Interaction of Soil Water and Nitrogen on the Photosynthesis and Growth in Pinustabulaeformis Seedlings
2017-06-05GuoWenxiaZhaoZhijiangZhengJiaoLiJunqing
Guo Wenxia Zhao Zhijiang Zheng Jiao Li Junqing,
(1. Key Laboratory for Silviculture and Conservation of Ministry of Education, Beijing Forestry University Beijing 100083; 2. Chinese Society of Forestry Beijing 100091; 3. Plant Functional Biology and Climate Change Cluster, University of Technology Sydney NSW 2007; 4. Fujian CIECC Engineering Consulting Co.Ltd Fuzhou 350003)
Interaction of Soil Water and Nitrogen on the Photosynthesis and Growth inPinustabulaeformisSeedlings
Guo Wenxia1, 2, 3Zhao Zhijiang4Zheng Jiao1Li Junqing1,
(1.KeyLaboratoryforSilvicultureandConservationofMinistryofEducation,BeijingForestryUniversityBeijing100083; 2.ChineseSocietyofForestryBeijing100091; 3.PlantFunctionalBiologyandClimateChangeCluster,UniversityofTechnologySydneyNSW2007; 4.FujianCIECCEngineeringConsultingCo.LtdFuzhou350003)
【Objective】Pinustabulaeformisis widely distributed over China, and a dominant species as an afforestation and reforestation tree species in ecological restoration and soil conservation programs. But as the climate change intensifies,P.tabulaeformisforests are experiencing soil water deficit and nitrogen deposition during growth season. Thus an experiment was carried out to investigate the interaction of different soil water content and N addition on the growth and photosynthesis ofP.tabulaeformisseedlings by measuring whole-plant growth, leaf area, biomass production and allocation, leaf photosynthesis, and chlorophyll fluorescence. 【Method】 Two-year-old seedlings ofP.tabulaeformiswere subjected to a nested design with four soil water regimes (W1, W2, W3, and W4) and four N addition levels (N1, N2, N3 and N4). 【Result】 The result showed that N addition significantly enhanced growth and biomass production of the seedlings under plentiful soil water conditions (W3 and W4), but aggregated the negative effect of low soil water treatments (W1 and W2) on plant growth. Moreover, N addition was able to lead to an increase in photosynthetic capacity under high soil water conditions (W3 and W4), but a decrease in low soil water treatments (W1 and W2), which was paralleled with the shifts of PSII actual efficiency and PSII photochemical quenching. Furthermore, W3N2 treatment was the appropriate N supply and optimum soil water conditions to growth, biomass production, and photosynthetic capacity ofP.tabulaeformisseedlings. 【Conclusion】 These results suggest N deposition might be beneficial to biomass production and photosynthesis ofP.tabulaeformisforests in the central and northeast areas in China with abundant rainfall, but harmful toP.tabulaeformisforests in the northwest arid and semi-arid regions. Thus, in the northwest arid and semi-arid regions in China,P.tabulaeformisshould no longer be used as afforestation and reforestation tree species in ecological restoration and soil conservation programs.
Pinustabulaeformis; leaf physiology; photosynthesis; chlorophyll fluorescence; growth; biomass
Human activities such as fossil fuel burning, forest disturbance and land-use conversion have increased the global atmospheric concentration of carbon dioxide CO2as well as atmospheric deposition of nitrogen (IPCC, 2007; Matsonetal., 2002). Increasing temperature can bring prolonged drought and increase the availability of N in many terrestrial ecosystems (Aberetal., 2003; IPCC, 2007). For such cases, how to response the impact by the drought and increasing N deposition forPinustabulaeformisbecome the focus of many researchers.
N is one of the most important nutrients affecting the growth, development, yield, fruit quality, and physiology of plants (Fernandesetal., 1995; Gerendásetal., 1997; Lebaueretal., 2008; Vitouseketal., 1991). It is required in the greatest quantities at each stage of plant growth during which N level markedly affects the amount of Rubisco content, and therefore photosynthesis (Evans, 1989; Evansetal., 1988). When the amount of available N in the soil cannot meet plant requirements, an increase in input of soil N may stimulate plant growth and photosynthesis (Hossainetal., 2010) by increasing leaf area and plant biomass (Liuetal., 1992), shoot/root ratio (Pregitzeretal., 1990), net photosynthetic rates (Mitchelletal., 1993; Van Hoveetal., 1989), leaf N content (Mitchelletal., 1993; Mulligan, 1989), chlorophyll content (Chandleretal., 1995; Ripulloneetal., 2003), and stomatal conductance (Dickmannetal., 1992). Similarly, N addition, such as atmospheric deposition, can enhance the plant tolerance to abiotic stresses such as water deficit, salt, and high temperature stresses (Lauteretal., 1981; Wuetal., 2008a). Conversely, an excess of soil nitrogen may limit plant growth and photosynthetic capacity, due to accelerated soil acidification, reducing the absorption of other mineral elements (Hossainetal., 2010).
Soil water is considered another key environmental factor that strongly affects plant growth and photosynthesis (Nemanietal., 2003), particularly so for soil water stress (Lawlor, 1995a). It is well known that one of the primary physiological consequences of water deficit is photosynthesis inhibition(Bresticetal., 1995; Lawlor, 1995b), attributed mainly to stomatal closure, reduced mesophyll conductance, and inhibition of Rubisco activity (Chavesetal., 2004; Earletal., 2005; Flexasetal., 2004; Foyeretal., 1998; Grassietal., 2005). While the major effects of water deficit on plant function are decreased shoot growth due to decreased leaf biomass and leaf area allocation, and increased leaf N content (Alvesetal., 2000).Soil water affects plant growth and photosynthesis directly on the one hand by influencing leaf phenology (Peuelasetal., 2009) and photosynthetic rate (Patricketal., 2009), and on the other hand indirectly by influencing the absorption of nutrients (Otsusetal., 2004; Peuelasetal., 2009), by providing the medium for nutrients uptake by roots (Ibrahimetal., 1998). So the effects of soil water and N nutrition on plant photosynthesis and growth are highly linked (Huetal., 2005).
Soil N availability can be affected by soil water availability (Engelbrechtetal., 2007; Quayeetal., 2009; Songetal., 2010) via several microbial-mediated pathways, such as litter decomposition (Liuetal., 2006) and N mineralization (Wangetal., 2006). Adequate soil water positively affects N input, decomposition, mineralization, and physical transport, thereby increasing N availability to plant growth and photosynthesis (Burkeetal., 1997), while water deficit reduces N uptake and plant growth (Misraetal., 2000). Many studies have shown that, under well-watered conditions, additional N supply significantly promoted plant growth and photosynthesis, but under water stress conditions, N addition had negative effects on plants (Songetal., 2010; Sunetal., 2011). Therefore, appropriate N supply under drought may stimulate plant growth and alleviate the effects of water stress by preventing cell membrane damage and enhancing osmoregulation (Bruecketal., 2010; Liuetal., 2012), whereas, excess N application reduced biomass allocation to root (Pattersonetal., 1997), increased leaf sensitivity to water stress (Tanetal., 1997), and decreased plant growth (Liuetal., 2012). So appropriate N supply is recommended to improve plant growth and photosynthetic efficiency under water stress (Shangguanetal., 2000). However, some other studies found no significant interactions between N and soil water to plant photosynthesis and growth (Eghballetal., 1993; Songetal., 2010; Wangetal., 2012; Wuetal., 2008b). Therefore, soil water and N co-act to regulate plant growth and photosynthesis may show different response to each other for diverse species.
P.tabulaeformis, an endemic evergreen coniferous species in China, is a key species of coniferous forests in arid, semi-arid and semi-humid regions of China, and widely used as afforestation and reforestation tree species in ecological restoration and soil conservation programs (Zhengetal., 1978). TheseP.tabulaeformisforests spread naturally from northeast to north and from central to west of China, across 10 provinces (Liaoning, Neimeng, Hebei, Shandong , Shanxi, Gansu, Shanxi, Qinghai, Henan and Sichuan) and 1 metropolis (Beijing), between 103°20′E to 124°45′E, 31°00′N to 43°33′N, and 100 to 2 600 m, a.s.l, with its distribution central in Shanxi (Liu, 2002; Zhengetal., 1978). There is a large difference over these regions in climatic conditions, as the average annual rainfall range from 400 mm to 1 000 mm and the average annual temperature range from 1 to 16 ℃. Therefore,P.tabulaeformisis assumed insensitive to water conditions. And as reported by Zhengetal. (1978)P.tabulaeformiswas a poor resistance plant. Thus, we hypothesize that the co-act of soil water and N will change the responses of growth, biomass production and photosynthesis forP.tabulaeformisto soil water and N.
The objective of this study was to investigate the effects of different soil water content and N addition on the growth and photosynthesis ofP.tabulaeformisseedlings by measuring whole-plant growth, leaf area, biomass production and allocation, leaf photosynthesis, and chlorophyll fluorescence. Better understanding of the interactions between soil water and N onP.tabulaeformismay provide critical insights on the potential responses of theP.tabulaeformisforest to climate change associated with increasing drought and atmospheric N deposition and therefore improve the management ofP.tabulaeformisplantations.
1 Materials and methods
1.1 Experimental design
The experiment was conducted in Xiaotangshan Experimental Site of Beijing Forestry University, located in the northern suburb of Beijing. A nested design was used with soil water as the primary factor and N addition as the secondary one. The experiment involved four adjacent greenhouse compartments, each 3.0 m×3.0 m×2.0 m (W×L×H) in size. Each greenhouse compartment was subjected to one of four soil water regimes: 1) 8% of soil water content (W1); 2) 12% soil water content (W2); 3) 16% of soil water content (W3); 4) 20% of soil water content (W4). And then each of the four greenhouse compartments was divided into four plots, each plot was subjected to one of four N addition levels: 1) 0 mg·kg-1(soil) (N1); 2) 31.25 mg·kg-1(soil) (N2); 3) 62.50 mg·kg-1(soil) (N3); 4) 93.75 mg·kg-1(soil) (N4). Each subplot replicate had 20 seedling pots, and the experiment 320 pots in all. 1.2 Plant culture and growth measurements
Two-year-oldP.tabulaeformisseedlings (average of 18 cm in height with about 50 new needles unfolded) were obtained from Container Tree Seedling Nursery in Luanping County, Heibei Province. In mid-March 2012, seedlings were transplanted into plastic cylindrical pots (25 cm diameter×30 cm depth) filled with field soil, with one seedling per pot. Field soil was collected from the Xiaotangshan Experimental Station, and was a mixture of sand and peat (1∶1 volume) with medium fertility (pH 7.8, N 19.6 mg·kg-1, P 4.6 mg·kg-1, K 135 mg·kg-1). The soil was air dried and 8 kg of the field soil was added to each pot.
In late May 2012, 320 healthy and similarly sized seedlings were selected from all seedlings and were randomly divided into 16 groups of 20 seedlings (one group per each water regime/ N level / replication). An additional 20 seedlings were used to determine the average initial dry mass. Soil water was monitored by weighting, supplementing water as needed (W1, 8%; W2, 12%; W3, 16%; W4, 20% soil water content) every two days. The four N levels were controlled by inputting urea (purity≥99.5% of CH4N2O, Urea Amresco0568, USA) 10 times to the potted soil and once every 10 days during the experiment after watering. Every time inputted N to the four N levels was: N1, 0 mg·kg-1; N2, 3.125 mg·kg-1; N3, 6.250 mg·kg-1; N4, 9.375 mg·kg-1. Treatments began on 15 June 2012, when seedlings were about 20 cm in height, and ended on 25 October 2012. Seedlings grew under each treatment for 130 days. The average growing season temperatures and relative humidity in the greenhouse was kept as ambient (20-36 ℃, 30%-76%). The experimental layout was surrounded with a single row of border plants to protect the experimental seedlings from external influences, and all subplots and main pots were rotated weekly to provide for random distribution.
One destructive harvest was conducted at the end of the experiment on 10 seedlings from each nitrogen supply treatment. During the harvest, we measured the heiqht of the main stem, diameter at stem base, and separated the seedling into roots (washed free of soil) and shoots by severing at the root collar, and the shoots were then further divided in stem (including branches and petioles) and leaf components. All harvested samples were oven-dried at 80 ℃ for 48 h, then weighed. During the experiment, we collected all dead leaves from each plant and the leaf mass was added to the final harvest data (Antenetal., 2001). For each plant, total leaf area was calculated as mean leaf area per leaf multiplied by the number of leaves per plant, the number of leaves per plant were counted at harvest and the mean leaf area per leaf was calculated as described by Lietal. (2007).1.3 Leaf gas exchange measurements and chlorophyll fluorescence emission
Spot measurements was carried out in the experimental field on four similarly clear days of 2 July, 23 July, 17 August and 21 September between 09:00 and 11:30 (the mean values of the four days used), using a portable photosynthesis analyzer (LI-6400, Li-Cor, Lincoln, NE, USA) supplying photosynthetic photon flux density by an red and blue leaf chamber. Net photosynthesis at saturating light (500±50 μmol·m-2s-1) (Asat), stomatal conductance (Gs), transpiration (Tr), intercellular CO2concentration (Ci), and ambient CO2concentration (Ca) were measured. UsingAsatandTr, the specific leaf water use efficiency (WUEL, defined as the ratio of net photosynthesis to transpiration) was calculated.

All above measurements were conducted on attached fully expanded, sunlit leaves exposed to ambient atmospheric pressure (110±0.3 kPa), temperature (30±1 ℃), and CO2concentration (360 μL·L-1). Three sample seedlings ofP.tabulaeformisfor each soil water regime/N level/replication treatment were used. And for each sample seedlings, three single attached leaves were used. Regularly, before measurements were recorded, each needle was allowed 5-10 min to equilibrate to chamber conditions, when readings were stable and the coefficient of variation was < 1%.
1.4 Statistical analyses
Statistical analyses were performed with the Statistical Software Package for the Social Science (SPSS, version 13.0). A two-way analysis of variance (ANOVA) was used to determine the differences of soil water treatments, nitrogen supply treatments and their interactions on mean variables (i.e.n=4). Means were compared using Duncan’s test. In all analyses, test results were considered significant ifP<0.05 and highly significant ifP<0.01.
2 Results
2.1 Plant biomass production and growth
An increase in soil water from W1 to W2 resulted in significant increases for both stem height and diameter (Fig.1a, b). The increase in soil water from W2 to W3 resulted in no significant effect on stem height and diameter (Fig.1a, b). When soil water increased to W4, stem height and diameter both dropped significantly (Fig.1a, b). Under lower soil water treatments (W1 and W2), N supply decreased both the main stem height and diameter, and the range of decrease increased with decreasing N supply; but under the highest soil water treatment (W4), N supply increased both the main stem height and diameter, and the range of increase with increasing N supply (Fig.1a, b). The treatment W3N2 was markedly stimulated both main stem height and diameter larger than the other treatments (Fig.1a, b). Additionally, under W3 soil water condition, N3 and N4 treatments had positive effects in main stem diameter, but had adverse effects in main stem height (Fig.1a, b).The soil water effects on plant biomass increased with the increasing of soil water content from W1 to W3 then decreased with the further increase of soil water content (Fig.2a). However, W3 treatment markedly increased both plant biomass and leaf area than other soil water treatments, but the effects of W1, W2, and W4 on leaf area were not significantly different (Fig.2a, b). Furthermore, under lower soil water treatments (W1, W2), N supply decreased both plant biomass and leaf area (Fig.2a, b). While under the highest soil water treatment (W4), N supply increased both plant biomass and leaf area (Fig.2a, b). Seedlings grown under the W3N2 treatment had the highest biomass and leaf area (Tab.1 and Fig.2a, b).

Fig.1 Main stem height and diameter of P. tabulaeformis grown at four levels of soil water content and four levels of N additionPlants were harvested 200 days after planting. Vertical error bars represent one standard error.The same below.
2.2 Biomass partitioning

Fig.2 Plant biomass and leaf area of P. tabulaeformis grown at four levels of soil water content and four levels of N addition
The decrease in soil water from W4 to W2 caused a slight increase in aboveground biomass (leaf biomass and stem biomass) but a decrease in root biomass, which led to the decrease in the ratio of root to shoot biomass (R/S) (Fig.3a-d). A further decrease in soil water to W1 caused a decrease in aboveground biomass and an increase in root biomass, resulting in increased R/S (Fig.3a-d). N treatments had different effects on leaf biomass, stem biomass, root biomass, and R/S under different soil water treatments. For the lowest soil water treatment (W1), N2, compared with N1, caused a significant decrease in R/S, but further increase in N supply improved R/S (Fig.3d). Under W2 soil water condition, N4 addition resulted in a significant (P<0.05) decrease in R/S, whereas no significant difference in R/S was observed between N2 and N3 addition (Fig.3d). Under W3 and W4 soil water treatments, N1 produced highest R/S over the other three N treatments and no significant difference in R/S was found among N2, N3 and N4 treatments (Fig.3d).
2.3 Leaf gas exchange

Fig.3 Biomass partitioning among root, stem and leaf and root/shoot (R/S) ratio of P. tabulaeformis grown under four levels of soil water content and four levels of N addition
The combination of lower soil water content (W1, W2) with different N supply didn’t result in significant changes to leafAsat, but in higher soil water treatments (W3, W4), N supply caused significant difference in leafAsat(Fig.4a). In high soil water treatment (W4),Asatincreased with increasing of N supply (Fig.4a). LeafAsatmarkedly increased by N2 under W3 but by N4 addition under W4 treatments (Fig.4a). And the W3N2 treatment had the highest value of leafAsatas 4.57 μmol· m-2s-1, significantly higher than the other treatments (Fig.4a).
Similarly to the responses of leafAsat,Gswas significantly enhanced by N2 and N4 addition under W3 and W4 treatments (Fig.4b). And under W3N2 treatment,Gsexhibited the highest as 0.032 mol·m-2s-1(Fig.4b). However, under lower soil water conditions (W1, W2), the effects of different N addition onGswere not significantly different, but with the increase of soil water content, the effects of different N addition onGswere increasingly significant (Fig.4b).
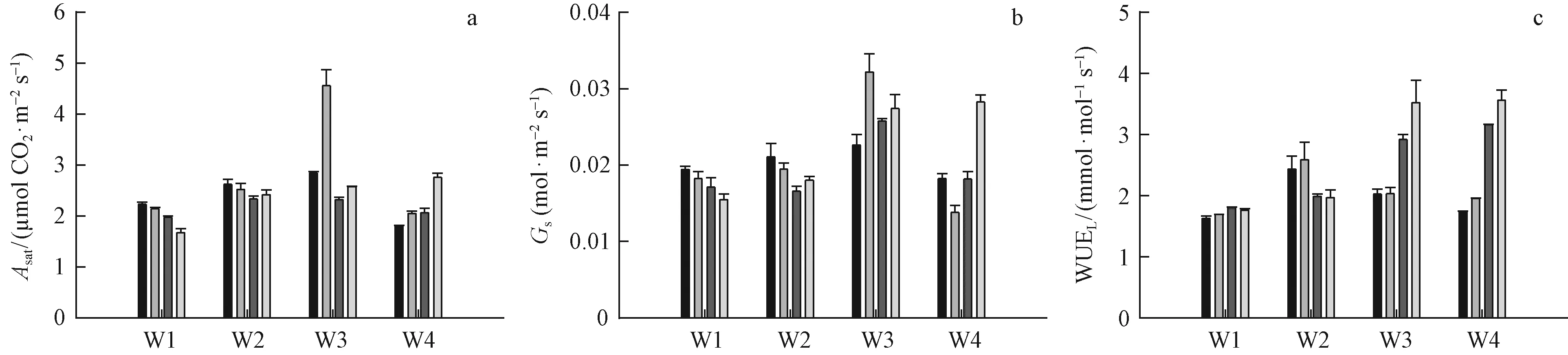
Fig.4 Light-saturated CO2 assimilation rates (Asat), stomatal conductance (Gs), and leaf water use efficiency (WUEL) of P. tabulaeformis grown at four levels of soil water content and four levels of N addition

Fig.5 Parameters derived from analyses of chlorophyll fluorescence emission at saturating photosynthetic photon flux density of P. tabulaeformis grown at four levels of soil water content and four levels of N addition
The responses of WUELto the interactive effects of soil water and N treatments were different toAsatandGs. With an increase in soil water, differences among the effects of the four N treatments increased (Fig.4c). N addition didn’t change WUELunder lower soil water condition (W1). But a significant increase in WUELwas found in W2N1 and W2N2 treatments, whereas N3 and N4 addition didn’t affect WUELin W2 treatment. However, under high soil water conditions (W3, W4), N3 and N4 addition markedly enhanced WUEL, while N1 and N2 addition didn’t affect WUEL. Seedlings grown in the W3N4 and W4N4 treatments had the highest WUELat 3.572 mmol·mol-1s-1.
2.4 Chlorophyll fluorescence
Actual PSⅡ efficiency and PSⅡ photochemical quenching were significantly influenced by soil water treatments inP.tabulaeformisseedlings (P<0.05) (Fig.5a, c). With the increase in soil water from W1 to W3, the values of actual PSⅡ efficiency tended to increase slightly, but the values of PSⅡ photochemical quenching increased slightly from treatments W1 to W2, and then decreased significantly at the highest water content (W4) (Fig.5a, c). Additional N significantly decreased actual PSⅡ efficiency and PSⅡ photochemical quenching under lower soil water conditions (W1, W2), but increased them under the highest soil water treatment (W4) (Fig.5a, c). In addition, markedly interactive effects of W3 and N2 were detected on actual PSⅡ efficiencies and PSⅡ photochemical quenching as their values were the highest (Fig.5a, c). However, PSⅡ maximum efficiency was not significantly affected by both soil water and N treatments, although it was also highest for the W3N2 treatment (Fig.5b).
3 Discussion3.1 Plant growth, biomass production and partitioning
In this study, we demonstrated significant interactive effects of N addition and soil water on the plant growth and biomass production ofP.tabulaeformisseedlings. We showed that the lowest and the highest soil water treatments (W1, W2 and W4) all decreased seedlings growth relative to the W3 treatments in terms of plant main stem height and diameter, plant total biomass, and leaf area. Stem height was reduced more than stem diameter under lower water conditions, suggesting that height growth is more sensitive to soil water than diameter growth. This agree with the result forSophoradavidiiseedlings (Wuetal., 2008a). The reduction of the seedlings growth induced by low soil water (W1, W2) was aggravated by N addition. This response ofP.tabulaeformisis contrary toFraxinusmandschuricaandSophoradavidiiseedlings, annual grass and wheat (Cabrera-Bosquetetal., 2007; Wangetal., 2012; Wuetal., 2008b; Zhouetal., 2011). However,P.tabulaeformis’s response to the highest soil water treatment (W4), is consistent with report forF.mandschuricaseedlings, apple trees, annual grass and wheat, that the reduction of the plant growth induced by high soil water supply was significantly attenuated by N addition and this tendency was partially diminished by N addition (Cabrera-Bosquetetal., 2007; Liuetal., 2012; Reichetal., 2006; Wangetal., 2012; Zhouetal., 2011). Similar result was also obtained by Ibrahimetal. (1998) in poplar. The growth responses of the seedlings to the interactive effects of soil water and N addition suggest that the effect of N supply is closely related to the soil water availability (Huetal., 2005; Reichetal., 2006; Wangetal., 2012), and N addition could amplify the negative effects of lower soil water (W1, W2) but could alleviate the negative effects of highest soil water (W4) manipulation onP.tabulaeformisseedlings growth. This result agrees with the hypothesis that there is co-act of soil water and N to the growth ofP.tabulaeformis, and it changed the responses ofP.tabulaeformisto soil water and N. In addition, the W3N2 treatment resulted in the greatest growth indicating an appropriate N supply and optimum soil water conditions to the growth ofP.tabulaeformisseedlings (Reichetal., 2006; Wangetal., 2012).
The shifts in biomass allocation also had an important impact on tree growth in the acclimation to changes of soil nutrient and water content (Domischetal., 2002; Reichetal., 1995). So the ratio of root to shoot biomass (R/S) is an indicator that represents the changes in belowground biomass and aboveground biomass allocation attributed by soil nutrient and water content changes (Lambersetal., 1998). Previous studies of Arndtetal. (2001), Marronetal. (2002), Lietal. (2003), Yinetal. (2005), Maetal. (2009) and Wangetal. (2012) found N limitation and drought stress increased carbon translocation from the leaves to the roots, thereby increased the R/S ratio. According to the resource depletion model of competition processes, increased root allocation is an adaptive response to belowground resource limitation, when belowground resources are the limiting factors, so the relative growth rates of all the individuals are not reduced by the same proportion, it would be more likely that plants will adopt different resource allocation strategies and alert the relative proportion of biomass allocation in order to allocate more resource to belowground components (Munsonetal., 1990; Newtonetal., 1993; Nilssonetal., 1993; Weineretal., 1986). But our result contrary to theirs, as soil water decrease not obviously increased carbon allocation from the leaves to the roots thereby increasing the R/S ratio. That may be because W1, W2, W3 and W4 have not obviously below the critical point of water stress.
It is also shown that N addition did not drive an alternation in the ratio of the aboveground and belowground biomass inP.tabulaeformisseedlings in W2, W3, and W4 treatments. This may be because N nutrition is not the limitation factor in W2, W3, and W4 treatments, as N addition had alleviated the N nutrient shortage in a short term, leading to non-significant differences in biomass allocation in different tree components. According to the resource depletion model of competition processes, when resources are adequate, the relative proportion of biomass allocation to different tree components might be unchanged (Changetal., 1996). However, under lower soil water condition (W1), N addition increasing stimulated an alternation in the ratio of the aboveground to belowground biomass, which indicated that biomass allocation ofP.tabulaeformisseedlings might be limited by N addition in lower soil water conditions. That may be because water deficit limited the availability of N, and soil water and N both became limiting factors, so plants allocated more biomass to belowground components as a way of response to belowground resource deficiencies (Changetal., 1996).
3.2 Leaf gas exchange
The leaf gas exchange study provides insight into the mechanism of the interactive effects of soil water and N addition on the photosynthesis ofP.tabulaeformisseedlings. Similar results were found forF.mandschuricaseedlings (Wangetal., 2012), durum wheat (Cabrera-Bosquetetal., 2007), and hybridized species (Campbelletal., 2010). Under the low soil water treatments (W1, W2) there was no significant difference between the effects of different N addition on leafAsat,Gs, and WUEL, but under high soil water treatments (W3, W4) the difference was significant. The photosynthetic responses to soil N and water availability indicated that the effects of N on the photosynthetic rate of the seedlings strongly depend on soil water content (Liuetal., 2012; Maetal., 2009; Nakajietal., 2001). In addition, N2 addition significantly enhanced the effect of W3 regime on leafAsat. That indicates the photosynthetic rate ofP.tabulaeformisseedlings might be dependent on soil N availability in high soil water conditions. Similar results also have been found in some hardwood tree species (Tyreeetal., 2009; Wangetal., 2012; Wangetal., 1998; Wendleretal., 1996).
We also investigated the changes ofGsresponse to different soil water and N addition to explain the potential mechanism in leaf photosynthesis. The results showed under lower soil water conditions (W1, W2), N addition had no significant effects onGs, but under high soil water conditions (W3, W4), N addition led to significantly enhancement ofGsforP.tabulaeformisseedlings. Similar results had been found in wheat,F.mandschuricaseedlings, and nine boreal tree species (Cabrera-Bosquetetal., 2007; Reichetal., 1998; Wangetal., 2012). In addition, N2 and N4 addition triggered a significant increase inGsof seedlings under W3 and W4 soil water treatment. It’s likely that N addition accelerate the transport of photosynthetic CO2in the leaves, leading to enhancedAsatof the seedlings (Wangetal., 2012).
Furthermore, we investigated the shifts of WUELresponse to different soil water and N addition, as WUELis a functional indicator strongly related to plant growth and health under water deficit condition, and is mostly dependent on the amount of water used for growth and biomass production (Liuetal., 2004; Monclusetal., 2006). Some studies reported WUELwas improved under water limitation (Liuetal., 2005), but some others have found the inverse case (Claveletal., 2005; Wuetal., 2008a). In this study,P.tabulaeformisseedlings employed both none of the above two strategies neglect N effects, it’s WUELdeclined with the increase of soil water under high soil water conditions (W3, W4), but increased with the increase of soil water under low soil water conditions (W1, W2). That might be attributed to the different soil water regimes applied in the experiments. In addition, we found the changes of WUELindicated that the effect of N addition on plant water use strongly depends on the availability of soil water, as the effects of N addition on WUELincreased with soil water increasing. This is likely because the increasing biomass production simulated by N addition under high soil water conditions. But this result consistent to that reported by Liuetal.(2012), Maetal.(2009), and Nakajietal.(2001), who found the effects of N addition on WUELincreased with soil water decreasing.
3.3 Chlorophyll fluorescence
The chlorophyll fluorescence parameters provide basic information on the function of the photosynthetic apparatus and on the capacity and performance of photosynthesis. In the present study, PSⅡ actual efficiency and PSⅡ photochemical quenching were obviously decreased by N addition in lower soil water regimes (W1, W2), but increased by N addition in the highest soil water regime (W4), which agree with previous study inS.davidiiseedlings (Wuetal., 2008b). These results once again indicate that the effects of soil water and N addition on the photosynthesis ofP.tabulaeformisseedlings highly interactive, as the N addition effects strongly depend on the soil water availability. Moreover, the W3N2 treatment markedly stimulated PSⅡ actual efficiency and photochemical quenching forP.tabulaeformisseedlings than other treatments. This indicate N2 and W3 treatments were the appropriate N supply and optimum soil water conditions to the photosynthetic capacity ofP.tabulaeformisseedlings. However, our result also shown PSⅡ maximum efficiency was not significantly affected by soil water and N addition. It’s likely that the efficiency of harvesting light byP.tabulaeformisseedlings isn’t affected by soil water and N availability but other environmental factors or the internal factors. But further research is needed to reveal what factors affect the efficiency of harvesting light byP.tabulaeformis.
4 Conclusion
In conclusion, this study evaluated the interactive effects of N addition and soil water on the growth and photosynthetic responses ofP.tabulaeformisseedlings, the forest of which widely distributed in the temperate ecosystem in China’s central and northern regions. We demonstratedP.tabulaeformishad different growth and photosynthetic responses to N addition in different soil water conditions. N addition significantly enhanced the growth and biomass production of the seedlings under plentiful soil water conditions (W3, W4), but aggregated the negative effect of low soil water treatments (W1, W2) on plant growth. Moreover, N addition could lead to an increase in the photosynthetic capacity under high soil water conditions (W3, W4), but a decrease in the low soil water treatments (W1, W2), which was paralleled with the shifts of PSⅡ actual efficiency and PSⅡ photochemical quenching. Furthermore, W3N2 treatment was the appropriate N supply and optimum soil water conditions to the growth, biomass production, and photosynthetic capacity ofP.tabulaeformisseedlings. Our data provide evidence that N deposition might be beneficial to biomass production and photosynthesis ofP.tabulaeformisforest in the central and northeast rainfall areas in China, but harmful toP.tabulaeformisforest in the northwest arid and semi-arid regions. So in the northwest arid and semi-arid regions in China,P.tabulaeformisshould no longer be used as afforestation and reforestation tree species in ecological restoration and soil conservation programs.
Reference
Aber J D, Goodale C L, Ollinger S V,etal. 2003. Is nitrogen deposition altering the nitrogen status of northeastern forests? BioScience, 53(4): 375-389.
Alves A A C, Setter T L. 2000. Response of cassava to water deficit: leaf area growth and abscisic acid. Crop Science, DOI:10.2135/cropsci2000.401131x.
Anten N P R, Ackerly D D. 2001. Canopy-level photosynthetic compensation after defoliation in a tropical understorey palm. Functional Ecology, 15(2): 252-262.
Arndt S K, Clifford S C, Wanek W,etal. 2001. Physiological and morphological adaptations of the fruit treeZiziphusrotundifoliain response to progressive drought stress. Tree Physiology, 21(11): 705-715.
Baker N R. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology, 59(1): 89-113.
Brestic M, Cornic G, Freyer M,etal. 1995. Does photorespiration protect the photosynthetic apparatus in french bean leaves from photoinhibition during drought stress? Planta, 196(3): 450-457.
Brueck H, Erdle K, Gao Y,etal. 2010. Effects of N and water supply on water use-efficiency of a semiarid grassland in Inner Mongolia. Plant & Soil, 328(1/2): 495-505.
Burke I C, Lauenroth W K, Parton W J. 1997. Regional and temporal variation in net primary production and nitrogen mineralization in grasslands. Ecology, 78(5): 1330-1340.
Cabrera-Bosquet L, Molero G, Bort J,etal. 2007. The combined effect of constant water deficit and nitrogen supply on WUE, NUE and Δ13C in durum wheat potted plants. Annals of Applied Biology, 151: 277-289.
Campbell D R, Travers S E, Wu C A. 2010. Photosynthetic and growth responses of reciprocal hybrids to variation in water and nitrogen availability. The American Journal of Botany, 97(6): 925-933.
Chandler J W, Dale J E. 1995. Nitrogen deficiency and fertilization effects on needle growth and photosynthesis in Sitka spruce (Piceasitchensis). Tree Physiology, 15(12): 813-817.
Chang S X, Weetman G F, Preston C M. 1996. Understory competition effect on tree growth and biomass allocation on a coastal old-growth forest cutover site in British Columbia. Forest Ecology and Management, 83(1/2): 1-11.
Chaves M M, Oliveira M M. 2004. Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. Journal of Experimental Botany, 55(407): 2365-2384.
Clavel D, Drame N K, Roy-Macauley H,etal. 2005. Analysis of early responses to drought associated with field drought adaptation in four Sahelian groundnut (ArachishypogaeaL.) cultivars. Environmental and Experimental Botany, 54(3): 219-230.
Dickmann D I, Liu Z, Nguyen P V,etal. 1992. Photosynthesis, water relations, and growth of two hybridPopulusgenotypesduring a severe drought. Canadian Journal of Forest Research, 22(8): 1094-1106.
Domisch T, Finer L, Lehto T. 2002. Growth, carbohydrate and nutrient allocation of Scots pine seedlings after exposure to simulated low soil temperature in spring. Plant and Soil, 246(1): 75-86.
Earl H J, Ennahli S. 2005. Physiological limitations to photosynthetic carbon assimilation in cotton under water stress. Crop Science, 45(6): 2374-2382.
Eghball B, Maranville J W. 1993. Root development and nitrogen influx of corn genotypes grown under combined drought and nitrogen stresses. Journal of the American Society of Agronomy, 85(1): 147-152.
Engelbrecht B M J, Comita L S, Condit R,etal. 2007. Drought sensitivity shapes species distribution patterns in tropical forests. Nature, 447(7140): 80-82.
Evans J R. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia, 78(1): 9-19.
Evans J R, Terashima I. 1988. Photosynthetic characteristics of spinach leaves grown with different nitrogen treatments. Plant and Cell Physiology, 29(1): 157-165.
Fernandes M S, Rossiello R O P. 1995. Mineral nitrogen in plant physiology and plant nutrition. Critical Reviews in Plant Sciences, 14(2): 111-148.
Flexas J, Bota J, Loreto F,etal. 2004. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biology, 6(3): 269-279.
Foyer C H, Valadier M H, Migge A,etal. 1998. Drought-induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and aarbon metabolism in maize leaves. Plant Physiology, 117(1): 283-292.
Gerendás J, Zhu Z, Bendixen R,etal. 1997. Physiological and biochemical processes related to ammonium toxicity in higher plants. Journal of Plant Nutrition and Soil Science, 160(2): 239-251.
Grassi G, Magnani F. 2005. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell & Environment, 28(7): 834-849.
Hossain M D, Musa M H, Talib J,etal. 2010. Effects of nitrogen, phosphorus and potassium levels on kenaf (HibiscuscannabinusL.) growth and photosynthesis under nutrient solution. Journal of Agricultural Science, 2(2): 49-57.
Hu Y C, Schmidhalter U. 2005. Drought and salinity: A comparison of their effects on mineral nutrition of plants. Journal of Plant Nutrition and Soil Science, 168(4): 541-549.
Ibrahim L, Proe M F, Cameron A D. 1998. Interactive effects of nitrogen and water availabilities on gas exchange and whole-plant carbon allocation in poplar. Tree Physiology, 18(7): 481-487.
IPCC. 2007. Climate change 2007: the physical science basis.Cambridge:Cambridge University Press.
Lambers H, Chapin F S, Pons T L. 1998. Plant physiological ecology. New York:Springer-Verlag.
Lauter D J, Munns D N, Clarkin K L. 1981. Salt response of chickpea as influenced by N deposition. Agronomy Journal, 73: 961-966.
Lawlor D W. 1995a. The effects of water deficit on photosynthesis∥Smirnoff N.Environment and plant metabolism: flexibility and acclimation.Oxford:DIOS Scientific Publishers, 129-160.
Lawlor D W. 1995b. Photosynthesis, productivity and environment. Journal of Experimental Botany, 46(sp): 1449-1461.
Lebauer D S, Treseder K K. 2008. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology, 89(2): 371-379.
Li C, Wang K. 2003. Differences in drought responses of three contrastingEucalyptusmicrothecaF. Muell. populations. Forest Ecology and Management, 179(1/3): 377-385.
Li X R, Liu Q J, Cai Z,etal. 2007. Specific leaf area and leaf area index of conifer plantations in Qianyanzhou station of subtropical China. Acta Phytoecology Sinica, 31: 93-101.
Liu B H, Cheng L, Li M J,etal. 2012. Interactive effects of water and nitrogen supply on growth, biomass partitioning, and water-use efficiency of young apple trees. African Journal of Agricultural Research, 7(6): 978-985.
Liu F, Stützel H. 2004. Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthusspp.) in response to drought stress. Scientia Horticulturae, 102(1): 15-27.
Liu F L, Andersen M N, Jacobsen S E,etal. 2005. Stomatal control and water use efficiency of soybean (GlycinemaxL. Merr.) during progressive soil drying. Environmental and Experimental Botany, 54(1): 33-40.
Liu P, Huang J, Han X,etal. 2006. Differential responses of litter decomposition to increased soil nutrients and water between two contrasting grassland plant species of Inner Mongolia, China. Applied Soil Ecology, 34(2/3): 266-275.
Liu Q. 2002. Ecological research on subalpine coniferous forests in China. Chengdu:Sichuan University Press,
Liu Z J, Dickmann D I. 1992. Abscisic acid accumulation in leaves of two contrasting hybrid poplar clones affected by nitrogen fertilization plus cyclic flooding and soil drying. Tree Physiology, 11(2): 109-122.
Ma F, Ji M F, Cheng L T,etal. 2009. Responses ofPinustabulaeformisseedlings to different soil water moistures in ecophysiological characteristics. Acta Botanica Boreali-Occidentalia Sinica, 29(3): 548-554.
Marron N, Delay D, Petit J M,etal. 2002. Physiological traits of twoPopulus×euramericanaclones, Luisa Avanzo and Dorskamp, during a water stress and re-watering cycle. Tree Physiology, 22(12): 849-858.
Matson P, Lohse K A, Hall S J. 2002. The globalization of nitrogen deposition: consequences for terrestrial ecosystems. AMBIO, 31(2): 113-119.
Misra A, Tyler G. 2000. Effect of wet and dry cycles in calcareous soil on mineral nutrient uptake of two grasses,AgrostisstoloniferaL. andFestucaovinaL. Plant and Soil, 224(2): 297-303.
Mitchell A K, Hinckley T M. 1993. Effects of foliar nitrogen concentration on photosynthesis and water use efficiency in Douglas-fir. Tree Physiology, 12(4): 403-410.
Monclus R, Dreyer E, Villar M,etal. 2006. Impact of drought on productivity and water use efficiency in 29 genotypes ofPopulusdeltoides×Populusnigra. New Phytologist, 169(4): 765-777.
Mulligan D R. 1989. Leaf phosphorus and nitrogen concentrations and net photosynthesis in Eucalyptus seedlings. Tree Physiology, 5(2): 149-157.
Munson A D, Timmer V R. 1990. Site-specific growth and nutrition of plantedPiceamarianain the Ontario Clay Belt: Ⅲ. Biomass and nutrient allocation. Canadian Journal of Forest Research, 20(8): 1165-1171.
Nakaji T, Fukami M, Dokiya Y,etal. 2001. Effects of high nitrogen load on growth, photosynthesis and nutrient status ofCryptomeriajaponicaandPinusdensifloraseedlings. Trees, 15(8): 453-461.
Nemani R R, Keeling C D, Hashimoto H,etal. 2003. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science, 300(5625): 1560-1563.
Newton P F, Jolliffe P A. 1993. Aboveground dry matter partitioning, size variation, and competitive processes within second-growth black spruce stands. Canadian Journal of Forest Research, 23(9): 1917-1929.
Nilsson U, Albrektson A. 1993. Productivity of needles and allocation of growth in young Scots pine trees of different competitive status. Forest Ecology and Management, 62(1/4): 173-187.
Otsus M, Zobel M. 2004. Moisture conditions and the presence of bryophytes determine fescue species abundance in a dry calcareous grassland. Oecologia, 138(2): 293-299.
Patrick L D, Ogle K, Tissue D T. 2009. A hierarchical Bayesian approach for estimation of photosynthetic parameters of C(3) plants. Plant, Cell & Environment, 32(12): 1695-1709.
Patterson T B, Guy R D, Dang Q L. 1997. Whole-plant nitrogen- and water-relations traits, and their associated trade-offs, in adjacent muskeg and upland boreal spruce species. Oecologia, 110(2): 160-168.
Pregitzer K S, Dickmann D I, Hendrick R,etal. 1990. Whole-tree carbon and nitrogen partitioning in young hybrid poplars. Tree Physiology, 7(1/4): 79-93.
Quaye A K, Laryea K B, Abeney-Mickson S. 2009. Soil water and nitrogen interaction effects on Maize (ZeamaysL.) grown on a vertisol. Jounal of Forestry, Horticulture, and Soil Science, 3(1): 1-11.
Reich P B, Walters M B, Tjoelker M G,etal. 1998. Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Functional Ecology, 12(3): 395-405.
Reich P B, Hungate B A, Luo Y Q. 2006. Carbon-nitrogen interactions in terrestrial ecosystems in response to rising atmospheric carbon dioxide. Annual Review of Ecology, Evolution, Systematics, 37: 611-636.
Reich P B, Walters M B, Kloeppel B D,etal. 1995. Different photosynthesis-nitrogen relations in deciduous hardwood and evergreen coniferous tree species. Oecologia, 104(1): 24-30.
Ripullone F, Grassi G, Lauteri M,etal. 2003. Photosynthesis-nitrogen relationships: interpretation of different patterns betweenPseudotsugamenziesiiandPopulus×euroamericanain a mini-stand experiment. Tree Physiology, 23(2): 137-144.
Shangguan Z P, Shao M A, Dyckmans J. 2000. Nitrogen nutrition and water stress effects on leaf photosynthetic gas exchange and water use efficiency in winter wheat. Environmental and Experimental Botany, 44(2): 141-149.
Song C J, Ma K M, Qu L Y,etal. 2010. Interactive effects of water, nitrogen and phosphorus on the growth, biomass partitioning and water-use efficiency ofBauhiniafaberiseedlings. Journal of Arid Environments, 74(9): 1003-1012.
Sun C X, Cao H X, Shao H B,etal. 2011. Growth and physiological responses to water and nutrient stress in oil palm. African Journal of Biotechnology, 10(51): 465-471.
Tan W X, Hogan G D. 1997. Physiological and morphological responses to nitrogen limitation in jack pine seedlings: potential implications for drought tolerance. New Forests, 14(1): 19-31.
Tyree M C, Seiler J R, Maier C A. 2009. Short-term impacts of nutrient manipulations on leaf gas exchange and biomass partitioning in contrasting 2-year-oldPinustaedaclones during seedling establishment. Forest Ecology and Management, 257(8): 1847-1858.
Van Hove L W A, Van K, Adema E H,etal. 1989. Physiological effects of long-term exposure to low and moderate concentrations of atmospheric NH3on poplar leaves. Plant, Cell & Environment, 12(9): 899-908.
Vitousek P, Howarth R. 1991. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry, 13(2): 87-115.
Wang C, Wan S, Xing X,etal. 2006. Temperature and soil moisture interactively affected soil net N mineralization in temperate grassland in Northern China. Soil Biology and Biochemistry, 38(5): 1101-1110.
Wang M, Shi S, Lin F,etal. 2012. Effects of soil water and nitrogen on growth and photosynthetic response of Manchurian ash (Fraxinusmandshurica) seedlings in northeastern China. PLoS ONE, 7(2): 1-12.
Wang Y P, Leuning R. 1998. A two-leaf model for canopy conductance, photosynthesis and partitioning of available energy I: Model description and comparison with a multi-layered model. Agricultural and Forest Meteorology, 91(1/2): 89-111.
Weiner J, Thomas S C. 1986. Size variability and competition in plant monocultures. Oikos,47: 211-222.
Wendler R, Millard P. 1996. Impacts of water and nitrogen supplies on the physiology, leaf demography and nitrogen dynamics ofBetulapendula. Tree Physiology, 16(1/2): 153-159.
Wu F Z, Bao W K, Li F L,etal. 2008a. Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency ofSophoradavidiiseedlings. Environmental and Experimental Botany, 63(1/3): 248-255.
Wu F Z, Bao W K, Li F L,etal. 2008b. Effects of water stress and nitrogen supply on leaf gas exchange and fluorescence parameters ofSophoradavidiiseedlings. Photosynthetica, 46(1): 40-48.
Yin C, Peng Y, Zang R,etal. 2005. Adaptive responses ofPopuluskangdingensisto drought stress. Physiologia Plantarum, 123(4): 445-451.
Zheng W J, Fu L G. 1978. Flora of China. Beijing:Science Press, 32-281.
Zhou X, Zhang Y, Ji X,etal. 2011. Combined effects of nitrogen deposition and water stress on growth and physiological responses of two annual desert plants in north-western China. Environmental and Experimental Botany, 74(1): 1-8.
(责任编辑 王艳娜 郭广荣)
土壤水分和氮素的交互作用对油松幼苗光合和生长的影响*
郭文霞1,2,3赵志江4郑 娇1李俊清1
(1.北京林业大学森林培育与保护省部共建教育部重点实验室 北京 100083; 2.中国林学会 北京 100091; 3.悉尼科技大学植物功能生物学和气候变化研究组 新南威尔士州 2007; 4.福建中咨工程咨询有限公司 福州 350003)
【目的】了解土壤水分亏缺和氮沉降对油松生长和光合特性的影响,为造林和再造林树种选择提供依据。【方法】 选取2年生油松幼苗,按照嵌套设计,设置4个土壤水分梯度(W1、W2、W3、W4)和4个施氮水平(N1、N2、N3、N4),调查土壤水分和施氮的交互作用对油松幼苗生长和光合特性的影响。【结果】 土壤水分充足条件下(W3和W4),施氮能够显著促进油松幼苗的生长和生物量积累,但在水分亏缺条件下(W1和W2)却会加剧缺水,对幼苗生长产生负面影响;土壤水分充足条件下,施氮能够提高油松幼苗的光合能力,但在水分亏缺状态下却会降低幼苗的光合能力,这与光系统Ⅱ的实际量子效率和光化学猝灭系数的变化规律一致; W3N2处理对提高油松幼苗生长、生物量积累和光合作用能力最有利。【结论】在我国中部和东北部湿润地区,氮沉降对油松的光合作用和干物质生产是有利的,而在西北干旱半干旱地区氮沉降却是有害的,因此,在西北干旱半干旱地区实施生态恢复或水土保持工程时应该谨慎选择树种,尽量不再使用油松。关键词:油松; 叶片生理; 光合作用; 叶绿素荧光; 生长; 生物量
S723.13
A
1001-7488(2017)04-0037-12
10.11707/j.1001-7488.20170405
Received date:2016-01-29; Revised date:2017-01-06.
Fund project: Beijing to Build Key Discipline “Ecology” Project (201401); High Level University Construction Project of China Scholarship Council (2012).
*Li Junqing is corresponding author. We thank Prof. Shen Yingbai for the advice in the design of the experiment, associate professor Lei Niya for her help in the purchase ofP.tabulaeformisseedlings, the staff (Yan Zhigang, Yan Zhiyong and Dong Xiaoyong) of Beijing Forestry University Nursery for their help during the experiment. Thank Professors Scott X Chang (University of Alberta), David T Tissue (University of Western Sydney) and Derek Eamus (University of Technology Sydney) for their comments on earlier drafts of this manuscript.
