the degree of tumor differentiation,tissue infiltration,lymph node metastasis,distant metastasis and clinical stage(P<0.05).The positive rates of IL-33 and VEGF-C in gastric cancer lymph node metastasis group were higher than those in non-lymph node metastasis group(P<0.05).The serum concentrations of IL-33 and VEGF-C in patients with gastric cancer were (50.24±13.08)pg/mL and(210.73±58.35)pg/mL,respectively,which were higher than those in healthy control group(P<0.05);the expressions of serum concentration of IL-33 and VEGF-C in the cases with lymph node metastasis were higher than those without lymph node metastasis and the difference was statistically significant(P<0.05).Conclusion High levels of IL-33 in gastric carcinoma patients might induce the secretion of VEGF-C,promote lymph node metastasis,and be applied as an important index of the appraisal to the prognosis of gastric cancer.
stomach neoplasms;vascular endothelial growth factor C;interleukins 33;lymph node metastasis
10.3969/j.issn.1671-8348.2017.15.014
国家自然科学基金面上项目(61371066); 江苏省医学重点人才资助项目(RC2011090);江苏省重点研发计划(社会发展-面上项目)(BE2015720);江苏省六大高峰人才项目(WSW-041)。 作者简介:夏兵祥(1988-),住院医师,在读硕士,主要从事普外肿瘤基础与临床研究方面研究。△
,E-mail:zhangyewei@njmu.edu.cn。
R735.2
A
1671-8348(2017)15-2056-04
2016-11-22
2017-01-10)
