Synthesis,gas adsorption and reliable pore size estimation of zeolitic imidazolate framework-7 using CO2 and water adsorption
2017-05-28MahdiNiknamShahrakMortezaNiknamShahrakAkbarShahsavandNasserKhazeniXiaofeiWuShuguangDeng
Mahdi Niknam Shahrak *,Morteza Niknam Shahrak ,Akbar Shahsavand ,Nasser Khazeni,Xiaofei Wu ,Shuguang Deng *
1 Chemical Engineering Department,Faculty of Engineering,Quchan University of Advanced Technology,Quchan,P.O.Box 84686-94717,Iran
2 Chemical Engineering Department,Faculty of Engineering,Ferdowsi University of Mashhad,Mashhad,P.O.Box 91775-1111,Iran
3 Chemical Engineering Department,New Mexico State University,Las Cruces,New Mexico 88003,USA
4 Chemical Engineering Department,Arizona State University,USA
1.Introduction
Extensive applications of various adsorption processes in numerous chemical engineering industries require much more efficient novel adsorbents.In 1965 Tomic[1]initially introduced the coordination polymers or supra-molecular structure and in 1999 Yaghiet al.fabricated the first MOF material known as MOF-5 in a laboratory scale batch[2].
Afterward,metal–organic frameworks(MOFs)have captured significant attention due to their outstanding properties,such as extremely large surface areas[3],high thermal and mechanical stabilities[4],low bulk densities[5],large micropore volumes and very high porosities[6].These materials have been used for numerous practical applications such as gas storage(e.g.methane,carbon dioxide and hydrogen),gas separation(e.g.nitrogen recovery from air),catalysis,drug delivery,membranes,luminescent and sensors fabrication[2,6,7–17].
Regarding the variety of the metals and ligands,over 38,000 MOF structures are listed up to now in the Cambridge Structure Database(CSD).Several reviews have also addressed this fast growing area,for example,over 2460 articles were recorded in MOF related subjects up to 2008[6,18].The most comprehensive ones are given by Kitagawa and Rowsell and Kuppleraet al.[6,19,20].More recently,in March 2015,a review article has explained fundamentals and wide array of potential applications of metal–organic frameworks[21].
In general,pore size distribution(PSD)is probably the most significant adsorbent specification that directly affects many other adsorption properties,such as surface area,bulk density and especially adsorption capacity[22–25].Bastos-Netoet al.related various textural properties(such as:PSD,micropore volume and solid surface area)to methane adsorption capacities for a number of activated carbon samples produced from different raw materials[22].They reported that“the textural parametersper sedo not unequivocally determine natural gas storage capacities.Surface chemistry and methane adsorption equilibria must be taken into account in the decision-making process of choosing an adsorbent for gas storage”.They also concluded that“for carbons produced from the same source,those which have higher surface area,higher micro-pore volume and narrower PSD within the range of 0.8–1.5 nm,show better methane adsorption properties”.Finally,they concluded that“although textural parameters provide an easy and useful tool for initial screening of activated carbons for natural gas storage,they do not allow ranking of these samples accurately”.
In 2007,Sahaet al.synthesized three different MOF-5s by dispersion in dimethylformamide(DMF),to investigate the effects of various synthesis operating conditions on their crystal structure,pore textural properties and the corresponding hydrogen adsorption performances[25].They also proposed a relationship between the PSDs of various porous MOF-5 materials and their hydrogen adsorption capacities.Finally they concluded that higher order of crystallinity in the MOF-5 materials leads to better adsorbent with larger crystal sizes,higher specific surface areas,more uniform PSDs,higher hydrogen adsorption capacities and faster hydrogen diffusions.
PSD estimation methods are usually classified into two main groups of independent and dependent methods.The former ones are more accurate but usually very cost demanding and are often used for comparison or validation purposes[26].Mercury porosimetry,X-ray diffraction(XRD),small angle X-ray scattering(SAXS),small angle neutron scattering(SANS)and nuclear magnetic response(NMR)are a few samples of such independent methods[27–33].Mercury porosimetry is more common for PSD determination of conventional adsorbents,such as active carbon and control-pore glasses(CPG)[27],while other methods are usually more adequate for extremely well structured materials such as MOFs and MCM-41(Mobil Composition of Matter No.41(mesoporous molecular sieves))[28,29,32,34].
The latter dependent group for PSD estimation is often less expensive and can be applied to almost all adsorbents.Almost most of these techniques[e.g.BJH(Barret,Joyner and Halenda),HK(Horvath–Kawzoe),KJS(Kruk–Jaroniec–Sayari),ND(Nguyen and Do(Do and co-workers)),DFT(Nonlocal density functional theory)and DBdB(Derjaguin–Broekhoff-de Boer)]require experimental adsorption(or condensation)isotherms coupled with some theoretical or analytical background[35–46].Most of these techniques suffer from some shortcomings and sometimes unrealistic assumptions some of which are discussed in more details elsewhere[47,48].Amongst the above methods,SAXS and HK procedures have received more attention for PSD estimation of MOF materialsviaindependent and dependent methods,respectively.
In 2007,Tsaoetal.employed SAXS method to determine various real structural details of MOF-5,including pore surface characteristics,pore shape,PSD,specific surface area,and pore-network structure[34].They con firmed that SAXS method provides adequate textural properties such as pore surface,pore shape,PSD and pore volume,while,BJH and HK methods may fail for some adsorbents.The HK method(presented in 1983),has been widely applied for several PSD estimations of various MOF materials[17,25,41,45,49].This method uses average potential function inside the slit-shape pores by employing the Kelvin equation[50]at the scale of molecule dimensions.Similar to other conventional PSD estimation techniques,HK method also requires a variety of detailed informations(such as diameter,polarizability and susceptibility of both adsorbent and adsorbate molecules and liquid density of adsorbate).Some of these data may not be readily available for many adsorption systems.Other conventional techniques(such as BJH)have also been extensively used to recover the PSD of various MOF materials[42,43].
In contrast to traditional dependent PSD recovery techniques,two recently proposed in-house methods[SHN1(Stands for Shahsavand–Niknam first method)and SHN2(Stands for Shahsavand–Niknam second method)]require only adsorbate surface tension and its liquid molar volume which are readily available for almost all adsorbates[51].It should be emphasized that in contrast to many conventional techniques,both SHN1 and SHN2 methods do not require any information about the form of local adsorption isotherm or kernel.A detailed comparison of various PSD estimation techniques can be found in our previous articles[47,48].
In the current article,a fast microwave technique is described for synthesis of a zeolitic imidazolate frameworks,ZIF-7.Afterwards,the collected CO2experimental isotherms data along with four other data sets of water adsorption borrowed from literature[52],over four different MOF materials(HKUST-1,ZIF-8,MIL-101 and MIL-100(Fe))are used to extract the corresponding PSDsviavarious in-house and conventional methods.The PSD prediction results are then validated by other independent techniques.It is worthwhile to mention that the most important reason for selection of ZIF-7 nano-porous material is related to its gate opening characteristic.The ZIF-7 sample can be opened or closed while it is faced with some gases such as CO2in different pressure ranges.So,because of this capability we selected this structure to investigate its PSD determinationviatheoretical methods.
2.Experimental
Synthesis of metal–organic frameworks can be carried out using several different methods such as solvothermal,microwave,sonochemical,electrochemical and mechanochemical[18,53,54].The microwave method has been employed in this work to produce ZIF-7 adsorbent as fast as possible.
2.1.Synthesis of ZIF-7
The ZIF-7 was synthesized by a microwave-assisted procedure,following the procedures given elsewhere[55,56].In this method,benzimidazole(C7H6N2,98%,from Aldrich)was used as linker,while,zinc nitrate(Zn(NO3)2·6H2O,from Fluka)was employed as metal source andN,N-dimethylformamide(DMF)(99%,Aldrich)is recruited as dispersant in a solvothermal reaction.
In a typical experiment,0.2347 g(2 mmol)benzimidazole and 0.8025 g(2.7 mmol)Zn(NO3)2·6H2O were dissolved in 75 ml DMF under sonication for 10 min.The homogeneous solution was then evenly transferred to two 80-ml reaction vessels,with about 37.5 ml each.The reaction vessels are capped tightly and kept in the microwave reaction system(Multiwave 3000/synthos 3000,Anton Paar).Synthesis was carried out at130°C with a heating rate of5°C·min−1.The reaction was performed under autogenous pressure for 200 min and,then,the product was removed from the reaction vessels and allowed to cool at room temperature.The mother liquor was carefully decanted from the product and replaced with methanol.Fresh methanol was used to exchange the DMF for 48 h at room temperature.After decanting the extra methanol and drying in air for 24 h,white crystals were obtained.The guest molecules in the crystals were removed under a dynamic vacuum at 150°C for 12 h.
2.2.Characterization of ZIF-7
The pore textural properties including the specific Langmuir area,Brunauer–Emmet–Teller(BET)surface area and pore volume were performed by analyzing adsorption and desorption N2isotherms at 77 K with Micromeritics_ASAP 2020 built-in software.Also Micromeritics_ASAP 2020 built-in software coupled with HK method was employed to calculate pore size of ZIF-7.
2.3.Adsorption of carbon dioxide on ZIF-7 at low pressures
Carbon dioxide adsorption equilibrium on ZIF-7 sample was measured in the Micromeritics_ASAP 2020 adsorption apparatus at different temperatures and CO2pressure up to 800 mm Hg(0.107 MPa).Ultrahigh purity carbon dioxide(99.999%)was utilized for the adsorption measurements.About 0.1 g of adsorbent was used for the gas adsorption studies.The initial degassing process was carried out at 150°C for 12 h under a 0.0001 mmHg(0.013 Pa)vacuum pressure.
3.Results and Discussion
The above experimental techniques and corresponding apparatuses were used to collect the following measurements of Physical properties of ZIF-7.
Scanning electron microscopy(SEM)images of the ZIF-7 crystals prepared in this work are shown in Fig.1.As it can be observed,the crystals are in flower shape.

Fig.1.SEM image of the synthesized ZIF-7.
Furthermore,the powder X-ray diffraction(XRD)patterns of the ZIF-7 sample are shown in Fig.2.The main peaks are identified by comparing the observed pattern with calculated pattern from the established crystal structure data.It can be seen from Fig.2 that the experimentally observed pattern agrees well with the simulated pattern,indicating that the bulk sample is the same as the single crystal.

Fig.2.The observed and simulated XRD patterns of ZIF-7.
Carbon dioxide adsorption was employed for the sample pore textural properties.The adsorption isotherms of carbon dioxide at three temperatures(273,298,and 323 K)and gas pressure up to 800 mmHg(1 mmHg=133.322 Pa)are demonstrated in Fig.3.
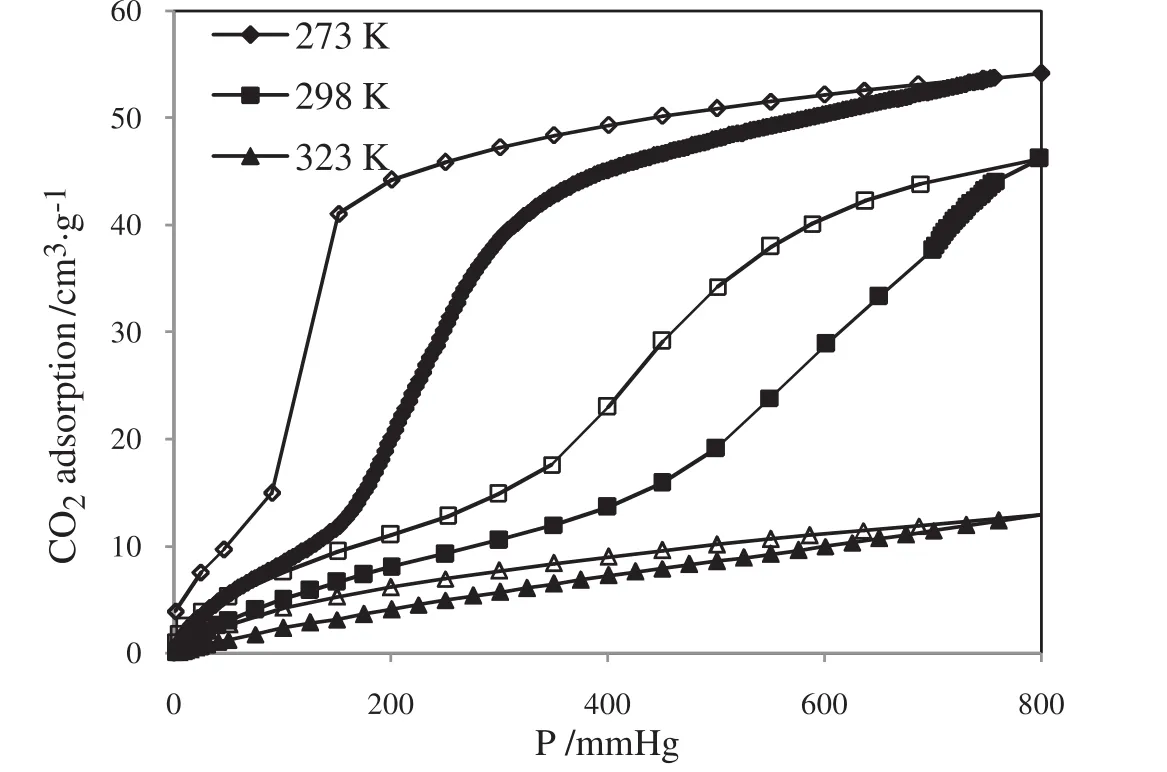
Fig.3.Carbon dioxide adsorption on ZIF-7 at 273,298 and 323 K(Filled mark:adsorption,open mark:desorption).
All temperatures are achieved by using a Dewar with a circulating jacket connected to a thermostatic bath with a precision of±0.01 °C.As it has been shown,CO2isotherm shapes at 273 and 298 K are like type IV BDDT(Brunauer,Deming,Denting,Teller)[50]or IUPAC[39]classification of adsorption isotherms.By using the adsorption isotherm of N2at77 K,the textural properties were calculated as it is demonstrated in Table 1.The Langmuir and BET specific surface area,maximum pore volume and HK-median pore size were calculated by the ASAP 2020 analyzer's built-in software.

Table 1Pore textural properties of ZIF-7
Evidently,the value of pore size of ZIF-7,shown in Table 1,suggests that the synthesized sample lies in micro-porous material category.The mean diameter of 0.4310 nm reported by Yaghiet al.[55]for ZIF-7 is in good agreement with our measurements presented in Table 1.These obtained and reported values using HK method and Yaghiet al.[55]article will be compared with the PSD estimationviaSHN methods in next sessions.
4.PSD Determination
As mentioned earlier,pore size distribution of adsorbents is one of the most significant textural properties that directly affects on the other adsorbent specifications.Various methods could be applied for PSD calculation however choosing the most convenient procedure is vital to achieve reliable size of pores.In this section at the first,SHN2 method would be utilized to extract the mean size of pores of the produced ZIF-7.Then,the obtained results will be compared with available performances of the HK method(presented in Table 1)and reported one by Yaghiet al.Subsequently,both SHN1 and SHN2 methods are applied for PSD determination of four different MOFs borrowed from literatureviaapplying water adsorption isotherm data[52].
4.1.Theoretical estimation of optimal PSD
Detailed descriptions of SHN1 and SHN2 are presented in our previous articles[47,48].A brief overview will be presented here to familiarize the reader with the essence of the proposed methods.
The following integrals are usually used to find the PSD of a heterogeneous solid adsorbent(f(r))from a set of noisy measured isothermal data available for the amount of adsorbed material at a given set of pressures(Pi;i=1,…,n):

After construction of matrix R and using proper order of regularization technique(with appropriate form of matrix B),the optimal level of regularization parameter(λ*)should be selected to establish the best stabilization of the solution vector f(r).Our other recently published article reviews various stabilization criteria(e.g.LOOCV(Leave One Out Cross Validation),LC(L-Curve method),MLC(Modified L-Curve method),UC(U-Curve method)and MUC(Modified U-Curve method))for automatic selection of optimum regularization parameter(λ*)[57].
Various real case studies will be employed to investigate the capability of SHN1 and SHN2 methods for determination of correct pore size distributions for various MOF materials.These methods are more suitable for types IV and II(SHN2)or V and III(SHN1)isotherms of BDDT or IUPAC classification.Various measured PSD's of ZIF-7 at different temperatures(as presented in Fig.3)and borrowed experimental PSD's[52]of HKUST-1,ZIF-8,MIL-101,MIL-100(Fe)will be used to validate the successful performances of SHN1 and SHN2 methods on capturing the true PSD from real adsorption isotherms.It is worthwhile to mention that the predicted PSD's reported by Yaghiet al.for ZIF-7[55],are computedviaindependent method of topology study.Finally,two isotherms of carbon dioxide adsorption over ZIF-7(Fig.3)at two different temperatures and four water adsorption isotherms on different MOF materials(Fig.4)are used to calculate the corresponding PSD's of five MOFs by resorting to SHN1 and/or SHN2 methods.
4.2.Optimal calculation of PSD
As SHN1 and SHN2 methods are based on regularization technique the order of regularization and also the value of regularization parameter(level)have crucial effect on the overall performances.Fig.5a and b illustrate the efficient effect of the order and level of regularization on PSD calculation of one of the studied samples(e.g.ZIF-8)respectively(The effect of these two parameters for other MOFs is depicted in Supplementary material).
As it has been shown in this figure,three orders of regularization,namely first,second and third show the same behavior.It proves that these methods can be readily used at various orders of regularization;however,the cost of time will increase with increasing the order of regularization.Furthermore,almost always the zero order of regularization technique provides relatively non-smooth solutions,while other regularization orders lead to very similar PSDs.It has to be noted,regularization orders more than three can be easily developed and successfully used for PSD recovery[57].
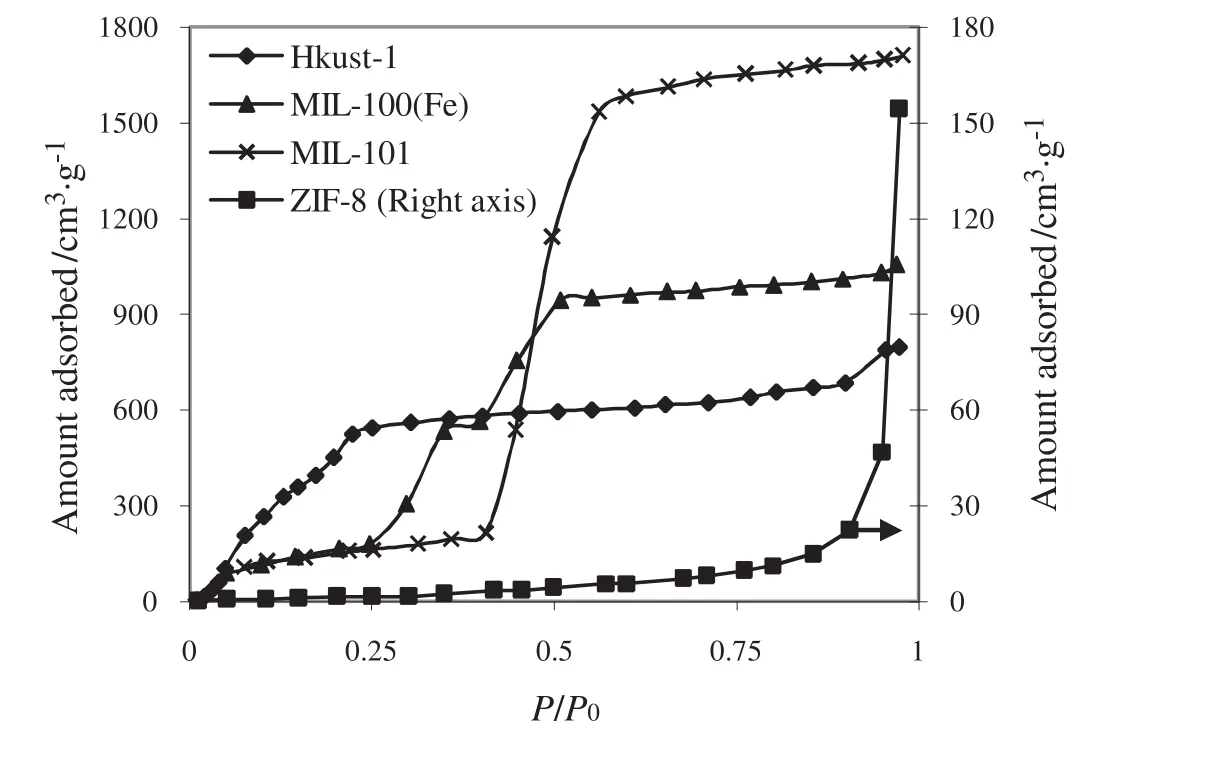
Fig.4.Water adsorption isotherms for different MOF materials at 298 K(ZIF-8 right axis,others left axis).All data are borrowed from literature[52].
On the other hand,regularization level is more important parameter that could adversely affect the produced results,if they are selected improperly.The effect of this factor on the PSD performanceviaSHN methods(Fig.5b),was reproduced in four levels(λ).Based on final conclusion of regularization order(last paragraph)and also to minimize the number of figures without missing any vital information,the effect of regularization level was investigated for the first order of regularization.
As it can be seen,the too small values of λ could not predict the accurate values of PSD and result in highly oscillatory solutions.On the other hand,extremely large values of regularization parameter ignore the information embedded in the coefficient matrix[47,48]used in SHN methods and pushes the final solution,f(r)towardaprioriinformation sought for the specific order of regularization(a constant for first order regularization).Therefore, finding the optimal regularization parameters(λ*)has important rule in accurate PSD recovery.Since the value of regularization level can vary in the entire domain of positive real numbers there should exist an optimal value of regularization parameter for each isotherm which provides the optimal PSD recovery performance.Evidently,the optimal value of λ*tends to zero for wellbehaved RTR(coefficient)matrices.On the contrary,the value of λ*will increase to infinity when the corresponding matrix RTR becomes severely ill-conditioned,i.e.singular[47,48].
Furthermore,the optimal regularization level can be selected by visual method when the actual PSD is known.Otherwise,more advanced techniques like leave one out cross validation(LOOCV),L-curve,U-curve and Modified U-curve are required for automatic selection of the optimal regularization level.This point has been received more attention in the literature by Shahsavand and Niknam[57].Finally some important notes are remembered as follows.(a)Shown results in Fig.5a,were depicted at the optimal regularization values(λ*)that are stated in parenthesis.(b)SHN1 was used for PSD determination in ZIF-8 sample.(c)Because of uniformity of pore sizes in MOF materials,the median point of calculated pore size distribution should be considered as a dominant size of pores.
Consequently,the first order of regularization and LOOCV criterion was employed for PSD estimation of all MOF samples using adsorption isotherms(Figs.3 and 4).Fig.6 shows the PSD of ZIF-7 extracted from SHN2 method.As expected,the obtained results from CO2isotherms at 273 and 298 K don't show significant difference.As a result,any adsorbate at any arbitrary temperature which exhibits isotherm shapes like type IV and II or V and III can be used for PSD recoveryviaSHN methods.Moreover,the obtained results from SHN2 and HK methods have been demonstrated and compared together in Fig.6.As mentioned in Introduction,the HKmethod isone ofthe most-applied procedures to calculate PSDofMOFs in which usually N2adsorption isotherm at77 Kis used[34].
Dashed line in Fig.6 illustrates the actual(real)pore diameter of ZIF-7,obtainedviastructure study[52].As it can be seen,recovered PSDviaSHN2 method is in good agreement with real diameter and estimated pore diameters using HK method.

Fig.5.Effect of order(a)and level(b)of regularization on PSD calculation calculating SHN methods.

Fig.6.The comparison of SHN2 and HK procedures with the actual measured pore size distributions for ZIF-7(Circle and rectangular:SHN2 method using CO2 isotherm at 273 K and 298 K respectively,dash dot:average pore size obtained using HK method,dashed line:actual PSD obtained via topology study[55]).
The calculated PSDs for other samples by using SHN methods are depicted in Fig.7.Water adsorption isotherms at 298 K(Fig.4)were used for their PSD recovery.
Evidently,Fig.7 con firms the remarkable performance of SHN methods to predict the actual pore sizes.It is important to know that the SHN1 method was employed for ZIF-8(because its water isotherm exhibits type V BDDT classification)and SHN2 was utilized for HKUST-1,MIL-101,MIL-100(Fe)(because their water isotherms show type IV BDDT classification).
As before,in this figure,dashed lines reflect the real(actual)PSDs extracted from literature(viatopology or morphology studies)[52].Obviously,all predicted PSDs by using SHN methods reveal the highest agreement with actual data.
As it was concluded,the first order of regularization was used for all PSD calculations.The optimum values of regularization levels for each adsorbent calculatedviaLOOCV criterion are presented in Table 2.If other regularization orders are employed,the optimum values change as shown in Table S.1 in Supporting information.
Table 3 also includes all required information necessary in PSD recoveryviaSHN methods.It should be pointed out that successful application of SHN methods for adsorbents like AC,CMC and CPG has already been proved[47,48].
5.Conclusions
A typical Zeolitic Imidazolate Frameworks,known as ZIF-7,was successfully synthesized using fast microwave fabrication technique.The collected ZIF-7 is then characterized using various experimental facilities such as SEM and CO2adsorption apparatus.Carbon dioxide isotherms at three different temperatures were used coupled with two in-house(SHN)methods to extract the unique PSD of ZIF-7.Furthermore,four other experimental isotherms for adsorption of water vapor at 298 K on various MOF materials were borrowed from literature and the corresponding PSDs were successfully predictedviasame SHN procedures.Both of these in-house methods do not require any restrictive pre-assumptions and only need minimum information to predict PSD for any shape of pores with any range of pore size.
Since both SHN1 and SHN2 techniques have deep roots in advanced mathematical topics such as inverse theory and linear regularization method,therefore,the effect of regularization order and the corresponding optimal regularization level were discussed in sufficient details.The computation results clearly indicated that the order of regularization has minor effect on the overall performance of the SHN methods.On the other hand,the optimal value of the regularization parameter has crucial effect on the final extracted PSD.The leave One Out Cross Validation(LOOCV)technique was successfully used for efficient calculation of the optimal regularization level for any given order of regularization.

Fig.7.Optimal PSD recovery for various MOFs calculated by SHN techniques(dashed line:actual PSD obtained via topology study[52]).

Table 2The optimum levels of regularization(λ*)for all adsorbents at first order of regularization
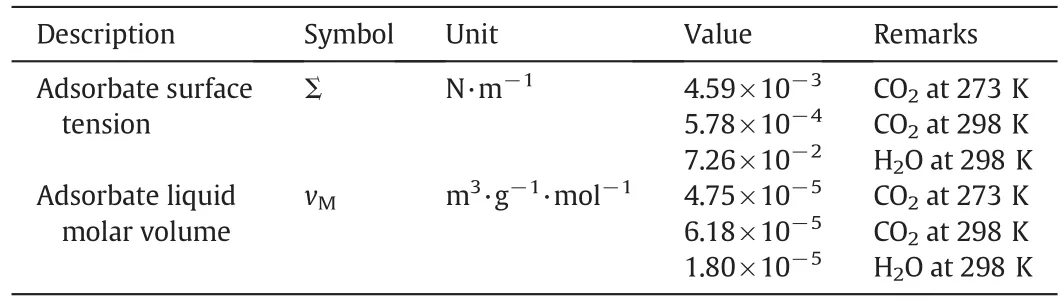
Table 3The only required physical properties for PSD recovery via SHN methods[51]
Supplementary Material
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cjche.2016.10.012.
[1]E.A.Tomic,Thermal stability of coordination polymers,J.Appl.Polym.Sci.9(1965)3745–3752.
[2]H.Li,M.Eddaoudi,M.O'Keeffe,O.M.Yaghi,Design and synthesis of an exceptionally stable and highly porous metal–organic framework,Nature402(1999)276–279.
[3]H.Furukawa,O.M.Yaghi,Storage of hydrogen,methane,and carbon dioxide in highly porous covalent organic frameworks for clean energy applications,J.Am.Chem.Soc.131(25)(2009)8875–8883.
[4]D.Britt,D.Tranchemontagne,O.M.Yaghi,Metal–organic frameworks with high capacity and selectivity for harmful gases,PNAS105(2008)11623–11627.
[5]H.Wu,W.Zhou,T.Yildirim,High-capacity methane storage in metal–organic frameworks M2(dhtp):The important role of open metal sites,J.Am.Chem.Soc.131(13)(2009)4995–5000.
[6]R.J.Kuppler,D.J.Timmons,Q.R.Fang,J.R.Li,T.A.Makal,M.D.Young,D.Yuan,D.Zhao,W.Zhuang,H.C.Zhou,Potential applications of metal–organic frameworks,Coord.Chem.Rev.253(2009)3042–3066.
[7]J.R.Long,O.M.Yaghi,The pervasive chemistry of metal–organic frameworks,Chem.Soc.Rev.38(2009)1213–1214.
[8]P.Chowdhury,C.Bikkina,S.Gumma,Gas adsorption properties of the chromiumbased metal organic framework MIL-101,J.Phys.Chem.C113(2009)6616–6621.
[9]U.Mueller,M.Schubert,F.Teich,H.Puetter,K.Schierle-Arndt,J.Pastre,Metal–organic frameworks—Prospective industrial applications,J.Mater.Chem.16(2006)626–636.
[10]R.Sabouni,H.Kazemian,S.Rohani,A novel combined manufacturing technique for rapid production of IRMOF-1 using ultrasound and microwave energies,Chem.Eng.J.165(2010)966–973.
[11]C.M.Lu,J.Liu,K.Xiao,A.T.Harris,Microwave enhanced synthesis of MOF-5 and its CO2capture ability at moderate temperatures across multiple capture and release cycles,Chem.Eng.J.156(2010)465–470.
[12]A.F.P.Ferreira,J.Santos,M.G.Plaza,N.Lamia,J.M.Loureiro,A.E.Rodrigues,Suitability of Cu-BTC extrudates for propane–propylene separation by adsorption processes,Chem.Eng.J.167(2011)1–12.
[13]A.R.Millward,O.M.Yaghi,Metal–organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature,J.Am.Chem.Soc.127(2005)17998–17999.
[14]R.E.Morris,P.S.Wheatley,Gas storage in nanoporous materials,Angew.Chem.Int.Ed.47(2008)4966–4981.
[15]L.G.Qiu,L.N.Gu,G.Hu,L.Zhang,Synthesis,structural characterization and selectively catalytic properties of metal–organic frameworks with nano-sized channels:A modular design strategy,J.Solid State Chem.182(2009)502–508.
[16]S.Wang,Q.Yang,C.Zhong,Adsorption and separation of binary mixtures in a metal–organic framework Cu-BTC:A computational study,Sep.Purif.Technol.60(2008)30–35.
[17]Y.Li,R.T.Yang,Gas adsorption and storage in metal–organic framework MOF-177,Langmuir23(2007)12937–12944.
[18]D.Tranchemontagne,J.R.Hunt,O.M.Yaghi,Room temperature synthesis of metal–organic frameworks:MOF-5,MOF-74,MOF-177,MOF-199,and IRMOF-0,Tetrahedron64(2008)8553–8557.
[19]S.Kitagawa,R.Kitaura,S.Noro,Functional porous coordination polymers,Angew.Chem.Int.Ed.43(2004)2334–2375.
[20]J.L.C.Rowsell,O.M.Yaghi,Metal–organic frameworks:A new class of porous materials,Microporous Mesoporous Mater.73(2004)3–14.
[21]S.Li,F.Huo,Metal–organic framework composites:From fundamentals to applications,Nanoscale7(2015)7482–7501.
[22]M.Bastos-Neto,D.V.Canabrava,A.E.B.Torres,E.Rodriguez-Castellon,A.Jimenez-Lopez,D.C.S.Azevedo,C.L.CavalcanteJr,Effects of textural and surface characteristics of microporous activated carbons on the methane adsorption capacity at high pressures,Appl.Surf.Sci.253(2007)5721–5725.
[23]D.Lozano-Castello,D.Cazorla-Amoros,A.Linares-Solano,D.F.Quinn,Influence of pore size distribution on methane storage at relatively low pressure:Preparation of activated carbon with optimum pore size,Carbon40(2002)989–1002.
[24]D.Lozano-Castello,J.Alcaniz-Monge,M.A.de la Casa-Lillo,D.Cazorla-Amoros,A.Linares-Solano,Advances in the study of methane storage in porous carbonaceous materials,Fuel81(2002)1777–1803.
[25]D.Saha,S.Deng,Z.Yang,Hydrogen adsorption on metal–organic framework(MOF-5)synthesized by DMF approach,J.Porous.Mater.16(2)(2009)141–149.
[26]P.Kowalczyk,M.Jaroniec,A.P.Terzyk,K.Kaneko,D.D.Do,Improvement of the Derjaguin–Broekhoff-de Boer theory for capillary condensation/evaporation of nitrogen in mesoporous systems and its implications for pore size analysis of MCM-41 silicas and related materials,Langmuir21(2005)1827–1833.
[27]O.Solcova,L.Matêjová,P.Schneider,Pore-size distributions from nitrogen adsorption revisited:Models comparison with controlled-pore glasses,Appl.Catal.A Gen.313(2006)167–176.
[28]M.Kruk,M.Jaroniec,A.Sayari,Relations between pore structure parameters and their implications for characterization of MCM-41 using gas adsorption and X-ray diffraction,Chem.Mater.11(1999)492–500.
[29]M.Kruk,M.Jaroniec,Y.Sakamoto,O.Terasaki,R.Ryoo,C.H.Ko,Determination of pore size and pore wall structure of MCM-41 by using nitrogen adsorption,transmission electron microscopy,and X-ray diffraction,J.Phys.Chem.B104(2000)292–301.
[30]A.P.Radlinski,M.Mastalerz,A.L.Hinde,M.Hainbuchner,H.Rauch,M.Baron,J.S.Lin,L.Fan,P.Thiyagarajan,Application of SAXS and SANS in evaluation of porosity,pore size distribution and surface area of coal,Int.J.Coal Geol.59(2004)245–271.
[31]E.Huang,M.F.Toney,W.Volksen,D.Mecerreyes,P.Brock,H.-C.Kim,C.J.Hawker,J.L.Hedrick,V.Y.Lee,T.Magbitang,R.D.Miller,Pore size distributions in nanoporous methyl silsesquioxane films as determined by small angle x-ray scattering,Appl.Phys.Lett.81(2002)2232–2234.
[32]R.Schmidt,E.W.Hansen,M.StiScker,D.Akporiaye,O.H.Ellestad,Pore size determination of MCM-41 mesoporous materials by means of1H NMR spectroscopy,N2adsorption,and HREM.A preliminary study,J.Am.Chem.Soc.117(1995)4049–4056.
[33]K.Kaneko,Determination of pore size and pore size distribution 1.Adsorbents and catalysts,J.Membr.Sci.96(1994)59–89.
[34]C.Tsao,M.Yu,T.Chung,H.Wu,C.Wang,K.Chang,H.Chen,Characterization of pore structure in metal–organic framework by small-angle X-ray scattering,J.Am.Chem.Soc.129(2007)15997–16004.
[35]E.P.Barrett,L.G.Joyner,P.P.Halenda,The determination of pore volume and area distributions in porous substances.I.Computation from nitrogen isotherms,J.Am.Chem.Soc.73(1951)373–380.
[36]G.Horvath,K.Kawazoe,Method for the calculation of effective pore size distribution in molecular sieve carbon,J.Chem.Eng.Jpn16(1983)470–475.
[37]M.Kruk,M.Jaroniec,A.Sayari,Application of large pore MCM-41 molecular sieves to improve pore size analysis using nitrogen adsorption measurements,Langmuir13(1997)6267–6273.
[38]C.Nguyen,D.D.Do,A new method for the characterization of porous materials,Langmuir15(1999)3608–3615.
[39]Z.Ryu,J.Zheng,M.Wang,B.Zhang,Characterization of pore size distributions on carbonaceous adsorbents by DFT,Carbon37(1999)1257–1264.
[40]J.C.P.Broekhoff,J.H.de Boer,Studies on pore systems in catalysts:IX.Calculation of pore distributions from the adsorption branch of nitrogen sorption isotherms in the case of open cylindrical pores A.Fundamental equations,J.Catal.9(1967)8–14.
[41]Z.Bao,L.Yu,Q.Ren,X.Lu,S.Deng,Adsorption of CO2and CH4on a magnesiumbased metal organic framework,J.Colloid Interface Sci.353(2011)549–556.
[42]D.Xuan-Dong,H.Vinh-Thang,S.Kaliaguine,MIL-53(Al)mesostructured metal–organic frameworks,Microporous Mesoporous Mater.141(2011)135–139.
[43]B.Liu,H.Shioyama,H.Jiang,X.Zhang,Q.Xu,Metal–organic framework(MOF)as a template for syntheses of nanoporous carbons as electrode materials for supercapacitor,Carbon48(2010)456–463.
[44]F.Shi,M.Hammoud,L.T.Thompson,Selective adsorption of dibenzothiophene by functionalized metal organic framework sorbents,Appl.Catal.B Environ.103(2011)261–265.
[45]D.Saha,Z.Wei,S.Deng,Equilibrium,kinetics and enthalpy of hydrogen adsorption in MOF-177,Int.J.Hydrog.Energy33(2008)7479–7488.
[46]B.Mu,P.M.Schoenecker,K.S.Walton,Gas adsorption study on mesoporous metal–organic framework UMCM-1,J.Phys.Chem.C114(2010)6464–6471.
[47]A.Shahsavand,M.Niknam Shahrak,Direct pore size distribution estimation of heterogeneous nano-structured solid adsorbents from condensation data:Condensation with no prior adsorption,Colloids Surf.A Physicochem.Eng.Asp.378(2011)1–13.
[48]A.Shahsavand,M.Niknam Shahrak,Reliable prediction of pore size distribution for nano-sized adsorbents with minimum information requirements,Chem.Eng.J.171(2011)69–80.
[49]D.Saha,S.Deng,Synthesis,characterization and hydrogen adsorption in mixed crystals of MOF-5 and MOF-177,Int.J.Hydrog.Energy34(2009)2670–2678.
[50]D.D.Do,Adsorption Analysis:Equilibria Kinetics,Imperial College Press,London,1999.
[51]C.L.Yaws,Chemical Properties Handbook,Mc-Graw-Hill,NY,1999.
[52]P.Küsgens,M.Rose,I.Senkovska,H.Frde,A.Henschel,S.Siegle,S.Kaskel,Characterization of metal–organic frameworks by water adsorption,Microporous Mesoporous Mater.120(2009)325–330.
[53]Z.Lin,D.S.Wragg,R.E.Morris,Microwave-assisted synthesis of anionic metal–organic frameworks under ionothermal conditions,Chem.Commun.19(2006)2021–2023.
[54]Y.R.Lee,J.Kim,W.S.Ahn,Synthesis of metal–organic framework:A mini review,Korean J.Chem.Eng.30(2013)1667–1680.
[55]K.S.Park,Z.Ni,A.P.Côté,J.Y.Choi,R.Huang,F.J.Uribe Romo,H.K.Chae,M.O'Keeffe,O.M.Yaghi,Exceptional chemical and thermal stability of zeolitic imidazolate frameworks,Proc.Natl.Acad.Sci.103(2006)10186–10191.
[56]X.Wu,M.Niknam Shahrak,B.Yuan,S.Deng,Synthesis and characterization of zeolitic imidazolate framework ZIF-7 for CO2and CH4separation,Microporous Mesoporous Mater.190(2014)189–196.
[57]M.NiknamShahrak,A.Shahsavand,A.Okhovat,Robust PSD determination of micro and meso-pore adsorbents via novel modified U curve method,Chem.Eng.Res.Des.91(2013)51–62.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Influence of Na+,K+,Mg2+,Ca2+,and Fe3+on filterability and settleability of drilling sludge☆
- An optimal filter based MPC for systems with arbitrary disturbances☆
- Measurement and calculation of solubility of quinine in supercritical carbon dioxide☆
- Solubility and metastable zone width measurement of 3,4-bis(3-nitrofurazan-4-yl)furoxan(DNTF)in ethanol+water
- Partition coefficient prediction of Baker's yeast invertase in aqueous two phase systems using hybrid group method data handling neural network
- The effect of transition metal ions(M2+=Mn2+,Ni2+,Co2+,Cu2+)on the chemical synthesis polyaniline as counter electrodes in dye-sensitized solar cells☆
