Synthesis of a novel functional group-bridged magnetized bentonite adsorbent:Characterization,kinetics,isotherm,thermodynamics and regeneration☆
2017-05-28ZhichaoLouWeiZhangXiaodanHuHaiqianZhang
Zhichao Lou *,Wei Zhang ,Xiaodan Hu ,Haiqian Zhang ,3
1 College of Materials Science and Engineering,Nanjing Forestry University,Nanjing 210037,China
2 College of Materials Science and Technology,Nanjing University of Aeronautics and Astronautics,Nanjing 210016,China
3 State Key Laboratory of Bioelectronics,Jiangsu Key Laboratory for Biomaterials and Devices,School of Biological Science and Medical Engineering,Southeast University,Nanjing 210096,China
1.Introduction
Colored wastewater,which is a consequence of batch processes both in the dye manufacturing industries and in the dye-consuming industries,has been a growing problem of water pollution[1].Along with the revolutionary developments of industrial and economic situation in China,massive dyes used in the textile coloration process are discharged directly in aqueous effluent with 3000–4000 kt per day.The abnormal coloration and reduction in photosynthesis caused by the dyes are destroying the aquatic ecosystem[2].Moreover,dyes can lead to carcinogenic and mutagenic effects on the organs of a variety of mammals,and can further harm human health through the food chain[3].Thus,the treatment of effluentcontaining dyes is of great significance.
In decades,numerous methods have been proposed to treat dye effluents.Though some of them,such as ozonation[4]and extraction[5],can significantly reduce the chemical oxygen demand(COD)value of wastewater,they need a large amount of oxidizing agent or organic solvent in the purification processes,causing secondary pollution.Most environmentally friendly methods are cumbered for widespread industrial use by the disadvantages of their own.For example,the membrane used in filtration is not resistant to corrosion,and its flux decreases with time goes by[6].The electrochemical treatments require high-energy consumption and expensive equipment[7].The microbial biological processes are now the most fledged and commonly-used in practical treatments,but their removal rates become lower with the presence of nonbiodegradable heterocyclic and aromatic components[8].These issues highlight the urgency of the field,and a continuous need to construct an environmentally friendly,efficient and low-cost method that can be used to treat the dye wastewaters.
Compared with the methods mentioned above,adsorption is proved to be a more effective treatment for dye effluents,owing to the initial cost, flexibility and simplicity of design,ease of operation and insensitivity to toxic pollutants[9].Natural bentonite has a specific surface area,high chemical and mechanical stability.It is one of the promising adsorbents for removing dye pollutants from wastewaters[10].Surface-functional Fe3O4nanoparticles have been developed and used as novel adsorbents for dye effluents due to their good properties,such as magnetic performance,recycling capability,specificity to certain dyes and ease of separation[11–13].Yanet al.utilized(3-Aminopropyl)triethoxysilane(APTES)modified Fe3O4nanoparticles as a magnetic adsorbent for the removal of C.I.Reactive Red 228 and Congo Red from aqueous solution[12].However,the magnetized bentonite by Fe3O4,which can be treated as compound adsorbents owning the advantages of both bentonite and Fe3O4,is barely studied.Previously,our group developed a kind of Fe3O4-magnetized bentonite(Fe3O4/bentonite)for methylene blue(MB)removal from aqueous solution[14].But this magnetic adsorbent is found to be unstable in continuous usage,with gradually lose of its magnetic part(Fe3O4nanoparticles on the bentonite surface).This will lead to magnetic weakening,as well as secondary pollution.The reason for this phenomenon is that the interaction between Fe3O4nanoparticles and bentonite is physical and weak.
Herein,a novel magnetized bentonite(APTES-Fe3O4/bentonite)was synthesized using APTESas a“bridge”to combine the magnetic particles(Fe3O4)with bentonite,viathe interactions between the amino groups(--NH3)of APTES-Fe3O4and the hydroxyl groups(--OH)on the bentonite surface.We chose MB as a model dye to investigate the adsorption ability of this magnetized bentonite for cationic dyes,because MB is not only a traditional industrial dye but also a handy,colorful biological dye used in the microscope experiments.The APTES-Fe3O4/bentonite was characterized using TEM,XRD,FT-IR,TGA,VSM,zeta potential analysis and BET.The effects of experimental conditions in adsorption process were explored.Regeneration of the adsorbent,which is a critical step contributing to the costs in industrial applications[15],was obtainedviagamma-irradiation in60Co sources[16].Additionally,the kinetic,thermodynamics and isotherm parameters were investigated.
2.Materials and Methods
2.1.Materials
Ferric chloride hexahydrate (FeCl3·6H2O),ferrous chloride tetrahydrate(FeCl2·4H2O),ammonium hydroxide,sodium hydroxide,hydrochloric acid,(3-aminopropyl)triethoxysilane and methylene blue were of analytical grade and purchased from Aldrich(Germany).All aqueous solutions were prepared with de-ionized water,which is purified with Milli-Q Plus water purification system.pH value of the mixture was adjusted using 0.1 mol·L−1HCl and 0.1 mol·L−1NaOH.All the samples were weighed by XPE26 provided by Mettler Toledo®in Switzerland,with the error of 0.001 mg.
2.2.Synthesis of APTES-Fe3O4/bentonite compound adsorbent
APTES-Fe3O4nanoparticles were prepared according to our previous publication[17].APTES-Fe3O4nanoparticles were then added into 100 ml bentonite solution,the mixture was stirred under N2at 80°C for 2 h.After the reaction,the products were isolated by magnetic separation and were rinsed with 80°C water three times.Finally,APTESFe3O4/bentonite were dried under vacuum at70°C.The general scheme for synthesis of APTES-Fe3O4/bentonite adsorbent is illustrated in Fig.1A.Since the APTES-Fe3O4nanoparticles possess plenty of amino functional groups,these groups can interact strongly with the hydroxylated edge groups surrounding bentoniteviaeither(i)hydrogen bonds or(ii)electrostatic interactions[18].
2.3.Adsorption experiments
The removal process of MB with the help of the APTES-Fe3O4/bentonite and an external magnetwas shown in Fig.1B.The batch sorption studies were performed by adding the obtained magnetic adsorbents into the MB solutions.After the mixture processviaultrasound treatment for half a minute,the mixtures were shaken in a thermostatic shaker at controlled temperature.The samples were taken at appropriate time intervals as necessary.The magnetic adsorbents with the adsorbed MB were separated from the solution by the aid of a magnet,and then the concentrations of the rest MB were calculated.Fig.1C is the structure of MB.The standard curve of MB solution was determined by measuring the optical density(OD)of MB aqueous solution at 665 nm with concentrations ranging from 0.5 mg·ml−1to 10 mg·ml−1.This curve was used to calculate the concentration of each experiment and convert absorbance data into concentrations for further studies.All the experimental adsorption data were collected from three independently repeated experiments.Unless extra notation,the adsorption processes were performed at 25°C and pH 7.0.The pH values of the MB solution were adjusted using 0.1 mol·L−1NaOH and 0.1 mol·L−1HCl solutions.

Fig.1.Schematic representation of(A)the synthesis of APTES-Fe3O4/bentonite adsorbent,and(B)its application in the adsorption of MB with the help of an external magnet.And(C)structure of MB.
2.3.1.Theadsorptioncapacityq(mg·g−1)andefficiencyη(%)valuesof the magnetic adsorbent
Theqand η values were calculated using Eqs.(1)and(2).

whereC0is the initial MB concentration andC1is the MB concentration in the supernatant solution(mg·L−1).V(L)is the volume of the solution.madsorbentis the mass of the adsorbent(g).
2.3.2.Effect of pH
The adsorption of MB(16 mg·L−1,5 ml)viaAPTES-Fe3O4/bentonite with the dosage of0.55 mg was investigated at pH values spanning from 2 to 10.The initial pH values of the MB solution were adjusted using 0.1 mol·L−1NaOH and 0.1 mol·L−1HCl solutions.In this study,MB solution was agitated with the magnetic adsorbent using ultrasound at room temperature.The samples were magnetic separated and the supernatants were analyzed.
2.3.3.Effect of temperature and adsorption isotherm
The effect of temperature on adsorption capacities was studied at pH 7.0,with certain amount of APTES-Fe3O4/bentonite and 50 ml MB solution(10,15,20,25,30 mg·L−1)at 293,303,313,323 and 333 K.After the equilibrium,the adsorbent was removed and the remaining concentration of MB was recorded.
2.3.4.Effect of contact time and kinetic studies
100 ml of MB solutions with 10 mg·L−1,12.5 mg·L−1,15 mg·L−1,17.5 mg·L−1,and 20 mg·L−1initial concentrations were gently shaken with a certain amount of the adsorbent(10 mg)without adjusting pH.The samples were taken at constant time intervals,and the aqueous phase was separatedviamagnetic decantation.
2.4.Characterization
UV–vis spectroscopy was carried out by a UV-3500 UV–Vis-NIR spectrophotometer produced by Shimadzu.Transmission electron microscopy(TEM)analysis was performed to study the morphology of APTES-Fe3O4and APTES-Fe3O4/bentonite composites.The particle sizes were measured and collected by a software program named Nano Measurer 1.2.X-ray diffraction(XRD)data were collected on an X-ray diffractometer employing CuKαradiation at 40 kV and 40 mA with 200 mg of each sample.The IR spectra were recorded by a Fourier transform infrared spectrophotometer(FT-IR),and each sample together with KBr was pressed to form a tablet.Magnetic measurements were carried out using a Lake Shore 7407 vibrating sample magnetometer(VSM)provided by East Changing Technologies,Inc.BET was measured by nitrogen adsorption and adsorption at77 K,using ASAP 2020 in Nanjing University.Zeta potential of both adsorbents were measured by Zeta Potential Analyzer in Southeast University.Thermal gravimetric analysis(TGA)was done by means of TG-DSC TAQ600.
2.5.Regeneration and reuse experiments
Regeneration of the adsorbents was carried outviagammairradiation in60Co sources[16].Firstly,we re-dispersed the collected saturated MB/APTES-Fe3O4/bentonite composites in waterviaultrasonic oscillations.Then the solution containing MB saturated magnetic adsorbents was exposed to the60Co sources at the dose rate of1 kGy·h−1.The required gamma-irradiation dose depends on the amount of the saturated magnetic adsorbents in the solution.Each gram of the saturated MB/APTES-Fe3O4/bentonite composites needs 6 kGy.All these parameters mentioned here are determined by pre-experiments,for the purpose to completely degrade the adsorbed MB but not to destroy the structural units surrounding the adsorbent surface.After irradiation,the composites were magnetically separated and redisposed for reuse.The adsorption capacities of the recycled magnetic adsorbent were assessed in nine consecutive cycles.
3.Results and Discussion
3.1.TEM,XRD,BET,VSM and TGA characterization
Fig.2A shows morphologies of APTES-Fe3O4and APTES-Fe3O4/bentonite.The TEM result shows that APTES-Fe3O4nanoparticles are uniformly dispersed and morphology unified.The diameter of APTES-Fe3O4nanoparticles was determined by measuring the long axis of each particle,indicating the average size of APTES-Fe3O4was(9.92±0.09)nm(insert in Fig.2A).From the TEM result of APTES-Fe3O4/bentonite,we may see that individual APTES-Fe3O4nanoparticles(smaller and darker grains,pointed by red arrows)are dispersed on the surface of bentonite(thinner and plate-like,pointed by yellow arrows).From the enlarged TEM image inserted,we can not only clearly see the APTES-Fe3O4nanoparticles modified on the surface of bentonite,but also clearly observe the laminated structures of the bentonite matrix.All the APTES-Fe3O4nanoparticles were observed to tightly bind to the bentonite surface.
The XRD patterns of as-prepared APTES-Fe3O4/bentonite are given in Fig.2B.From Fig.2B,we may observe the diffraction peak positions at 2θ =30.0°,35.3°,43.0°,56.9°,and 62.5°corresponding to the(220),(311),(400),(511),and(440)planes of Fe3O4in a cubic phase[19].The peaks at 2θ=26.6°and 27.8°which are attributed to quartz of bentonite can also be observed.Since these patterns were obtained by drying the magnetic fractions from the samples,the results here indicated that the APTES-Fe3O4nanoparticles and bentonite were evidently combined and attracted by a magnetic field.
The surface nature of these materials was con firmed by five-point BET surface measurements.The resulting BET equation is

whereP0andPare the saturation and the equilibrium pressure ofadsorbates at the temperature of adsorption,Qis the adsorbed gas quantity,andAandIare the slope andy-intercept values of the straight line with 1/Q[(P0/P)−1]on they-axis andP0/Pon thex-axis according to the nitrogen adsorption–desorption isotherm experiments we used here.The adsorption–desorption isotherms and calculated data used for the five-point BET surface area analysis are plotted in Fig.2C.The BET surface area of natural bentonite and APTES-Fe3O4/bentonite is 39.60 and 140.62 m2·g−1,respectively.This high surface area of APTES-Fe3O4/bentonite generated is mainly by the interstitial space between the combined bentonite and APTES-Fe3O4,and its high value reflects its open mesoporous architecture.The high BET surface area is beneficial for adsorption reactions.
As shown in Fig.2D,the saturation magnetization value of APTESFe3O4/bentonite is 30.16 emu·g−1,indicating that the as-prepared magnetic adsorbent has a fast response to an external magnetic field.
Fig.2E shows a representative set of TGA data for APTES-Fe3O4/bentonite.From the TGA curve,we could conclude that the as-prepared magnetic adsorbent exhibited three steps of weight loss.The adsorbed water was lost from the clay atT<200 °C,corresponding to 2.90%.After this,physicochemical-adsorbed loss occurred in two steps.The magnetic adsorbent showed a mass lose(6.01%)in the temperature range of~415 °C due to the decomposition of APTES[20].Another mass loss(2.60%)centered in the range of 500–900 °C due to the dehydroxylation of the OH units[16].

Fig.2.(A)TEMimages ofAPTES-Fe3O4,and APTES-Fe3O4/bentonite.Insert:histogram indicating the particle size distributions ofAPTES-Fe3O4 nanoparticles and the enlarged TEM image of the squared area by blue dashed lines,respectively.(B)XRD patterns for APTES-Fe3O4/bentonite obtained by drying the fractions.(C)The five-point BET surface area analysis for APTESFe3O4/bentonite and natural bentonite.(D)Magnetization hysteresis for the APTES-Fe3O4/bentonite.(E)TGA data for APTES-Fe3O4/bentonite.
3.2.FT-IR characterization of APTES-Fe3O4/bentonite differs through adsorption process
FT-IR spectra of APTES-Fe3O4/bentonite(A)and MB/APTESFe3O4/bentonite(B)are shown in Fig.3.For APTES-Fe3O4/bentonite,the broad and intense adsorption peak at around 3415.1 cm−1corresponds to the HO--H vibration of the water molecules absorbed on the silicate surface.The peak at 3735.4 cm−1is due to the O--H stretching vibration of the silanol groups(Si-OH).The peak at 1630.9 cm−1is attributed to the bending HO-H bond of water molecules retained in the silicate matrix.The bands representing Al--Al--OH and Al--Mg--OH bending vibrations are combined in one peak at 879.4 cm−1.The absorption bands at 576.6 cm−1,520.7 cm−1and 468.6 cm−1result from the split of the v1 band at 570 cm−1corresponding to the Fe--O bond of bulk magnetite and the shifting of the v2 band of the Fe--Obond of bulk magnetite to a higher wavenumber.The two weak adsorptions at 2929.3 cm−1and 2977.6 cm−1represent the C--H group of APTES.The representative peak for Fe--O--Si binding present between the Fe3O4and APTES,which is supposed to be at around 584 cm−1,is overlapped by the band of Fe--O of Fe3O4nanoparticles.The peak that appeared at796.5 cm−1is ascribed to the characteristic peak of Si--O--Sigroupsofthe tetrahedral.All these results indicate the presence of APTES-Fe3O4and bentonite in the as-prepared magnetic adsorbent.Besides,the absorption band due to Si--OH groups is shifted to higher frequencies(1043.3 cm−1).This observed shift suggests the presence of interactions between amino groups of APTES-Fe3O4and the hydroxyl groups on the bentonite surface[18].
However,in the case of APTES-Fe3O4/bentonite after adsorption process,several new peaks are formed.Among these peaks,the bands corresponding to the symmetric and asymmetric C--H stretching vibration of--CH3can be seen at 1390.4 cm−1and 1336.4 cm−1.The additional adsorption bands at1442.5 cm−1,1490.7 cm−1and 1602.6 cm−1are due to the C=C stretching vibration from the aromatic rings of methylene.It is worth to mentioning that the typical adsorption peak at 3000–3100 cm−1corresponding to the aromatic rings has been covered by the broaden peak at 3425.69 cm−1in the adsorbent.The changes in FT-IR spectra con firm the adsorption of MB on the as-prepared magnetic adsorbent in this work since all these additional peaks mentioned above represent the functional groups present in MB structure(Fig.1C).

Fig.3.FT-IR spectra of obtained APTES-Fe3O4/bentonite before(A)and after(B)the adsorption process of MB.
3.3.pH effects on MB adsorption by APTES-Fe3O4/bentonite
Batch experiments,investigating the effect of solution pH on the adsorption of MB by the as-obtained adsorbent,were carried out to enhance comprehensive evaluation of the adsorption process since pH is a non-ignorable factor of aqueous solution studies.As we know,the charges of adsorbent and adsorbate play an important role in the reaction of adsorption and the heterocharge between the adsorbent and adsorbate is in favor of adsorbing reaction.Fig.4A shows the effect of adsorption of MB on the magnetic adsorbent as a function of solution pH.As observed from the graph,the equilibrium removal proportion firstly increases continuously with the increase in pH,and it reaches the maximum at pH 8.0,and then decreases.
Atlower pH,the reaction solution would be positively charged,where hydrogen ions compete dramatically with positive MB cations.In addition,at lower pH values,the number of negatively charged adsorbent sites decreases and the number of positively charged sites increases,which impedes the adsorption of positively charged MB cations due to electrostatic repulsion.Otherwise,the highest increase of removal proportion is around 18%,which is weak yet pronounced.This may be attributed to the fact that there are a considerable number of Si-OH and Al-OH bonds along the surface of the bentonite crystals,which indicates hydrogen-bonding formation is also a possible mechanism of this adsorption process.
When pH values continue increasing,the reduction of MB removal is ascribed to the increase of the hydroxide ions in the dye solution.The hydroxide ions may deprotonate the MB cations and decrease the electrostatic force between the adsorbent and adsorbate.Similarly,the degree of the decrease is 3%,which is mild but obvious.In the alkaline medium,the basal oxygen surface of the bentonite crystals contains excess hydroxide ions.The electrostatic repulsion between the negatively charged surface and the free hydroxyl ions in MB solution leads to better dispersion of the adsorbent,which avoids the removal proportion decreasing dramatically.These results are also con firmed by zeta potential results in Fig.4B.
3.4.Effect of contact time and adsorption kinetics
Fig.5A shows the effect of contact time on the adsorption efficiency of MB.The equilibrium is established within 120 min.
In order to evaluate the adsorption kinetics of MB onto APTES-Fe3O4/bentonite magnetic adsorbent,Lagergren pseudo- first order,pseudosecond order,Elovich and intra-particle diffusion kinetics models were applied to fit the experimental data[21].The data was calculated and recorded in Table 1.
For pseudo first order kinetics,the rate of the adsorption interactions can be evaluated by Eq.(5)in Table 1,whereqeandqtare the values of amount adsorbed per unit mass at equilibrium and atany timet,respectively,andk1is the pseudo first order sorption rate constant.The calculated data are represented in Fig.6A,and the corresponding values ofk1and correlation coefficient(R2)obtained are listed in Table 1.The plots showed a poor linearity andR2is less than 0.9 for some concentrations.Moreover,the theoreticalqevalues differ a lot from the corresponding experimental values.Thus,the pseudo first order is not appropriate to express the adsorption kinetic data in this work.
In order to find a more reliable description of the kinetics,pseudo second order kinetics was applied,which can be represented by the linear Eq.(6)in Table 1,wherek2is the second order rate constant andhis the initial adsorption rate.The calculated data are represented in Fig.6B,and the corresponding values ofk2,handR2obtained are listed in Table 1.From these data,it is observed that the calculatedR2is higher and closer to unity for pseudo second order kinetics model than for pseudo first order kinetics model.Besides,the calculatedqeis consistent with the experimental data.These results suggest that a pseudo second order adsorption is the predominant mechanism and the overall rate constant is controlled by the chemisorption process.
The Elovich model was adopted to fit the experimental data to investigate the predominantly chemical adsorption in this work,which can be represented by the linear Eq.(7)in Table 1,where α and β are the Elovich coefficients represent the initial sorption rate and the adsorption constant,respectively.The calculated data are represented in Fig.6C,and the corresponding calculated values of the parameters obtained are listed in Table 1.Although the plots exhibit poorer linear relations than those for pseudo second order kinetics,the calculatedqeis consistent with the experimental data and the calculatedR2is higher than that of pseudo first order kinetics.Thus,it is still predicted that MB molecules are held strongly to the surface of the magnetic adsorbents by chemisorptive bonds.
The possibility of intra-particle diffusion was explored by using the intra-particle diffusion model given by Eq.(8)in Table 1,wherek3is the intra-particle diffusion rate constant andCis a constant gives an idea about the thickness of the boundary layer,which is indicative for the boundary layer effect.The calculated data are represented in Fig.6D,and the corresponding calculated values of the parameters obtained are listed in Table 1.It is obvious from Table 1 and Fig.6D that the plots do not have a zero intercept as proposed by Eq.(8),indicating that the adsorption process may not be mainly controlled by intra-particle diffusion.

Fig.5.The kinetic data for MB adsorption by APTES-Fe3O4/bentonite at different MB concentrations.
3.5.Temperature effect and adsorption isotherm
The effect of temperature on adsorption efficiency is shown in Fig.7A.It is obvious that adsorption capacity is highest at 333 K and lowest at 293 K in each initial concentration,indicating high temperature is beneficial for MB sorption.

Fig.4.(A)Effect of pH on the adsorption of MB by APTES-Fe3O4/bentonite.(B)The zeta potential of the adsorbent at different pH values.
Two available isotherm models,Freundlich and Langmuir,are for predicting the adsorption uptake of the adsorbent[22].Fig.7B shows the plotted models including the fitted models,and the linear forms of these two models along with the calculated isotherm parameter values are summarized in Table 2.Based on the highestR2,it is clear that Langmuir model provided a better fit for the experimental data,assuming a complete monolayer of adsorption with no transmigration of MB on the adsorbent surface plan.A dimensionless constant separation factor,RL=1/(1+KLC0),was calculated to be from 0.1294 to 0.04728,indicating the adsorption of MB is favorable because the value falls between 0 and 1.

Table 1Mathematical equations in MB adsorption kinetics and kinetic parameters of various models fitted to experimental data
3.6.Adsorption thermodynamics
The thermodynamic parameters of MB adsorption onto APTESFe3O4/bentonite were assessed from the slope and intercept of the plots of lgkdvs.1/Tusing Van't Hoff equation(Fig.7C):


Fig.6.Fitting of Lagergren pseudo- first order(A),pseudo-second order(B),Elovich(C)and intra-particle diffusion(D)kinetic models for MB adsorption,respectively.

Fig.7.(A)Effect of temperature on the adsorption of MB onto APTES-Fe3O4/bentonite at different initial concentrations.(B)Freundlich and Langmuir isotherm plots of the adsorption.(C)Van't Hoff plots for adsorption of MB onto APTES-Fe3O4/bentonite.
wherekdis the distribution coefficient of MB and equals toqe/Ce,ΔS0is the entropy change,ΔH0is the enthalpy change,Ris the ideal gas constant andTis the temperature in Kelvin.
The free energy change(ΔG0)was calculated as bellow:

The values are summarized in Table 3.The positive values of ΔH0indicate that the interaction of MB adsorption is endothermic,which is in accordance with the obtained data in Fig.7A.The negative values of ΔG0suggest the spontaneous nature of the adsorption process.The positive values of ΔS0reflect the affinity of the adsorbent towards MB in solutions,and suggest an increased randomness at the solid–liquid interface during the adsorption process[23].
3.7.Regeneration and reuse
From Fig.8,it can be seen that the adsorption capacities of both asprepared and recycled APTES-Fe3O4/bentonite remained at a high level even after the 9th recycling,implying the high stability and regeneration performance of APTES-Fe3O4/bentonite.Thus,the as-prepared APTES-Fe3O4/bentonite adsorbent can be used for repeated treatment of wastewaters consisting MB.


On the other hand,electrons are trapped at surface sites of the magnetic adsorbent and removed by reactions with adsorbed molecular O2to form superoxide anion radical ·O2−.

Both •OH and ·O2−are the most powerful oxidizing species in the process of the decomposition of MB adsorbed on the surface of MBF.These oxidants can further convert organic compounds into end products(water and CO2).[25].
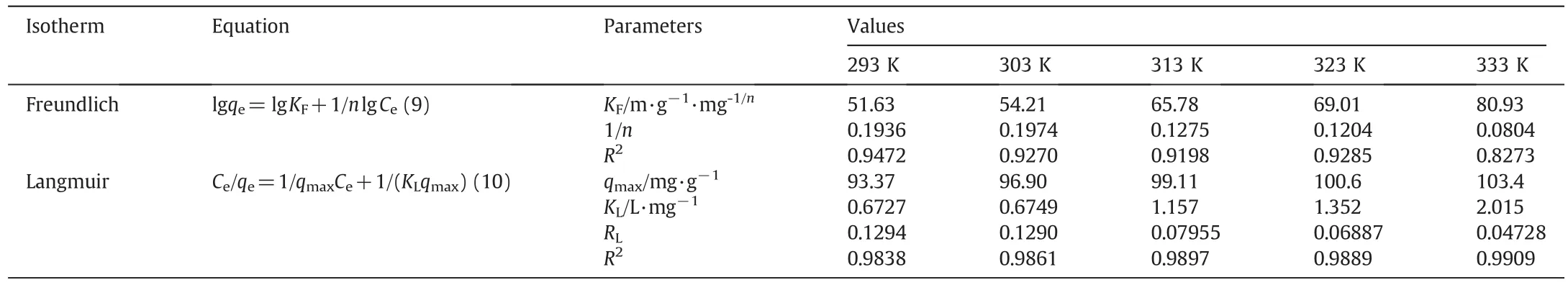
Table 2Freundlich and Langmuir isotherm parameters for adsorption of MB by APTES-Fe3O4/bentonite

Table 3Thermodynamic parameters for adsorption of MB by APTES-Fe3O4/bentonite

Fig.8.Adsorption capacities of both as-prepared and recycled adsorbents.
3.8.Comparison of the capacities of the APTES-Fe3O4/bentonite and other low-cost adsorbents reported previously
In general,a sorbent can be assumed to be“low cost”if it requires little processing and is abundant in nature,or waste material from another industry,which has lost its economic or is a by-product or further processing values.Based on this,our as-prepared magnetic adsorbent in this work is low-cost.
Many low-cost adsorbents are studied previously.[26]It is easily to see that,the adsorption capacity of the as-obtained APTES-Fe3O4/bentonite for MB is much higher than that of the majority of other adsorbents.Furthermore,the APTES-Fe3O4/bentonite shows a fast MB removal performance to reach complete removal after 120 min,which was much quicker than some other adsorbents.And compared with the adsorbents,which are with short equilibrium time and with high adsorption capacity,APTES-Fe3O4/bentonite could be easily separated from aqueous solution by a magnetic field and the used one can be easily regenerated with gamma-irradiation in60Co sources.
4.Conclusions
In this study,a low-cost magnetic adsorbent,APTES-Fe3O4/bentonite was synthesized for the adsorption of MB.The equilibrium was established within 120 min and the adsorption capacity of the adsorbent reached maximum at pH 8.The adsorbent shows a higher adsorption capacity and a more efficient adsorption towards MB.The adsorption trend of the adsorbents follows the pseudo-second order kinetics model.The adsorption data gives good fits with Langmuir isotherm model.The parameter factorRLfalls between 0 and 1,indicating the adsorption of MB is favorable.The adsorption process is endothermic with positive ΔH0values.The positive values ofΔG0confirm the affinity of the adsorbent towards MB in solutions,and suggest that the randomness increases at the solid–liquid interface during the adsorption process.Regeneration of the saturated adsorbent was easily carried out with gamma-irradiation of a certain dose.The adsorption capacity of the recycled APTES-Fe3O4/bentonite remains at a high level even after the 9th recycling.These results indicate that the adsorbent has a great potential in the treatment of colored wastewaters.
[1]B.D.Waters,The Dye regulator's View,Society of Dyers and Colourists,Bradford,UK,1995.
[2]M.D.Chengalroyen,E.R.Dabbs,The microbial degradation of azo dyes:Minireview,World J.Microbiol.Biotechnol.29(2012)389–399.
[3]C.Y.Li,K.Q.Lai,Y.Y.Zhang,L.Pei,Y.Q.Huang,Use of surface-enhanced Raman spectroscopy for the test of residuals of prohibited and restricted drugs in fish muscle,Acta Chim.Sin.71(2013)221–226.
[4]W.Pratarn,T.Pornsiri,S.Thanit,C.Tawatchai,T.Wiwut,Adsorption and ozonation kinetic model for phenolic wastewater treatment,Chin.J.Chem.Eng.19(1)(2011)76–82.
[5]H.Hu,M.Yang,J.Dang,Treatment of strong acid dye wastewater by solvent extraction,Sep.Purif.Technol.42(2005)129–136.
[6]A.D.Dhale,V.V.Mahajani,Reactive dye house wastewater treatment.Use of hybrid technology:Membrane,sonication followed by wet oxidation,Ind.Eng.Chem.Res.38(1999)2058–2064.
[7]N.Djafarzadeh,M.Safarpour,A.Khataee,Electrochemical degradation of three reactive dyes using carbon paper cathode modified with carbon nanotubes and their simultaneous determination by partial least square method,Korean J.Chem.Eng.31(2014)785–793.
[8]H.Zhuang,H.Han,S.Shan,Treatment of British gas/Lurgi coal gasification wastewater using a novel integration of heterogeneous Fenton oxidation on coal fly ash/sewage sludge carbon composite and anaerobic biological process,Fuel178(2016)155–162.
[9]M.Sharma,R.K.Vyas,K.Singh,A review on reactive adsorption for potential environmental applications,Adsorpt.J.Int.Adsorpt.Soc.19(2013)161–188.
[10]T.A.Wolfe,T.Demirel,E.R.Baumann,Interaction of aliphatic amines with montmorillonite to enhance adsorption of organic pollutants,ClayClayMiner.33(1985)301–311.
[11]L.Lian,X.Cao,Y.Wu,D.Lou,D.Han,Synthesis of organo-functionalized magnetic microspheres and application for anionic dye removal,J.Taiwan Inst.Chem.Eng.44(2013)67–73.
[12]T.Yan,L.Wang,Adsorption of C.I.Reactive red 228 and Congo red dye from aqueous solution by amino-functionalized Fe3O4particles:kinetics,equilibrium,and thermodynamics,Water Sci.Technol.69(2014)612–621.
[13]A.A.Atia,A.M.Donia,W.A.Al-Amrani,Adsorption/desorption behavior of acid orange 10 on magnetic silica modified with amine groups,Chem.Eng.J.150(2009)55–62.
[14]Z.Lou,Z.Zhou,W.Zhang,X.Zhang,X.Hu,P.Liu,H.Zhang,Magnetized bentonite by Fe3O4nanoparticles treated as adsorbent for methylene blue removal from aqueous solution:synthesis,characterization,mechanism,kinetics and regeneration,J.Taiwan Inst.Chem.Eng.49(2014)199–205.
[15]J.Gomez-Pastora,E.Bringas,I.Ortiz,Recent progress and future challenges on the use of high performance magnetic nano-adsorbents in environmental applications,Chem.Eng.J.256(2014)187–204.
[16]S.Jan,A.N.Kamili,T.Parween,R.Hamid,J.A.Parray,T.O.Siddiqi,etal.,Feasibility of radiation technology for wastewater treatment,Desalin.WaterTreat.55(2015)2053–2068.
[17]Z.Lou,Y.Zhang,X.Zhang,Z.Zhou,X.Hu,H.Zhang,A facile approach to monodisperse Au nanoparticles on Fe3O4nanostructures with surface Plasmon resonance amplification,J.Nanosci.Nanotechnol.15(2015)2371–2378.
[18]N.V.Afanas'eva,V.A.Petrova,E.N.Vlasova,S.V.Gladchenko,A.R.Khayrullin,B.Z.Volchek,et al.,Molecular mobility of chitosan and its interaction with montmorillonite in composite films:dielectric spectroscopy and FTIR studies,Polym.Sci.Ser.A55(2013)738–748.
[19]F.J.Zhang,J.Liu,K.Zhang,W.Zhao,W.K.Jang,W.C.Oh,Anoveland simple approach for the synthesis of Fe3O4-graphene composite,Korean J.Chem.Eng.29(2012)989–993.
[20]M.Espinosa,S.Pacheco,R.Rodriguez,Synthesis and characterization of NH2-porphyrins covalently immobilized on modified-SBA-15,J.Non-Cryst.Solids353(2007)2573–2581.
[21]P.C.C.Siu,L.F.Koong,J.Saleem,J.Barford,G.McKay,Equilibrium and kinetics of copper ions removal from wastewater by ion exchange,Chin.J.Chem.Eng.24(1)(2016)94–100.
[22]S.Wang,S.S.Xu,C.B.Liu,F.Chen,D.T.Wang,S.Q.Liu,Z.G.Chen,Z.Y.Wu,Characterization and adsorption behaviors of a novel synthesized mesoporous silica coated carbon composite,Chin.J.Chem.Eng.24(1)(2016)190–195.
[23]M.Soleimani,Z.H.Siahpoosh,Ghezeljeh nanoclay as a new natural adsorbent for the removal of copper and mercury ions:Equilibrium,kinetics and thermodynamics studies,Chin.J.Chem.Eng.23(11)(2015)1819–1833.
[24]S.I.Borrely,A.C.Cruz,N.L.Del Mastro,M.H.O.Sampa,E.S.Somessari,Radiation processing of sewage and sludge.A review,Prog.Nucl.Energy33(1998)3–21.
[25]M.R.Hoffmann,S.T.Martin,W.Choi,D.W.Bahnemann,Environmental applications of semiconductor photocatalysis,Chem.Rev.95(1995)69–96.
[26]M.T.Yagub,T.K.Sen,S.Afroze,H.M.Ang,Dye and its removal from aqueous solution by adsorption:A review,Adv.Colloid Interf.Sci.209(2014)172–184.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Influence of Na+,K+,Mg2+,Ca2+,and Fe3+on filterability and settleability of drilling sludge☆
- An optimal filter based MPC for systems with arbitrary disturbances☆
- Measurement and calculation of solubility of quinine in supercritical carbon dioxide☆
- Solubility and metastable zone width measurement of 3,4-bis(3-nitrofurazan-4-yl)furoxan(DNTF)in ethanol+water
- Partition coefficient prediction of Baker's yeast invertase in aqueous two phase systems using hybrid group method data handling neural network
- The effect of transition metal ions(M2+=Mn2+,Ni2+,Co2+,Cu2+)on the chemical synthesis polyaniline as counter electrodes in dye-sensitized solar cells☆
