Catalytic ozonation of thymol in reverse osmosis concentrate with core/shell Fe3O4@SiO2@Yb2O3 catalyst:Parameter optimization and degradation pathway☆
2017-05-28LiangWangAnqiLiuZhaohuiZhangBinZhaoYingmingXiaYunTan
Liang Wang ,Anqi Liu ,Zhaohui Zhang ,,*,Bin Zhao ,,Yingming Xia ,Yun Tan
1 State Key Laboratory of Separation Membranes and Membrane Processes,Tianjin Polytechnic University,Tianjin 300387,China
2 Department of Environmental Engineering,Tianjin Polytechnic University,Tianjin 300387,China
1.Introduction
Concerns about the fresh water are growing due to its limitation on quantity and distribution,especially in arid areas.The reuse of the effluent from the municipal wastewater treatment plants(WWTPs)and chemical pharmaceutical industries has become a hot spot in environmental sciences and technologies[1].Reverse osmosis(RO)has been con firmed to be a highly effective and reliable technology for desalination and advanced wastewater treatment for water reuse.However,the RO concentrates remain an unsolved problem.
The salinity level of the RO concentrates is high,which markedly inhibits the growth and activity of microorganism.Therefore,the organic matters concentrated in the RO concentrates,including pesticides,pharmaceutical products and other refractory chemicals[2,3],can hardly be removed by biodegradation processes[4–7].Direct discharge of RO concentrates into the aqueous environment without effective treatment could be hazardous to the aquatic ecosystem.Therefore,further treatment of the RO concentrates is necessary and crucial[8,9].
Thymol is a common phenolic antiseptic.It is widely used in cosmetics,food,and pharmaceuticals[10].Thymol has often been detected in municipal and pharmaceutical wastewater[11].Even though the ratio between the measured environmental concentrations(MEC)and the predicted no effect concentration(PNEC)indicated the urgent risk of thymol to the aquatic organisms was limited,its longterm and chronic adverse effect,such as endocrine disruption,was not negligible[12].Thymol concentrations in the RO concentrates are several-fold higher than those in the original wastewater,and thus the harm will be markedly increased.
Techniques based on hydroxyl radicals(•OH)are promising for effective degradation of organic contaminants in RO concentrates.Eversloh[13]used electrochemical oxidation with boron-doped diamond electrodes to degrade iopromide in reverse osmosis concentrate.Greenlee[14]used O3/H2O2to decompose the phosphonate antiscalants used for reverse osmosis desalination,and results showed that ozone dosage,accompanying components,pH,and H2O2concentration had great impacts on the degradation kinetics.Justo[15]used UV/H2O2to dispose the RO concentrates and demonstrated that high oxidant dosage was necessary to ensure the complete removal of all the target micro-pollutants from the brines.
Due to the high oxidative activity,ozonation has been extensively employed as pre-treatment or post-treatment technology for wastewater treatment[16,17].The removal of organic substances by ozonation is mainly through their direct reaction with O3as well as through the oxidation with•OH produced by ozone decomposition in aqueous solution[18–20].The direct reaction with O3is selective to certain organic pollutants in aqueous solution,while•OH is regarded as a nonselective oxidant with much stronger oxidation capacity.•OH can oxidize organic substances rapidly and produce oxygenated products.Furthermore,these products can be ultimately degraded to carbon dioxide or other low molecular organics[21–23].•OH production due to the O3self-decomposition preferentially occurs at alkaline condition.Therefore,solution pH has a great impact on the ozonation efficiency.Catalytic ozonation is another effective method to improve the efficiency of ozonation.Besides homogeneous catalysis,a variety of solids has also exhibited activity for catalytic ozonation,such as main group element oxides(Al2O3)and transition metal oxides(TiO2,Fe2O3,MnO2)[24].Due to the properties of the hybridization of unoccupied 4f levels,rare earth metals expressed an excellent catalytic activity and were widely used as catalysts in the catalytic synthetic industries and catalytic degradation of environmental contaminants[25–27].Some rare earth metals,such as La,Ce,and Gd,have been studied,and it was found that each of them had a positive effect on the improvement of catalytic activity[28–30].As a typical rare earth element,Yb has been used for the improvement of TiO2photocatalytic activity under visible light[31].However,study on the effect of Yb on catalytic ozonation is still limited.
This study focused on the degradation of thymol in RO concentrates using ozonation with core/shell Fe3O4@SiO2@Yb2O3catalyst.The effects of initial thymol concentration,ozone dosage,initial solution pH,and catalyst dosage were studied.A possible pathway of thymol degradation in this process was proposed based on the intermediates detected.This work can provide basic data and theoretical support to the treatment of pharmaceutical RO concentrates.
2.Materials and Methods
2.1.Materials and chemicals
All the chemicals and solvents used for COD measurement were of analytical grade,and were purchased from Shanghai Chemical Reagent Co.,Ltd.(Shanghai,China).The main characteristics of the simulated RO concentrates are shown in Table 1 based on the reference[13].Thymol purchased from Aladdin Reagent(China)Co.,Ltd.was spiked into the simulated RO concentrates.Deionized water was used throughout this study.

Table 1Main characteristics of the simulated RO concentrates
2.2.Preparation and characterization of Fe3O4@SiO2@Yb2O3
The core/shell nanomagnetic catalyst Fe3O4@SiO2@Yb2O3was prepared according to the chemical precipitation and subsequent calcination process[32,33](Fig.1).Firstly,the Fe3O4magnetic nanoparticles were prepared based on Matijević's method[32].In the procedure,KNO3and KOH were dissolved in water.FeSO4was added at constant temperature and under nitrogen protection.After stirring,black precipitate was generated.It was separated by magnetic force,and washed with absolute ethanol and distilled water for several times.The magnetic nanoparticles were then dried for use.Secondly,Fe3O4@SiO2nanoparticles were prepared[33].Fe3O4nanoparticles were dispersed in ethanol and distilled water for modification.After adding NH3·H2O and TEOS,the slurry was mechanically stirred continuously.The prepared Fe3O4@SiO2nanoparticles were collected and cleaned,and then dried.At last,Fe3O4@SiO2@Yb2O3nanoparticles were preparedviaa chemical precipitation and subsequent calcination process.Fe3O4@SiO2nanoparticles were dispersed in Yb nitrate solution.Then KOH was added in a four-neck flask.After vacuum filtration,the precipitate was washed with ethanol and dried.The dried powder was heated in a tube furnace.After calcination,Fe3O4@SiO2@Yb2O3nanoparticles were obtained.
The morphologies of the Fe3O4@SiO2@Yb2O3nanoparticles were characterized by transmission electronic microscopy(TEM)(Tecnai G2 F30 S-Twin,Philips-FEI,Netherlands)and scanning electronic microscopy(SEM)(Hitachi-s570,Hitachi,Japan).
2.3.Experimental procedures
Experimental setup for thymol removal by ozonation is shown in Fig.2.O3was obtained from a CFY-3 ozone generator which used pure oxygen as the source.All the degradation experiments were carried out at25°C in a 1.5 L cylindrical Pyrex glass reactor which was equipped with a spherical coarse microporous disperser in the centre of the reactor bottom.O3was bubbled into the solution through the disperser and the residual ozone was emitted and absorbed by KI solution.
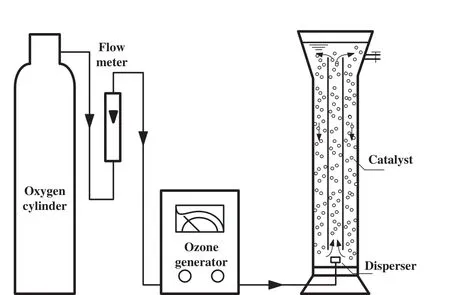
Fig.2.Experimental setup for catalytic ozonation.

Fig.1.Schematic diagram of the Fe3O4@SiO2@Yb2O3 preparation.
2.4.Analytical methods
A high performance liquid chromatograph(HPLC,1200 Agilent)was used to determine the concentration of thymol in aqueous solution,which was equipped with a C18 reversed phase column(Eclipse XDB,Agilent Technologies,USA)and a UV detector.The temperature of the HPLC system was kept at 30 °C.The injection volume was 5 μl and the mobile phase was water and acetonitrile(35:65,V/V).The flow rate of the eluent was 0.8 ml·min−1.The absorbance at 280 nm was detected.
In order to obtain the concentrations of intermediates generated in the thymol ozonation process,experiment was conducted at a high thymol initial concentration(100 mg·L−1).The intermediates were extracted by dichloromethane several times and enriched before analysis.They were identified by gas chromatography–mass spectrometry(GC–MS)(Agilent 7890–5975)which was equipped with a HP-5(30 m × 0.32 mm × 0.25 μm)chromatographic column.The column temperature was kept at 65 °C for 1 min and increased to 190 °C at a rate of 20 °C·min−1,and then increased to 280 °C at a rate of 40 °C and kept at 280 °C for 5 min.The injector temperature was 270 °C and the source temperature was 230°C.The EI impact ionization was 70 eV and the m/z scanning ranges were from 50 to 600.Helium was used as the carrier gas at a flow rate of 1 ml·min−1.
Acetic acid and maleic acid produced in the catalytic ozonation process were identified by an ion chromatography(IC)(Dionex model ICS2000)which was equipped with a Diones IonPac AS19 analytical column and an electrical conductivity detector.An IonPac AG19 analytical column was used as the guard column.
The chemicaloxygen demand(COD)was determined with a standard potassium dichromate oxidation method according to the National Standard Method of China(GB11914-89).

Fig.3.TEM(a)and SEM(b)images of the Fe3O4@SiO2@Yb2O3 catalyst.
3.Results and Discussion
3.1.Catalyst analysis
TEM and SEM were useful analytical techniques for understanding the morphologies and structures of the prepared catalysts.Fig.3(a)shows the TEM image of Fe3O4@SiO2@Yb2O3.The prepared Fe3O4@SiO2@Yb2O3catalyst was nanoparticles with clear core/shell structure.The Fe3O4@SiO2particles were successfully encapsulated into the Yb2O3layer.Fig.3(b)shows the SEM image of Fe3O4@SiO2@Yb2O3.Due to the aggregation of partial Fe3O4@SiO2@Yb2O3nanoparticles,the diameters of the prepared catalyst ranged from 200 to 500 nm.
3.2.Effect of initial thymol concentration
Initial concentration of contaminant has a great impact on the contaminant removal as well as COD removal[34].In order to investigate the effect of initial concentration,experiments were conducted at initial pH value of 9 and ozone dosage of 16 mg·min−1with different thymol concentrations(200,100,50,and 20 mg·L−1),respectively.

Fig.4.Effect of initial thymol concentration on thymol degradation(a)and COD removal(b)(O3 dosage=16 mg·min−1,pH=9).
Fig.4(a)shows that with the increase in the initial thymol concentration,longer time was required to completely eradicate thymol.When the initial thymol concentration was 20 mg·L−1,it took only 5 min to remove all the thymol.However,when the initial thymol concentration was 200 mg·L−1,it cost 30 min to remove all the thymol.As shown in Fig.4(b),the removal of COD was much slower and less complete than that of the thymol.When the initial thymol concentration was 20 mg·L−1,the COD removal efficiency was 92%after 1 h.When the initial thymol concentration was 200 mg·L−1,the COD removal efficiency was 28%after 1 h.The difference in the removal efficiency between thymol and COD indicated that by-products were formed during the thymol degradation,and the by-products were more resistant to ozonation than the thymol.
3.3.Effect of ozone dosage
As shown in Fig.5,under the conditions of initial thymol concentration of 100 mg·L−1and initial pH value of 9,four different O3dosages,namely 8,16,32,and 48 mg·min−1,were employed to investigate the effect of O3dosage on the thymol degradation and COD removal.
The thymol degradation rate significantly increased with the increase in the O3dosage.92%of thymol was degraded at the O3dosage of 8 mg·min−1after 30 min while thymol was completely disappeared within 10 min at the O3dosage of 48 mg·min−1.At the O3dosage of 8,16,32,and 48 mg·min−1,COD removals after 1 h were 27%,38%,46%,and 46%,respectively.By-products were more resistant to ozonation.The maximum COD removal by ozonation seemed less than 50%all the time when the initial thymol concentration was 100 mg·L−1,and excess O3was helpless with respect to the COD removal.
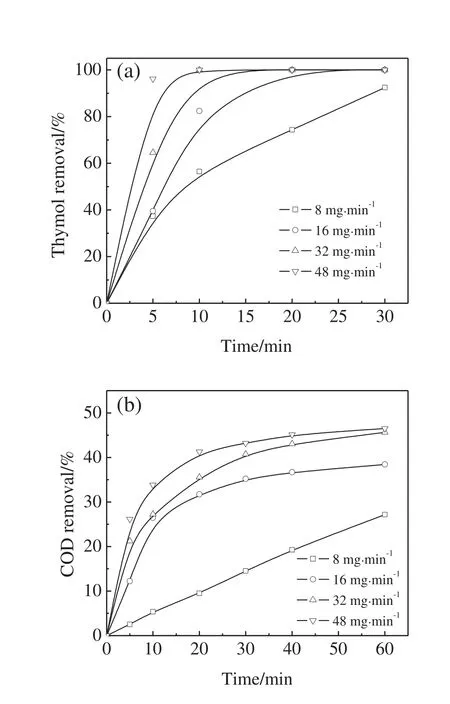
Fig.5.Effect of ozone dosage on thymol degradation(a)and COD removal(b)(initial thymol concentration=100 mg·L−1,pH=9).
3.4.Effect of initial pH value
O3is a strong oxidant but its oxidation is selective.Direct reaction with O3is not strong enough to oxidize organic matters into CO2and H2O within a reasonable time[35].Alkaline pH is generally superior to acidic pH for O3decomposition in aqueous solution.O3decomposition yielded•OH,which exhibited much stronger oxidative activity than O3[36,37].In order to investigate the effect of initial pH value on the thymol degradation and COD removal,experiments were conducted at the initial thymol concentration of 100 mg·L−1and O3dosage of 16 mg·min−1with different initial solution pH,namely 3,6,9,and 11.
As shown in Fig.6(a),with the increase in the initial solution pH value,the thymol removal rate increased significantly.This is because in the alkaline solution more•OH were yielded.Similar trend was also found for the COD removal in Fig.6(b).At the initial pH values of 3,6,9,and 11,the COD removals were 34%,36%,38%,and 49%after 1 h.It should be noted that the COD removals markedly increased when the pH increased from 9 to 11.Therefore,it could be concluded that,even though the by-products of the thymol ozonation were resistant to ozonation,they could be further oxidized or even mineralized by•OH generated from the O3decomposition.Increase in the•OH yield during the ozonation process was an effective way to improve the COD removal.

Fig.6.Effect of initial pH value on thymol degradation(a)and COD removal(b)(O3 dosage=16 mg·min−1,initial thymol concentration=100 mg·L−1).
3.5.Effect of catalyst dosage
Due to the resonance structure of O3,one of the oxygen atoms with high electron density shows high basicity resulting in strong affinity to Lewis acid sites on the surface of metal oxides[38].Therefore,the presence of Fe3O4@SiO2@Yb2O3catalyst might accelerate the decomposition of O3into•OH.In order to investigate the effects of the catalyst dosage on the thymol degradation and COD removal,experiments with the Fe3O4@SiO2@Yb2O3catalyst of 0.2,0.5,and 1.0 g as well as without catalyst were carried out at the initial thymol concentration of 100 mg·L−1,O3dosage of 16 mg·min−1,and initial pH value of 11(Fig.7).
The presence of the Fe3O4@SiO2@Yb2O3catalyst enhanced the process of both thymol degradation and COD removal.However,compared with the thymol degradation,the presence of catalyst and its increase were much more effective for the COD removal.Since the by-products were resistant to the ozonation,the maximum COD removal without catalyst was less than 50%.When the catalyst of 0.2 g was added into the system,the COD removal increased to 51%.Moreover,the COD removal increased with the increase in catalyst dosage,and 57%of COD was removed at the catalyst dosage of 1.0 g.This was because the presence of Fe3O4@SiO2@Yb2O3catalyst accelerated the decomposition of O3into•OH and the increase in the catalyst dosage provided more surface active sites for this conversion.The improvement of COD removal was attributed to the increase in the produced•OH as a result of the catalytic ozonation.

Fig.7.Effect of catalyst dosage on thymol degradation(a)and COD removal(b)(O3 dosage=16 mg·min−1,initial thymol concentration=100 mg·L−1,pH=11).
3.6.Mechanism analysis
Maleic acid,acetic acid,and fumaric acid were identified as the main acidic by-products generated in the thymol degradation by ozonation.Their evolutions are shown in Fig.8.Acetic acid was the major byproduct.Its concentration increased during the first 30 min,but slightly decreased hereafter.The concentration of maleic acid linearly increased with time,and it was much higher than that of fumaric acid.
Based on the intermediates identified by GC/MS,a degradation pathway of the thymol degradation by ozonation was proposed as shown in Fig.9.The para-position and ortho-position of the hydroxyl on the thymol molecular were vulnerable to the attack of O3and•OH.As a result,thymol transformed top-cymene-2,5-diol andp-cymene-2,3-diol.p-Cymene-2,5-diol andp-cymene-2,3-diol were then oxidized top-cymene-2,5-dione.The benzene ring ofp-cymene-2,5-dione was cracked and produced acidic by-products with lower molecular weight.These acidic by-products were finally mineralized to H2O and CO2.
4.Conclusions
A novel catalyst of Fe3O4@SiO2@Yb2O3was prepared and the degradation of thymol in RO concentrates by ozonation was investigated.The results indicated that initial thymol concentration,O3dosage,initial pH value,and catalyst dosage had great impacts on the thymol degradation by ozonation.The increase in the O3dosage,initial pH value,and catalyst dosage would accelerate the thymol degradation and COD removal;however,higher initial thymol concentration required longer time to complete the thymol removal.The optimum initial pH value in terms of the COD removal and thymol degradation was 11.The addition of Fe3O4@SiO2@Yb2O3catalyst could significantly improve the COD removal due to the enhancement in the•OH yield.Maleic acid,acetic acid and fumaric acid were the main acidic by-products of the thymol degradation by ozonation,and the degradation pathway was proposed.

Fig.8.Time pro files of maleic acid,acetic acid,and fumaric acid concentrations during the thymol degradation by ozonation(O3 dosage=16 mg·min−1,initial thymol concentration=100 mg·L−1,pH=11,Fe3O4@SiO2@Yb2O3 dosage=1.0 g).

Fig.9.The pathway of thymol degradation by catalytic ozonation(O3 dosage=16 mg·min−1,initial thymol concentration=100 mg·L−1,pH=11,Fe3O4@SiO2@Yb2O3 dosage=1.0 g).
[1]S.B.Grant,J.D.Saphores,D.L.Feldman,A.J.Hamilton,T.D.Fletcher,P.L.M.Cook,M.Stewardson,B.F.Sanders,L.A.Levin,R.F.Ambrose,A.Deletic,R.Brown,S.C.Jiang,D.Rosso,W.J.Cooper,I.Marusic,Taking the “waste”out of “wastewater”for human water security and ecosystem sustainability,Science337(2012)681–686.
[2]T.Y.Liu,C.K.Li,B.Pang,B.Van der Bruggen,X.L.Wang,Fabrication of a dual-layer(CA/PVDF)hollow fiber membrane for RO concentrate treatment,Desalination365(2015)57–69.
[3]S.Pradhan,L.H.Fan,F.A.Roddick,Removing organic and nitrogen content from a highly saline municipal wastewater reverse osmosis concentrate by UV/H2O2-BAC treatment,Chemosphere136(2015)198–203.
[4]Y.X.Sun,Y.Gao,H.Y.Hu,F.Tang,Z.Yang,Characterization and biotoxicity assessment of dissolved organic matter in RO concentrate from a municipal wastewater reclamation reverse osmosis system,Chemosphere117(2014)545–551.
[5]S.Malamis,E.Katsou,K.Takopoulos,P.Demetriou,M.Loizidou,Assessment of metal removal,biomass activity and RO concentrate treatment in an MBR–RO system,J.Hazard.Mater.209(2012)1–8.
[6]X.H.Lin,S.F.Y.Li,Determination of organic pollutants in municipal reverse osmosis concentrate by electrospray ionization–quadrupole time-of- flight tandem mass spectrometry and photocalaytic degradation methods,Desalination344(2014)206–211.
[7]A.M.Urtiaga,G.Perez,R.Ibanez,I.Ortiz,Removal of pharmaceuticals from a WWTP secondary effluent by ultra filtration/reverse osmosis followed by electrochemical oxidation of the RO concentrate,Desalination331(2013)26–34.
[8]S.Ben Abdelmelek,J.Greaves,K.P.Ishida,W.J.Cooper,W.H.Song,Removal of pharmaceutical and personal care products from reverse osmosis retentate using advanced oxidation processes,Environ.Sci.Technol.45(2011)3665–3671.
[9]Y.Zhang,K.Ghyselbrecht,R.Vanherpe,B.Meesschaert,L.Pinoy,B.Van der Bruggen,RO concentrate minimization by electrodialysis:Techno-economic analysis and environmental concerns,J.Environ.Manag.107(2012)28–36.
[10]V.Hahn,K.Sunwoldt,A.Mikolasch,F.Schauer,Two different primary oxidation mechanisms during biotransformation of thymol by gram-positive bacteria of the genera Nocardia and Mycobacterium,Appl.Microbiol.Biotechnol.97(2013)1289–1297.
[11]N.Nakada,T.Tanishima,H.Shinohara,K.Kiri,H.Takada,Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment,Water Res.40(2006)3297–3303.
[12]I.Tamura,K.Kagota,Y.Yasuda,S.Yoneda,J.Morita,N.Nakada,Y.Kameda,K.Kimura,N.Tatarazako,H.Yamamoto,Ecotoxicity and screening level ecotoxicological risk assessment of five antimicrobial agents:Triclosan,triclocarban,resorcinol,phenoxyethanol and p-thymol,J.Appl.Toxicol.33(2013)1222–1229.
[13]C.L.Eversloh,N.Henning,M.Schulz,T.A.Ternes,Electrochemical treatment of iopromide under conditions of reverse osmosis concentrates—Elucidation of the degradation pathway,Water Res.48(2014)237–246.
[14]L.F.Greenlee,B.D.Freeman,D.F.Lawler,Ozonation of phosphonate antiscalants used for reverse osmosis desalination:Parameter effects on the extent of oxidation,Chem.Eng.J.244(2014)505–513.
[15]A.Justo,O.Gonzalez,J.Acena,S.Perez,D.Barcelo,C.Sans,S.Esplugas,Pharmaceuticals and organic pollution mitigation in reclamation osmosis brines by UV/H2O2and ozone,J.Hazard.Mater.263(2013)268–274.
[16]A.D.Coelho,C.Sans,A.Aguera,M.J.Gomez,S.Esplugas,M.Dezotti,Effects of ozone pre-treatment on diclofenac:Intermediates,biodegradability and toxicity assessment,Sci.Total Environ.407(2009)3572–3578.
[17]D.B.Mawhinney,B.J.Vanderford,S.A.Snyder,Transformation of 1H-benzotriazole by ozone in aqueous solution,Environ.Sci.Technol.46(2012)7102–7111.
[18]L.Zhao,J.Ma,Z.Z.Sun,X.D.Zhai,Mechanism of influence of initial pH on the degradation of nitrobenzene in aqueous solution by ceramic honeycomb catalytic ozonation,Environ.Sci.Technol.42(2008)4002–4007.
[19]Q.Z.Dai,L.L.Chen,W.Chen,J.M.Chen,Degradation and kinetics of phenoxyacetic acid in aqueous solution by ozonation,Sep.Purif.Technol.142(2015)287–292.
[20]T.Nothe,H.Fahlenkamp,C.von Sonntag,Ozonation of wastewater:Rate of ozone consumption and hydroxyl radical yield,Environ.Sci.Technol.43(2009)5990–5995.
[21]R.Rosal,A.Rodriguez,J.A.Perdigon-Melon,A.Petre,E.Garcia-Calvo,M.J.Gomez,A.Aguera,A.R.Fernandez-Alba,Degradation of caffeine and identification of the transformation products generated by ozonation,Chemosphere74(2009)825–831.
[22]M.J.Quero-Pastor,M.C.Garrido-Perez,A.Acevedo,J.M.Quiroga,Ozonation of ibuprofen:A degradation and toxicity study,Sci.Total Environ.466(2014)957–964.
[23]Z.Jeirani,A.Sadeghi,J.Soltan,B.Roshani,B.Rindall,Effectiveness of advanced oxidation processes for the removal of manganese and organic compounds in membrane concentrate,Sep.Purif.Technol.149(2015)110–115.
[24]J.Nawrocki,Catalytic ozonation in water:Controversies and questions.Discussion paper,Appl.Catal.B Environ.142(2013)465–471.
[25]H.Y.Ma,T.P.Spaniol,J.Okuda,Rare earth metal complexes supported by 1,omegadithiaalkanediyl-bridged,bis(phenolato)ligands:Synthesis,characterization and ring-opening polymerization catalysis of L-lactide,Dalton Trans.(2003)4770–4780.
[26]Y.J.Feng,Y.H.Cui,B.Logan,Z.Q.Liu,Performance of Gd-doped Ti-based Sb-SnO2anodes for electrochemical destruction of phenol,Chemosphere70(2008)1629–1636.
[27]S.Bingham,W.A.Daoud,Recent advances in making nano-sized TiO2visible-light active through rare-earth metal doping,J.Mater.Chem.21(2011)2041–2050.
[28]Q.Y.Wang,G.F.Li,B.Zhao,M.Q.Shen,R.X.Zhou,The effect of La doping on the structure of Ce0.2Zr0.8O2and the catalytic performance of its supported Pd-only three-way catalyst,Appl.Catal.B Environ.101(2010)150–159.
[29]R.C.Martins,R.M.Quinta-Ferreira,Catalytic ozonation of phenolic acids over a Mn–Ce–O catalyst,Appl.Catal.B Environ.90(2009)268–277.
[30]M.Farbod,M.Kajbafvala,Effect of nanoparticle surface modification on the adsorption-enhanced photocatalysis of Gd/TiO2nanocomposite,Powder Technol.239(2013)434–440.
[31]Y.F.Ma,M.Y.Xing,J.L.Zhang,B.Z.Tian,F.Chen,Synthesis of well ordered mesoporous Yb,N co-doped TiO2with superior visible photocatalytic activity,Microporous Mesoporous Mater.156(2012)145–152.
[32]T.Sugimoto,E.Matijević,Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels,J.Colloid Interface Sci.74(1980)227–243.
[33]Q.Z.Dai,J.Y.Wang,J.Yu,J.Chen,J.M.Chen,Catalytic ozonation for the degradation of acetylsalicylic acid in aqueous solution by magnetic CeO2nanometer catalyst particles,Appl.Catal.B Environ.144(2014)686–693.
[34]T.Garoma,S.Matsumoto,Ozonation of aqueous solution containing bisphenol A:Effect of operational parameters,J.Hazard.Mater.167(2009)1185–1191.
[35]H.H.Yan,P.Lu,Z.Q.Pan,X.Wang,Q.Y.Zhang,L.S.Li,Ce/SBA-15 as a heterogeneous ozonation catalyst for efficient mineralization of dimethyl phthalate,J.Mol.Catal.A Chem.377(2013)57–64.
[36]M.Kuosa,J.Kallas,A.Häkkinen,Ozonation ofp-nitrophenol at different pH values of water and the influence of radicals at acidic conditions,J.Environ.Chem.Eng.3(2015)325–332.
[37]J.Ma,M.H.Sui,T.Zhang,C.Y.Guan,Effect of pH on MnOx/GAC catalyzed ozonation for degradation of nitrobenzene,Water Res.39(2005)779–786.
[38]L.Zhao,Z.Z.Sun,J.Ma,H.L.Liu,Enhancement mechanism of heterogeneous catalytic ozonation by cordierite-supported copper for the degradation of nitrobenzene in aqueous solution,Environ.Sci.Technol.43(2009)2047–2053.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Partition coefficient prediction of Baker's yeast invertase in aqueous two phase systems using hybrid group method data handling neural network
- An improved flexible tolerance method for solving nonlinear constrained optimization problems:Application in mass integration
- An optimal filter based MPC for systems with arbitrary disturbances☆
- Measurement and calculation of solubility of quinine in supercritical carbon dioxide☆
- Solubility and metastable zone width measurement of 3,4-bis(3-nitrofurazan-4-yl)furoxan(DNTF)in ethanol+water
- The effect of transition metal ions(M2+=Mn2+,Ni2+,Co2+,Cu2+)on the chemical synthesis polyaniline as counter electrodes in dye-sensitized solar cells☆
