An ecofriendly approach for corrosion control of 6061 Al-15%(v)SiC(P)composite and its base alloy
2017-05-28CharithaPadmalathaRao
Charitha B.P.,Padmalatha Rao*
Department of chemistry,Manipal Institute of Technology,Manipal,Udupi,Karnataka,India
1.Introduction
Aluminum and aluminum alloys have large number of industrial and domestic applications mainly due to the presence of highly protective passivating oxide film[1,2].However,due to lower strength and stiffness their applications are limited.Reinforcing 6061 Al alloy with SiC particles results in better performance,by enhancement of strength and stiffness[3].Reinforced aluminum metal matrix composites(AMCs) find large number of applications in automobile and military applications[4,5].Reinforcement of aluminum with SiC results in decrease in the corrosion resistance due to the rupture of continuous protective layer[6].Due to wide applications,these composite materials frequently come in contact with mineral acids mainly during pickling process and there will be considerable material loss.Addition of synthetic organic heterocyclic compounds as inhibitor to the corrosive is reported to be one of the effective methods to control corrosion.However,because of environmental regulations,nowadays' research is more focused towards the utility of environmentally benign green inhibitors.
Biopolymers are the class of compounds which are naturally available,cheap,nontoxic and environmentally acceptable and some of them showed to function as effective inhibitors for metal corrosion in different acid medium[7].As a part of our studies with corrosion control of metals and alloys using green inhibitors[8,9]we report herein the results of use of biopolymer dextran for the corrosion control of 6061 Al and 6061 Al-15%(v)SiC(p)composite in acid medium.
2.Experimental
2.1.Materials
The experiment was performed by using 6061 aluminum alloy and 6061 Al-15%(v)SiC(P)composite.The composition of the base alloy is given in Table 1.
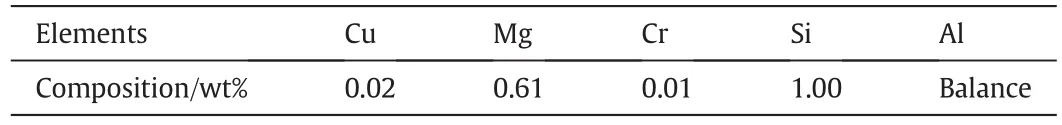
Table 1The composition of the base metal 6061 Al-alloy
Surface area of 1 cm2cylindrical test coupon of aluminum and its composite metal sealed with resin material.It was polished with different graded emery papers.Further polishing was done with disk polisher using levigated alumina to get the mirror surface.
The stock solution of HCl of higher concentration was prepared by using 37%HCl and double distilled water,standardized by volumetric titration.1 mol·L−1solution was prepared by appropriate dilution.
Dextran(Sigma Aldrich)was used as such.The inhibitor stock solution was prepared by dissolving dextran(1 g)in 1 Lof1 mol·L−1hydrochloric acid solution.
2.2.Electrochemical measurements
Corrosion behavior of 6061 aluminum and 6061 Al-15%(v)SiC(P)composite were studied by using electrochemical work station(CH600 D-series US model with CH instrument with beta software).The electrochemical cell used was a conventional three electrode compartment glass vessel containing saturated calomel electrode(SCE)as reference electrode and Platinum as auxiliary electrode.6061 aluminum and 6061 Al-15%(v)SiC(P)composite were used as working electrode.All the potential values were recorded with respect to SCE.
Cell set up used was immersed in a thermostat and all measurements were done under unstirred conditions.
2.2.1.Potentiodynamic polarization(PDP)measurement
The potentiodynamic polarization studies were carried out in 1 mol·L−1HCl solution at different temperatures ranging from 303 to 323 K by varying the concentrations of inhibitor.With reference to SCE the steady states open circuit potential(OCP)was recorded at the end of 1800 s.The potentiodynamic currentvs.potential plots were obtained by polarizing the working electrode from−250 mV cathodically to+250 mV anodically and with respect to OCP at the scan rate of 1 mV·s−1.
2.2.2.Electrochemical impedance spectroscopy(EIS)studies
The EIS measurement for 6061 aluminum and 6061 Al-15%(v)SiC(P)composite was carried out by using small amplitude of AC signal of 10 mV,at the OCP with a frequency ranging from 10000 Hz to 0.01 Hz.Resulted Nyquist plots was used to obtain impedance parameters.Suitable equivalent circuit was developed using Zimpwin 3.1 software.
Potentiodynamic polarization was carried out immediately after electrochemical impedance spectroscopy,without further surface cleaning.All the studies were done minimum of 3–4 times and the average of best three agreeing values was reported.
2.3.Surface morphology and elemental analysis
Surface morphology studies of 6061 aluminum and 6061 Al-15%(v)SiC(P)composite was carried out by using analytical scanning electron microscope(JEOL JSM-6380L)and elemental mapping was done by energy dispersive X-ray analysis.
3.Results and discussion
3.1.Potentiodynamic polarization(PDP)measurements
Plots of potentiodynamic polarization studies for the corrosion of 6061aluminum and 6061 Al-15%(v)SiC(P)composite in 1 mol·L−1HCl solution containing various concentrations of dextran are shown in Fig.1(a)and(b)respectively.Corresponding results are tabulated in Table 2.
Potentiodynamic polarization plots indicates active dissolution of 6061 aluminum and 6061 Al-15%(v)SiC(P)composite in acidic environment.Various parameters such as corrosion potential(Ecorr),corrosion current density(icorr),cathodic Tafel slopes(−βc)anodic Tafel slopes(−βa)values were obtained by potentiodynamic polarization studies.By usingicorrvalues percentage inhibition efficiency[I.E(%)]of inhibitor was calculated by using Eq.(1)[10].
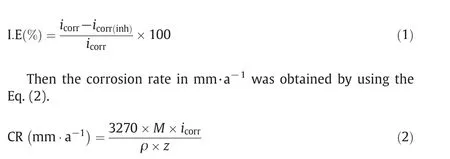
where,3270 is a constant which revels the unit of corrosion rate,ρ is the corroding material density of aluminum and aluminum compositei.e.,2.7 g·cm−3and 2.66 g·cm−3respectively,Mis the atomic mass of aluminum(27)and aluminum composite(9.15),Zis the electrons transferred per metal atom(3)[11].
Due to the passivation of the metal in the range of−0.6 to−0.5 V,significant change in the anodic Tafel slopes(−βa)was observed[12].Since the plateau of anodic current is not well defined the corrosion current density was determined by extrapolation of cathodic slopes.Further,there was no remarkable change in the value of cathodic Tafel slopes after the addition of inhibitor.This suggests the simple blocking effect of inhibitor on the surface of the metal.The added inhibitor results in surface inactivation and do not alter the mechanism of corrosion process[13].
Corrosion potential(Ecorr)did not change remarkably after the addition of inhibitor[14].As per the reported literature,if the shift inEcorrexceeds±85 mV in comparison with uninhibited solutions,the inhibitor behaves distinctly as either cathodic or anodic inhibitor.In the present investigation,displacement ofEcorrwas less than+85 mV[15].This observation indicates that dextran may act as mixed inhibitor for both aluminum and aluminum composite material corrosion.Thus dextran controls the corrosion process by forming a protective film on both anodic and cathodic area and thereby behaves as mixed inhibitor.
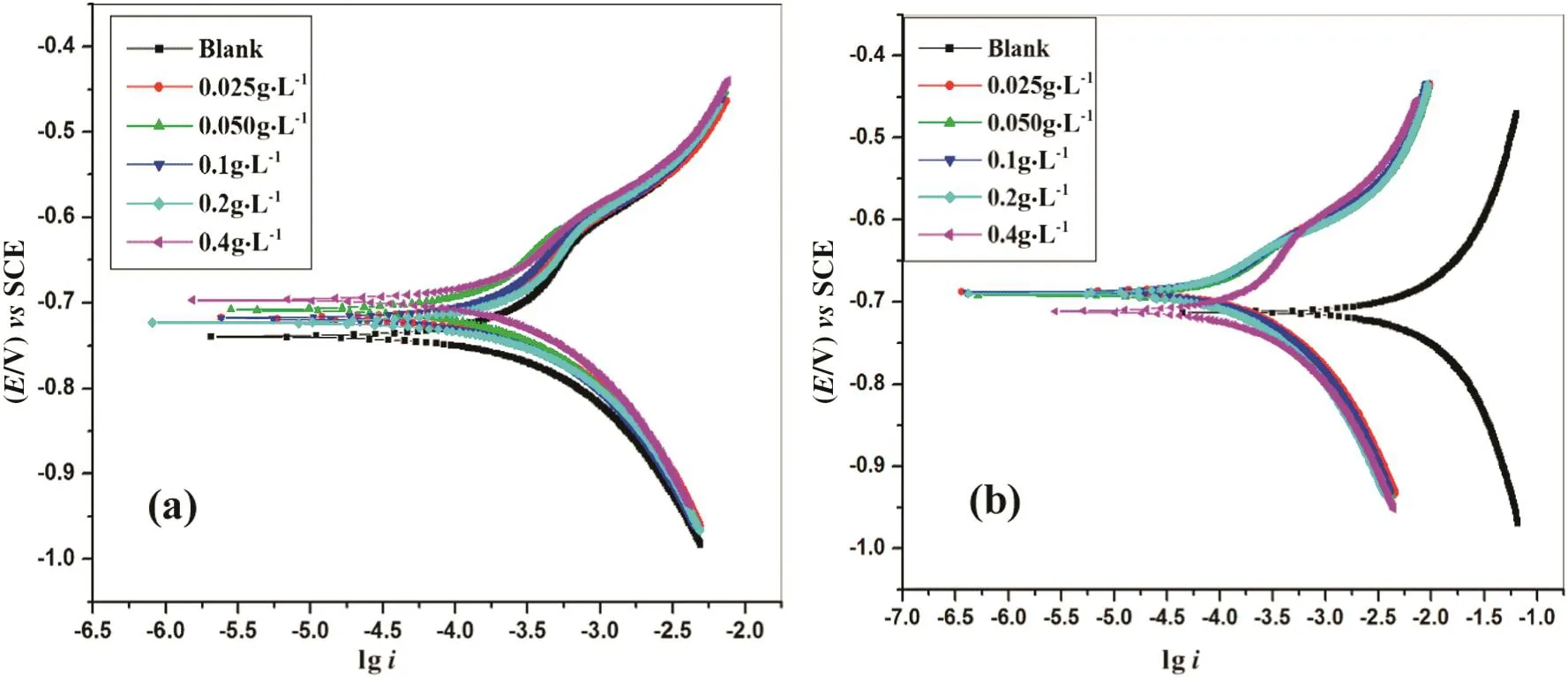
Fig.1.Potentiodynamic polarization plots in 1 mol·L−1 hydrochloric acid containing different concentrations of dextran at 308 K(a)6061 aluminum alloy(b)6061Al-15%(V)SiC(P).
The maximum inhibition efficiency was found to be 74.6%for 6061 aluminum alloy and 91%for 6061 Al 15%(V)SiC(P)composite for 0.4g·L−1at 303 K.Corrosion rate of composite material is many folds higher than that of base alloy.This is mainly because of the presence of reinforcing element SiC.Addition of reinforcing agent breaks the continuous protective layer of oxides and increases the corrosion sites.Further,electrode potential of Silicon(Si)is−0.14 V and electrode potential of aluminum(Al)is−1.67 V.Thus,Si having high electrode potential behaves as cathode compared with aluminum matrix.Hence,aluminum matrix behaves as anode and it will undergo corrosion.
3.2.Electrochemical Impedance Spectroscopy(EIS)studies
Nyquistplots of6061 aluminum alloy and 6061 Al15%(V)SiC(P)composite for uninhibited and inhibited medium in 1 mol·L−1HCl medium are shown in Fig.2.
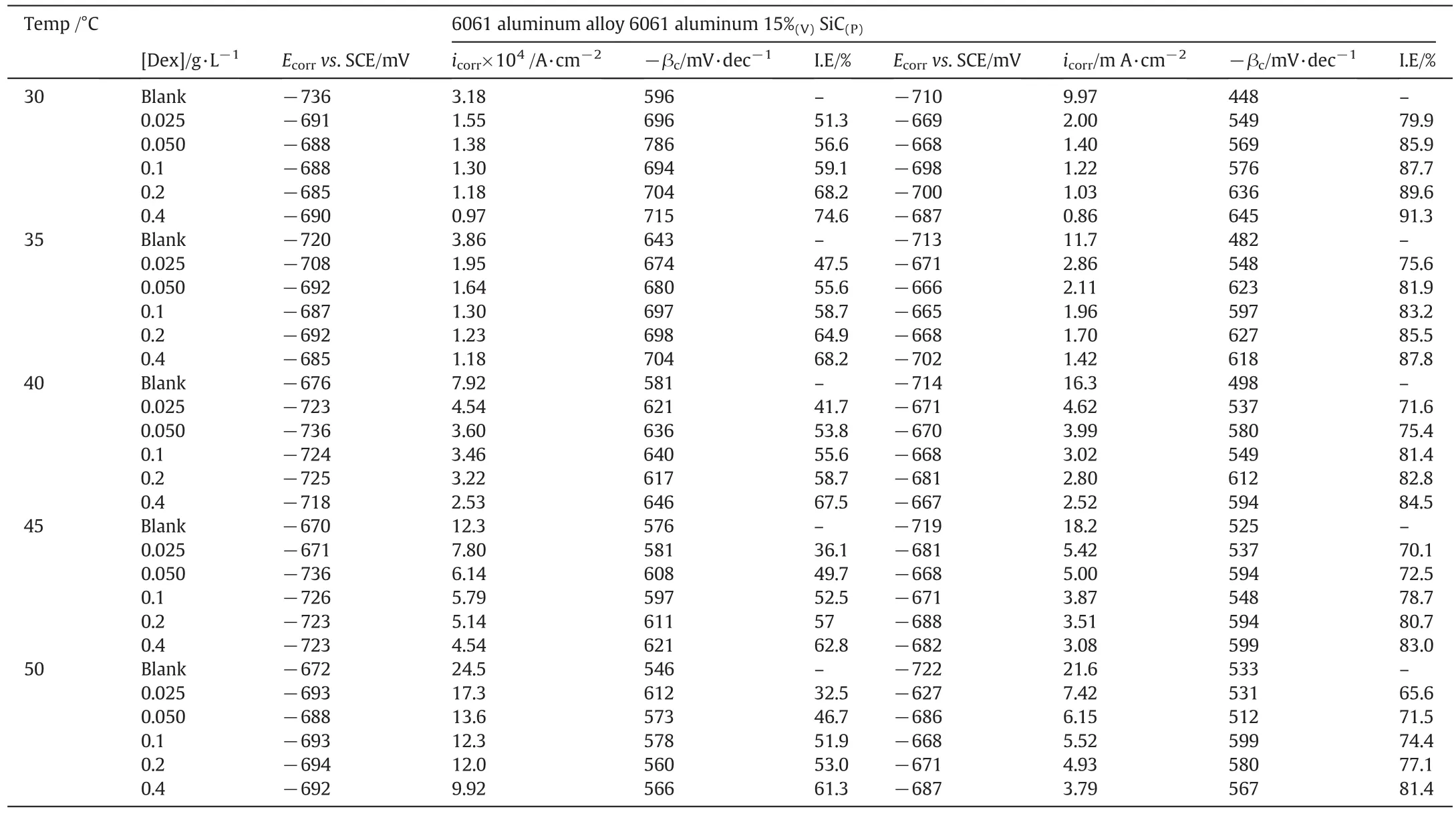
Table 2Results of potentiodynamic polarization measurements in 1 mol·L−1 hydrochloric acid containing different concentrations of dextran at different temperatures for the corrosion of 6061 aluminum alloy and 6061 Al 15%(V)SiC(P)
Semicircle impedance plots were obtained for both 6061 aluminum alloy and 6061Al-15%(V)SiC(P)in uninhibited and inhibited 1 mol·L−1hydrochloric acid medium.The plots obtained consists of three frequency regions,namely,High frequency(HF),Intermediate frequency(IF)and Low frequency(LF)region.Three loops of the impedance plots were assigned to these three frequency regions.That is first capacitive loop for HF region,small inductive loop for IF region and another capacitive loop for LF region.These Nyquist plots for aluminum corrosion in acidic medium were very well agreed with the reported Nyquist plots[16,17].
6061 aluminum alloy and 6061 Al 15%(V)SiC(P)composite oxidation at M+/oxide/solution interface corresponds to HF capacitive loop.During corrosion process,Al+1getoxidized to Al+3.Through oxide/electrolyte interface Al+1will migrates from M+/oxide interface and get oxidized to Al+3.At oxide/solution interface OH−and O2−ions are formed.The capacitive loop at HF region is attributed for the formation of passivating oxide layer on the surface of the metal.The bulk or surface species relaxation in the protective oxide layer leads to inductive loop at IF[18]and it also may be due to the adsorbed inhibitor molecule relaxation over the aluminum surface or due to the incorporation of Cl−ions,charged intermediates and oxide ions on and into the protective oxide layer.The LF capacitive loop is attributed to dissolution of M+ions[19].
As the concentration of the inhibitor increased,the capacitive loop diameter increased,this indicates the resistance to corrosion attack by the adsorption of inhibitor molecule.Due to the electrode surface inhomogeneity,the semicircle of the impedance plots were depressed[20].
For circuit fitment 3.21 version of Zimpwin software was used.Table 3 shows the results obtained from the circuit fitment.In the present study two different equivalent circuits were used to simulate for the obtained impedance values.One of the equivalent circuits consists of nine elements;they are solution resistance(RS),charge transfer resistance(Rct),inductive resistance(RL)and inductive element(L).It also consist of CPE(constant phase element,Q),which is parallel to the series of capacitanceC1andC2and also parallel to the series of resistorR1,R2,RLandRct.RLis parallel to inductor(L).The circuit with parallel resistor and capacitance were attributed for conduction of ions in oxide layer and to its dielectric constant respectively shown in Fig.3a.This equivalent circuit was used for the impedance values obtained for aluminum alloy.
Another equivalent circuit consists of five elements;they are solution resistance(RS),charge transfer resistance(Rct),inductive resistance(RL)and inductive element(L).It also consist of CPE(Constant Phase Element,Q),which is parallel to resistors and inductive element shown in Fig.3b.
Double layer capacitance(Cdl)and polarization resistance(RP)for nine element or five element circuit fitment can be calculated by using the Eqs.(3)and(4)respectively,
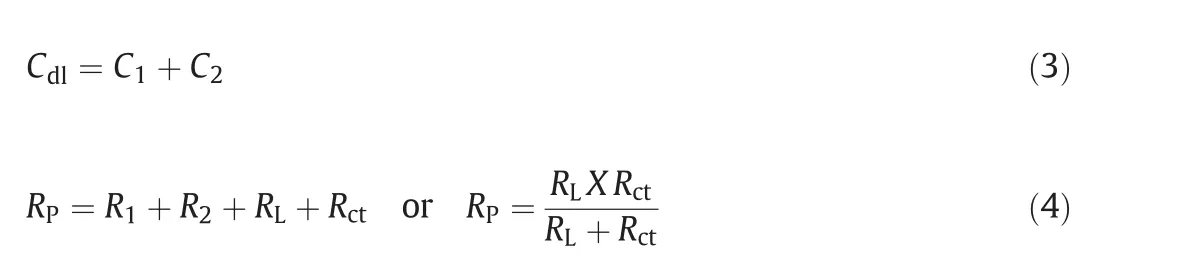
Nyquist plots are depressed.Thus,in the circuit fitment,the componentQdl,and coefficient‘a’ofQ(CPE)quantifies some physical phenomena like inhomogeneous of surface of the metal,binding of the inhibitor molecule and formation of porous layeretc.[21]

The polarization resistance(RP)values are inversely proportional to the corrosion current density(icorr).The inhibition efficiency can be calculated by using the Eq.(6)

RPandRP(inh)are the polarization resistance in the absence and in the presence of the inhibitor.
Results of EIS studies are tabulated in Table 3.RPvalues were increased with enhancing the concentration of inhibitor butCdlvalue decreased because of increase in the thickness of electrical double layer at M+/solution interface.
RPvalue obtained from Potentiodynamic polarization measurement is in good agreement withRPvalue obtained from Electrochemical Impedance Spectroscopy studies.
3.3.Effect of temperature
Studies were performed at different temperatures to evaluate kinetic parameters.The energy of activation(Ea)was calculated from Arrhenius law equation[22],

where,Bis Arrhenius constant which depends upon the metal type andRis equal to 8.314 J·K−1·mol−1(universal gas constant),Tis the absolute temperature.Fig.4 is the Arrhenius plots for the aluminum alloy and Al-composite material for various concentrations of dextran inhibitor in 1 mol·L-1hydrochloric acid.The plots of ln(CR)versus1/Tgave collinear lines with a slope corresponding to–Ea/Rfrom which energy of activation(Ea)values was obtained for the aluminum and aluminum composite corrosion and its inhibition process.Average correlation co-efficient(R2)was found to be 0.98.
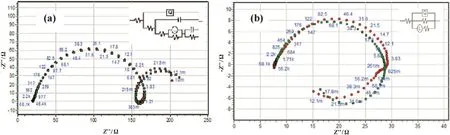
Fig.3.Equivalent circuit of(a)Nine elements and(b) five elements used to simulate for the obtained impedance values.
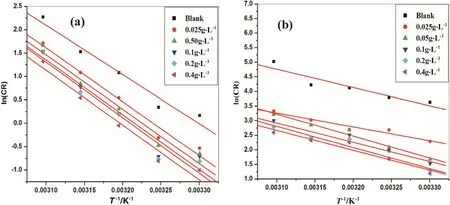
Fig.4.Arrhenius plots in 1 mol·L−1 hydrochloric acid containing different concentrations of dextran for the corrosion of(a)6061 aluminum alloy and(b)6061 Aluminum 15%(V)SiC(P).
The transition state equation was used to calculate the enthalpy(ΔH#)and entropy of activation(ΔS#)for the dissolution of metal.The transition state equation is[23],

Energy of activation(Ea)values of the inhibited solutions was more when compared with that of uninhibited solution.This increase in theEavalue of the inhibited solution is suggestive of physical adsorption of the dextran inhibitor on the surface of the metal[24].The inhibitor molecule gets physically adsorbed on the metal surface and reduces the electrochemical corrosion process by creating a barrier[25,26].The ΔH#values are approximately equal toEavalues of aluminum and Alcomposite metal dissolution process;this supports the physical adsorption of inhibitor[27].The entropy of activation(ΔS#)values is negative which indicates that,in rate determining step the activated complex is association not dissociationi.e.,decrease in the disorderness on going from reactants towards the activated complex[28].
3.4.Adsorption isotherm
The mechanism of inhibition of corrosion by the addition of dextran can be understood by studying the adsorption behavior of inhibitor molecule on 6061 aluminum alloy and 6061 Al-15%(v)SiC(P)composite metal surface.Information regarding how the inhibitor molecule interacted with aluminum alloy and composite surface can be obtained by adsorption isotherm.(θ)Degree of surface coverage values for different concentration was obtained from potentiodynamic polarization studies were applied to various adsorption isotherms such as Langmuir,Freundlich,Temkin and Frumkin.The data was best fitted with Langmuir adsorption isotherm which can be related by the relationship(9),

where,Kis the adsorption/desorption equilibrium constant(L·g−1),Cis the concentration of inhibitor molecule in the electrolyte,then θ is given by the Eq.(10)

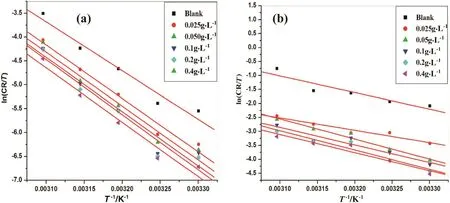
Fig.5.Plots of ln(CR/T)vs.(1/T)in 1 mol·L-1 hydrochloric acid containing different concentrations of dextran for the corrosion of(a)6061 aluminum alloy and(b)6061 Al 15%(V)SiC(P).

Table 4Activation parameters for different concentrations of dextran in hydrochloric acid for the corrosion of 6061 aluminum alloy and 6061 Al 15%(V)SiC(P)
The plot ofCθversus Cgave straight line;from the interceptK1values were obtained.Fig.6 indicates the Langmuir adsorption isotherm plot for aluminum and its composite material.The slopes were close to unity,and the correlation coefficient(R2)was found to be 0.99.This indicated that the adsorption of Dextran on the metal surface obeyed the Langmuir's adsorption isotherm.
The standard free energy of adsorption(ΔG0ads)is related to adsorption/desorption constant(K)by the following equation,

whereRis universal gas constant,Tis the absolute temperature;Khaving the unit L·g−1leads to the concentration of water unit as g·L−1,thus the value will become 1 g× 103[29].

Fig.6.Langmuir adsorption isotherm in 1 mol·L−1 HCl containing different concentrations of dextran for(a)6061 Al alloy(b)6061 Al 15%(v)SiC(P)composite.
3.5.Mechanism of corrosion
3.5.1.Anodic dissolution reaction
In HClmedium,the protective layer of aluminumgets destroyed and corrosion attack takes place.Due to more negative potential of aluminum in galvanic series(E=−1.66 Vvs.SHE)following reaction takes place(Eq.(13)),

Dextran inhibitors containing plenty of–OH groups(shown in Fig.8)get interacts with metal and protects the metal from undergoing corrosion.
3.5.2.Hydrogen evolution reaction
General cathodic reduction reaction in acidic environment is given below,

The large polymer dextran competes with protons and occupy cathodic region,forming Al-dextran(Eq.(15)).The proton size is negligible compared to large molecular weight compound polymer dextran.Therefore Dextran as it is larger molecule;it covers almost all the parts of the metal and brings down both cathodic and anodic reactions under control through physical adsorption.

3.6.Surface morphology studies
SEM image of aluminum and aluminum composite in contact with hydrochloric acid is shown in Fig.9(a)and(b)respectively.Aluminum alloy containing magnesium when comes in contact with corrosive environment;the formation of Al3Mg2takes place at grain boundaries and leads to inter-granular corrosion.

Fig.7.Plot of ΔG0ad s vs.temperatures in 1 mol·L−1 hydrochloric acid for(a)6061 Al alloy(b)6061 Al 15%(v)SiC(P)composite.

Table 5Thermodynamic parameters for the adsorption of dextran in hydrochloric acid at different temperatures on(a)6061 aluminum alloy and(b)6061 Al 15%(V)SiC(P)
Fig.9b shows the rough surface.Here localized attack occurs preferentially at the reinforcement/matrix interface.Attack at the interface has been attributed to galvanic corrosion between the reinforcement and matrix.Crevice corrosion is also initiated at voids at the interface formed during processing.The formation of voids on the surface of composite is mainly due to the detachment of Al4C3interphase,which is likely to be formed as a result of reaction between Al and SiC during the processing.
Fig.10 shows the smooth surface of 6061 Al-and 6061 Al-15%(v)SiC(P)immersed in hydrochloric medium containing dextran inhibitor.
After the addition of dextran inhibitor surface of the aluminum alloy became smoother,this is due to the adsorption of the inhibitor molecule onto surface of the aluminum alloy.In aluminum composite,the zones of the detached SiC particulates were blocked by the dextran inhibitor and get adsorbed onto the metal surface thus creating the protective barrier between metal surface and the medium.This leads to the smooth surface of the aluminum composite material.
EDX spectrum corresponds to un-corroded,corroded and inhibited samples were analyzed.The data of elemental mapping is given in Tables 6 and 7.
Fig.8.Structure of dextran.
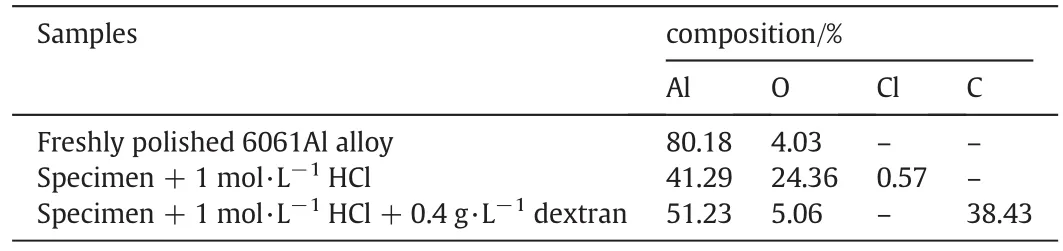
Table 6EDX data for 6061Al surface analysis
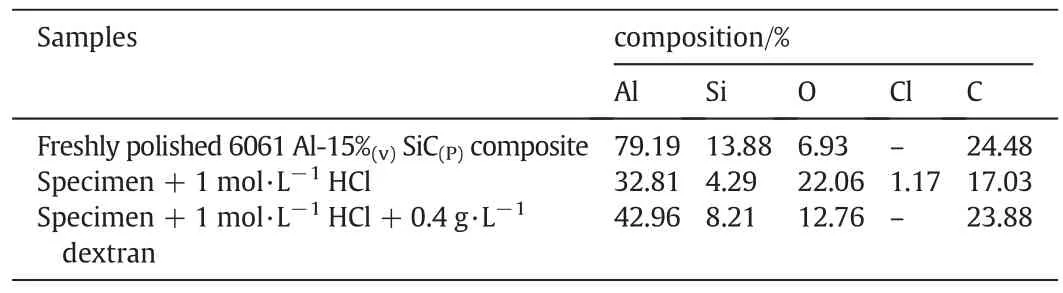
Table 7EDX data for 6061 Al-15%(v)SiC(P)composite surface analysis
Increase in composition of oxygen and chlorine in the corroded sample is indicative of dissolution of stable oxide film layer.Peak due to carbon in the inhibited sample con firms the adsorption of dextran on the surface of the material.

Fig.9.SEM images of(a)6061 aluminum alloy and(b)6061 Al-15%(v)SiC(p)composite material surface immersed in hydrochloric acid medium.
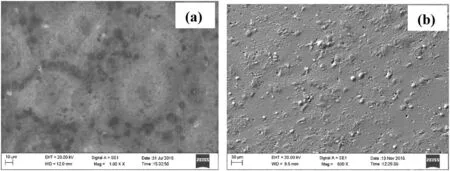
Fig.10.SEM image of(a)6061 aluminum alloy and(b)6061 Al-15%(v)SiC(p)composite material surface immersed in hydrochloric acid medium containing 0.4 g ·L−1 of dextran inhibitor.
3.7.FTIR analysis
FTIR spectrum for dextran shows the peak for–OH stretching frequency at 3600 cm−1and 3422 cm−1respectively(Fig.11a).After the corrosion,when the products were scraped and FTIR was taken,there was shift in the stretching frequency of–OH group to 3764 cm−1and 3622 cm−1for aluminum base alloy(Fig.11b).Similarly shift in the stretching frequency of–OH group to 3739 cm−1and 3614 cm−1for 6061 Al 15%(V)SiC(P))was observed(Fig.11c).These shift in the–OH stretching frequency interaction of–OH group with the material and adsorption of dextran on the surface of base alloy and composite materials.
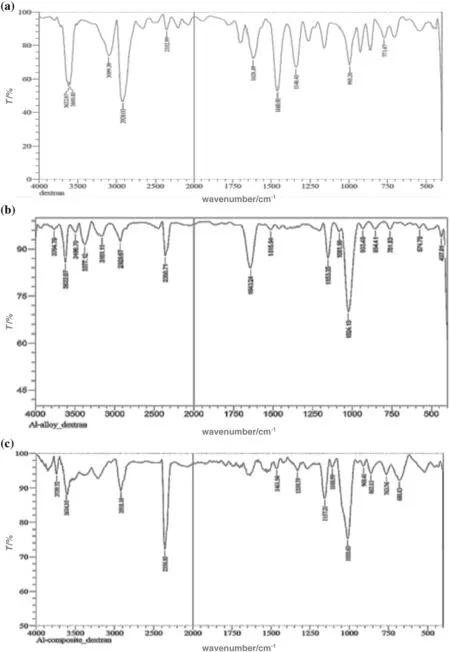
Fig.11.(a)FTIR spectra of Dextran.(b)FTIR spectra of scrapped corrosion product of 6061 Al-alloy and dextran.(c)FTIR spectra of scrapped corrosion product of 6061 Al-composite material and dextran.
4.Conclusions
Based upon the corrosion inhibition studies carried out following conclusions were drawn:
•Corrosion rate for composite material is higher than that of base alloy
•Anticorrosive performance of dextran is more effective in case of composite material than alloy
•Inhibition efficiency of dextran increases with increase in the concentration of dextran and decreases with increase temperature.
•Dextran acts as a mixed inhibitor,it gets physically adsorbed both on composite material and base alloy and follows Langmuir adsorption isotherm.
•Surface studies and elemental mapping con firm the adsorption of dextran molecule onto the surface composite material and base alloy.
Acknowledgements
Ms.Charitha B P is very much thankful to lManipal University for Research fellowship,and Department of Chemistry,MIT Manipal for Laboratory facilities.
[1]E.S.M.Sharif,Effect of graphite on the corrosion behavior of aluminum-graphite composite in sodium chloride solutions,Int.J.Electrochem.Sci.6(2011)1085–1099.
[2]J.Vereceken,Corrosion control of aluminum forms of corrosion and prevention,Talat Lecturers5103(1994)6.
[3]M.Abdulla,Inhibition of aluminum corrosion in HCl by cellulose and chitosan,J.Am.Sci.9(4)(2013)580–586.
[4]M.M.Schwartz,Composite material processing fabrication and application,Prentice Hall,USA,1997.
[5]J.E.Binshop,V.K.Kinar,Anlaysis of electro thermodynamic damping in particle reinforced metal matrix composites,Mcfall-Metall.Trans.26(a)(1995)2773–2782.
[6]P.P.C.Pandey,Composite materials,web based course,2004.
[7]H.J.Koepsell,H.M.Tsuchiya,N.N.Hellman,A.Kazenko,C.A.Hoffman,E.S.Sharpe,R.W.Jackson,Enzymatic synthesis of dextran acceptor specificity and chain initiation,J.Biol.Chem.200(2)(1953)793–801.
[8]D.Prabhu,P.Rao,Coriandrum sativumL.—A novel green inhibitor for the corrosion inhibition of aluminum in 1 M phosphoric acid solution,J.Environ.Chem.Eng.1(2013)676–683.
[9]D.Prabhu,P.Rao,Garcinia indicaas an environmentally safe corrosion inhibitors for aluminum in 0.5 M phosphoric acid,Int.J.Corros.(2013)1–11.
[10]W.H.Li,Q.He,S.Zhang,C.Pei,B.Hou,Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium,J.Appl.Electrochem.38(2008)289–295.
[11]M.G.Fontana,Corrosion Engineering,McGraw-hill,Singapore,1987.
[12]B.O.Oni,N.O.Egiebor,N.J.Ekekwe,A.Chuku,Corrosion behavior of tin plated carbon steel and aluminum in NaCl solutions using electrochemical impedance spectroscopy,JMMCE7(2008)331–346.
[13]W.H.Li,Q.He,S.Zhang,C.Pei,B.Hou,Experimental and theoretical investigation of the adsorption behavior of new triazole derivatives as inhibitors for mild steel corrosion in acid media,Electrochim.Acta52(2007)6386–6394.
[14]E.Stupnisek-Lisac,A.Gazivoda,M.Madzarac,Evaluation of non-toxic corrosion inhibitors for copper in sulphuric acid,Electrochim.Acta47(2002)4189–4194.
[15]M.Shahin,S.Bilgic,H.Yilmaz,The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl medium,Appl.Surf.Sci.195(2003)1–7.
[16]H.J.W.Lenderink,M.V.D.Linden,J.H.W.De Wit,Corrosion of aluminum in acidic and neutral solutions,Electrochim.Acta38(1993)1989–1992.
[17]H.De Wit,H.J.W.Lenderink,Electrochemical impendence spectroscopy as a tool to obtain mechanistic information on the passive behavior of commercial sample of aluminium,J.Electrochim.Acta41(1996)1111–1119.
[18]A.Aytac,U.Ozmen,M.Kabasakaloglu,Investigation of some Schiff bases as acidic corrosion of alloy AA3102,Mater.Chem.Phys.89(2005)176–181.
[19]M.M.Frers,C.Stefenel,Mayer,T.Chierchie,AC-impedance measurements on aluminium in chloride containing solutions and below the pitting potential,J.Appl.Electrochem.20(1990)996–999.
[20]F.Mansfeld,S.Lin,K.Kim,H.Shih,Pitting and surface modification of SiC/Al,J.Corros.Sci.27(1987)997–1000.
[21]F.Mansfeld,S.Lin,S.Kim,H.Shih,Electrochemical impedance spectroscopy as a monitoring tool for passivation and localized corrosion of Al alloys,Werkst.Korros.39(1988)487–492.
[22]B.S.Sanat Kumar,J.Nayak,A.N.Shetty,The corrosion inhibition of maraging steel under weld aged condition by 1(2E)-1-(4-aminophenyl)-3-(2-thienyl)-prop-2-en-1-one in 1.5 M hydrochloric acid medium,J.Coat.Technol.Res.4(2011)483–493.
[23]T.Poornima,N.Jagannatha,A.Nityananda Shetty,Studies on corrosion of annealed and aged 18Ni 250 grade maraging steel in sulphuric acid medium,Port.Electrochim.Acta28(2010)173–188.
[24]J.Yahalom,The significance of the energy of activation for the dissolution reaction of metal in acids,Corros.Sci.12(1972)867–868.
[25]S.S.Abdel Rehim,A.M.Magdy,K.F.Ibrahim,4-Aminoantipyrine as an inhibitor of mild steel corrosion in HCl solution,J.Appl.Electrochem.29(1999)593–599.
[26]M.A.Ameer,E.Khamis,G.Al-Senani,Effect of temperature on stability of adsorbed inhibitors on steel in phosphoric acid solution,J.Appl.Electrochem.32(2002)149–156.
[27]E.E.Oguzie,V.O.Njoku,C.K.Enenebeaku,C.O.Akalezi,C.Obi,Effect of hexamethylpararosaniline chloride(crystal violet)on mild steel corrosion in acidic media,J.Corros.Sci.50(2008)3481–3486.
[28]N.Soltani,M.Behpour,S.M.Ghoreishi,H.Naeimi,Corrosion inhibition of mild steel in hydrochloric acid solution by double Schiff bases,Corros.Sci.52(2010)1351–1361.
[29]X.Li,S.Deng,Inhibition effect ofDendrocalamus brandisiileaves extract on aluminum in HCl,H3PO4solutions,Corros.Sci.65(2012)299.
[30]F.Bentiss,M.Traisnel,M.Lagrenee,Influence of 2,5-bis(4-imethylaminophenyl)-1,3,4-thiadiazole on corrosion inhibition of mild steel in acidic media,J.Appl.Electrochem.31(2001)41–48.
[31]M.A.Quraishi,J.Rawat,M.Ajmal,Dithiobiurets:A novel class of acid corrosion inhibitors for mild steel,Appl.Electrochem.30(2000)745–751.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- A novel green inhibitor for C-steel corrosion in 2.0 mol·L−1 hydrochloric acid solution
- Polymorphism of D-mannitol:Crystal structure and the crystal growth mechanism☆
- Bulk and bubble-scale experimental studies of influence of nanoparticles on foam stability
- Simulation and optimization of an industrial gas condensate stabilization unit to modify LPGand NGL production with minimizing CO2 emission to the environment
- Efficient decolorization of dye-containing wastewater using mycelial pellets formed of marine-derived Aspergillus niger☆
- Characterization of pyrolytic lignins with different activities obtained from bio-oil☆
