Bulk and bubble-scale experimental studies of influence of nanoparticles on foam stability
2017-05-28NurudeenYekeenAhmadKamalIdrisMuhammadMananAliMohamedSaminAbdulRahimRisalTanXinKun
Nurudeen Yekeen*,Ahmad Kamal Idris,Muhammad A.Manan,Ali Mohamed Samin,Abdul Rahim Risal,Tan Xin Kun
Department of Petroleum Engineering,Faculty of Chemical and Energy Engineering,Universiti Teknologi Malaysia,81310 Johor Bahru,Malaysia
1.Introduction
Foams are dispersion of gas in liquid[1],with versatile applications in several engineering and industrial processes such as the food industry,firefighting and enhanced oilrecovery[2–5].However,foamsare thermodynamically unstable[6],thus,surface active agents such as surfactants are required for foam generation and its lamellae stability.Foam stability depends on the stability of the thin liquid films(lamellae)at the gas–liquid interface of foams.The stability of foam lamellae is very important,it influences foam performance in several applications such as fire control and foam flooding where foams interact with air,water and oil[6–8].Generally,surfactants have been used as the conventional foaming and stabilizing agents for several decades[9].In surfactant-stabilized foam,gaseous bubbles are prevented from coalescing by the adsorption of surfactant molecules at the gas–liquid interface of the foam[2].
However,surfactant-stabilized foams are unable to maintain their stability for a long time at high salinity,temperatures,and in the presence of external disturbances such as air,water and oil.This is due to surfactant high propensity to degrade at unfavorable conditions and the low adhesion energy of surfactants at foam interface.Low surfactant adhesion at foam lamellae promotes easy surfactant desorption and rapid film thinning of surfactant foam films[10,11].The film thinning increases and the foam becomes drier as a result of liquid drainage from the foam films[12].The thinning of the foam films eventually results in foam coalescence,that is,the breaking of smaller unstable bubbles to form bigger bubbles[12,13].
Recently,there is an emerging interest in foam stabilized by a mixture of nanoparticles and surfactant.The synergistic advantage of surfactant as foaming agent and nanoparticles as the stabilizing agent is utilized in nanoparticles/surfactant foams.Results of some previous studies show that,foams stabilized by nanoparticles–surfactant mixtures demonstrated high initial foamability and long term stability[14–18].This can be attributed to the remarkable stability of the foam films due to the irreversible adsorption of nanoparticles on the surface of their bubbles.Nanoparticles as the stabilizing components of the foam are solids;therefore foams stabilized by nanoparticles/surfactant mixtures are likely to be more resistant to high temperatures and the destabilizing effect of external forces like air,water and oil[19].Moreover,nanoparticles/surfactant foams improved oil recovery through interfacial tension and capillary forces reduction by the foaming agents[20,21].
Despite these advantages of nanoparticles/surfactant foam,the potential applications of silicon oxide(SiO2)and aluminum oxide(Al2O3)nanoparticles for improving the stability of conventional air and CO2foams have not been fully investigated.Likewise,the influence of nanoparticle concentration and hydrophobicity on the performance of these foams is not yet explicit due to limited studies.The study of these two critical parameters will provide valuable information regarding the optimum nanoparticle concentration and hydrophobicity required for effective foam generation,propagation and stability at static and dynamic conditions.Moreover,in several previous studies,bulk foam stability was only examined by monitoring the changes in the height of foam in vertical columns over time.There is still paucity of information on the bubble-scale dynamics of nanoparticles/surfactant foams.Bubble-scale information is required to fully understand the mechanisms of nanoparticles/surfactant foam generation and stability.
The main objective of this study is to experimentally investigate the influence of SiO2and Al2O3nanoparticle concentration and hydrophobicity on the stability of nanoparticles/SDS air and CO2foams at bulk and bubble scale.In order to investigate and compare the foam static behaviors to its behaviors in porous media,the apparent viscosity of the nanoparticles/surfactant foams was determined in Hele-Shaw cell porous media and compared with SDS foams.
2.Experimental
2.1.Materials
An anionic surfactant,sodiumdodecyl sulfate(SDS),two kinds of silicon oxide(SiO2)nanoparticles powder and a hydrophilic aluminum oxide(Al2O3)nanoparticles were used as the foaming and stabilizing agents in this study.The SDS was bought from Scharlau Chemie S.A.with a molecular weight of 288.38 g·mol−1and a purity of 95%.The two kinds of silica nanoparticles are:the hydrophilic silica nanoparticles obtained from US Research Nanomaterials Inc.,USA and a modified silica nanoparticles(50%methylsilyl capped,50%SiOH)supplied by Walker Chemicals Co.,Ltd.,Germany.The modification of the silica nanoparticles was performed by the manufacturer by reaction with dichlorodimethylsilane in order to increase its hydrophobicity.The hydrophilic aluminum oxide(Al2O3)nanoparticles were supplied by SkySpring Nanomaterials,Inc.,USA.
The specific surface area(SSA),the average sizes and the watercontact angle of the nanoparticles are given in Table 1.The CO2gas used for the foam generation was supplied by Mega Mount Industrial Gases.Sdn.Bhd with the maximum purity of 99%.All the solutions were prepared with sodium chloride(NaCl)supplied by Sigma USA(>99.0 wt%pure)at 0.5 wt%concentration in order to simulate the formation water.The viscosities of the foaming agent solutions were measured with RST rheometer(Brook field Engineering,USA).The average size distributions of aqueous dispersed nanoparticles at different SDS/nanoparticles ratios were measured by Malvern zetasizer(Zetasizer Nano-ZS instrument).All properties measurements were carried out at 25°C.
2.2.Experimental methods
2.2.1.Determination of nanoparticles hydrophobicity and surfactants adsorption on different particles
The nanoparticles–surfactant dispersion was prepared by dispersing a certain mass of the nanoparticles at different concentrations(0.0 wt%,0.05 wt%,0.1 wt%,0.5 wt%,1.0 wt%,1.5 wt%2.0 wt%,5.0 wt%)and SDS(0.3 wt%)into brine(0.5 wt%NaCl).The prepared dispersion was stirred for 12 h followed by hours of sonication[16].The nanoparticles hydrophobicity was determined by contact angle measurement.To measure the contact angle,several drops of the nanoparticles dispersion were dispersed on a glass slide and dried at room temperature.Then a water drop was placed on the modified glass slide.The shape of the water drop was captured by Nikon D 5100(NIKON Corporation)camera and the contact angle was measured with Image J software.The contact angles of the nanoparticles are shown in Table 1.
Surfactants adsorption on different nanoparticles was determined by measuring the conductivity of the surfactants,nanoparticles and nanoparticles/surfactant mixed solutions.The nanoparticles dispersions for hydrophilic SiO2and Al2O3were prepared by dispersing a certain mass of nanoparticles dispersion into brine(0.5 wt%NaCl)followed by hours of sonication.For the modified silica nanoparticles,aqueous dispersions were prepared by dispersing the nanoparticles into ethanol,centrifuging the dispersion,decanting the supernatant,and re-dispersing the particles into 0.5 wt%NaCl.The nanoparticles/surfactant mixed solutions were prepared as previously described.For conductivity measurement,the conductivity meter electrode(Jenway 3540)was placed into the dispersion,and the equilibrium conductivity of the dispersion was measured when the reading becomes stable.This test was performed with 0.05 wt%,0.1 wt%and 1 wt%nanoparticle concentrations and varying surfactant concentrations from0.01 to 1.00 wt.%.The adsorption index was calculated using Eq.(1)[22]

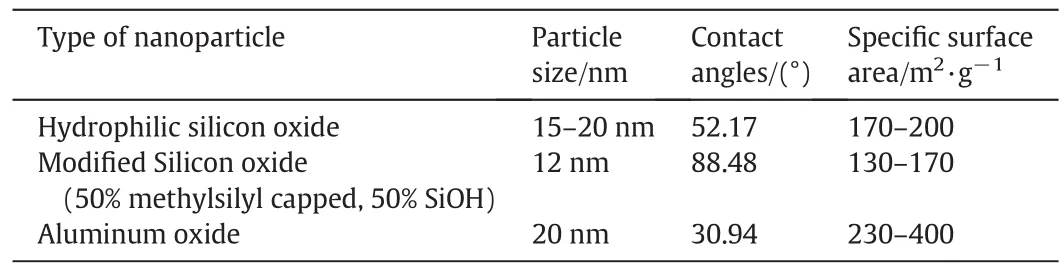
Table 1Properties of the SiO2 and Al2O3 nanoparticles used for the experiment
where λ represents conductivity andNP,SandNPSstand for nanoparticle dispersion,surfactant solution and nanoparticle/surfactant mixtures.
2.2.2.Air-foam bulk and bubble-scale foam stability experiments
The bulk and bubble scale foam stability experiment for air-foam was performed using KRÜSS dynamic foam analyzer DFA100(KRÜSS GmbH—Germany)as shown in Fig.1.Foam was generated in a tempered glass column with a height of 250 mm and a diameter of 40 mm by sparging air(from electronic gas flow control)through a porous filter plate(40–100μm)in a fixed amount of surfactant or nanoparticles/surfactant mixed solutions(50 cm3)at a fixed gas flow rate of 0.3 L·min−1.The pump time was set for 12 s and the gas flow stopped automatically after 12 s.The foam generation setup was connected to a computer which serves as the data acquisition and monitoring unit.The entire measurement,results evaluation and analysis were controlled by installed foam analysis software.Foam structure with regard to the bubble size and its distribution was measured with foam structure modules.The bulk foam stability was evaluated from the half-life,the bubble size distribution and the histograms of bubble size distribution obtained directly from the foam analyzer.
Fig.1.Krüss foam analyzer(DFA100)for air-foam bulk and bubble-scale stability experiment.
2.2.3.CO2foam bulk-scale foam stability
The experimental set-up for the CO2foam bulk stability experiment comprises of the vertical foam column which is a glass column with nominal internal diameter of 50 mm and height of 700 mm.Foam was generated in the glass column by dispersing CO2gas through a similar porous filter plate used for air-foam(40–100 μm)in a fixed amount of surfactant or nanoparticles/surfactant solution(100 cm3)at a fixed gas flow rate of 3 ml·min−1for 5 min.Foaming capacity was determined from the maximum height of the generated foam in the glass column.Foam stability was determined from the foam half-life,that is,the time taken to reach half of the foam original height after generation.
2.2.4.CO2foam bubble-scale foam stability
The CO2foam bubble-scale experiment was conducted in a Hele-Shaw cell shown in Fig.2.The cell consists of two parallel glass plates with a length of 25 cm and width of 15 cm.The glass plates were separated by a gasket of 0.04 cm which helps to prevent leakage from the cell.The foam was generated by dispersing CO2gas through a porous filter plate(40–100 μm)in a similar glass column(with a height of 11 cm and internal diameter of 5 cm)used for the CO2bulk stability experiment.In order to investigate the influence of the three critical mechanisms that influence foam aging process[23],which are drainage,coalescence and coarsening.The Hele-Shaw was placed vertically while the foam was injected into the vertical Hele-Shaw cell horizontally.The foam was generated and injected into the cell at similar conditions to the bulk stability experiment.The foam injection into the cell was stopped after the foam completely filled the cell.The foam aging process was monitored as a function of time.A Nikon D90 camera with maximum resolution of 4288×2848(12.3 effective megapixels)and 420 pixel RGBG CMOS sensor was used to capture the foam image in the cell at different time intervals.

Fig.2.2D Hele-Shaw cell used to investigate CO2 foam stability at bubble-scale.
2.2.5.The properties of the foamfilms
The properties of the foam films were evaluated from the bubbles' morphology, film thickness and the rate of liquid drainage from the foam.The rate of liquid drainage from the foam was determined from the time taken for 25%,50%and 75%of the liquid to drain from the foam directly from the foam analyzer.The morphology of the bubbles was analyzed with Leica EZ4 HDstereo microscope(Leica Microsystems Limited).The generated foam microbubbles were allowed to stabilize and the bubble suspension was placed on a microscope slide.Image from the microscope was captured with a CMOS camera integrated into the microscope and connected to a computer.The film thickness of the foam image in the presence and absence of nanoparticles was observed and measured as function of time to explain the mechanisms of foam stabilization by nanoparticles.
2.2.6.Apparent viscosity of foam
In order to be able to relate the static behaviors of the nanoparticles/surfactant foams to its behaviors in porous media,the apparent viscosity of the CO2foam was quantified in a 2D Hele-Shaw cell with respect to changing flow rate and foam quality.The cell was constructed from two glass plates with a dimension of 35×25×0.5 cm.A similar gasket used for CO2foam bubble-scale stability experiment(with thickness of 0.04 cm)was used to prevent leakage and create gap between the glass plates.Foam was generated by simultaneous injection of CO2and foaming solution(using Harvard Apparatus,model 704500 syringe pump)into the similar foam generator described in Section 2.2.4 above.The differential pressure created by the foam as it propagates through the Hele-shaw cell was measured with the aid of pressure transducers connected to the inlet and outlet.The foam apparent viscosity was calculated using Eq.(2)[24]


3.Results and Discussion
3.1.Nanoparticle hydrophobicity and surfactants adsorption on different nanoparticles
The extent of particles hydrophobicity depends on the percentage of silanol(SiOH)groups on their surface.As shown in Table 1,the contact angles of the modified SiO2nanoparticles(88.48°)with 50%silanol(SiOH)groups on their surfaces,show that the nanoparticles are more hydrophobic than the hydrophilic SiO2and Al2O3nanoparticles with contact angles of 52.17°and 30.94°respectively.The relationship between the energy required to remove the particle from the interface,E,the radius of the particle,r,the interface surface tension γawand the particle contact angle at the interface,θ,is given by[25]

Eq.(3)shows that at favorable contact angles and particles diameter,the detachment energy will be very large.The adsorption of nanoparticles at the gas–liquid interface of the foam increases with increasing nanoparticles hydrophobicity.Hence,the modified silica nanoparticles(with about50%SiOH)are expected to be strongly attached to the foam gas–liquid interface more than the unmodified SiO2and Al2O3nanoparticles(with 100%SiOH)[17].
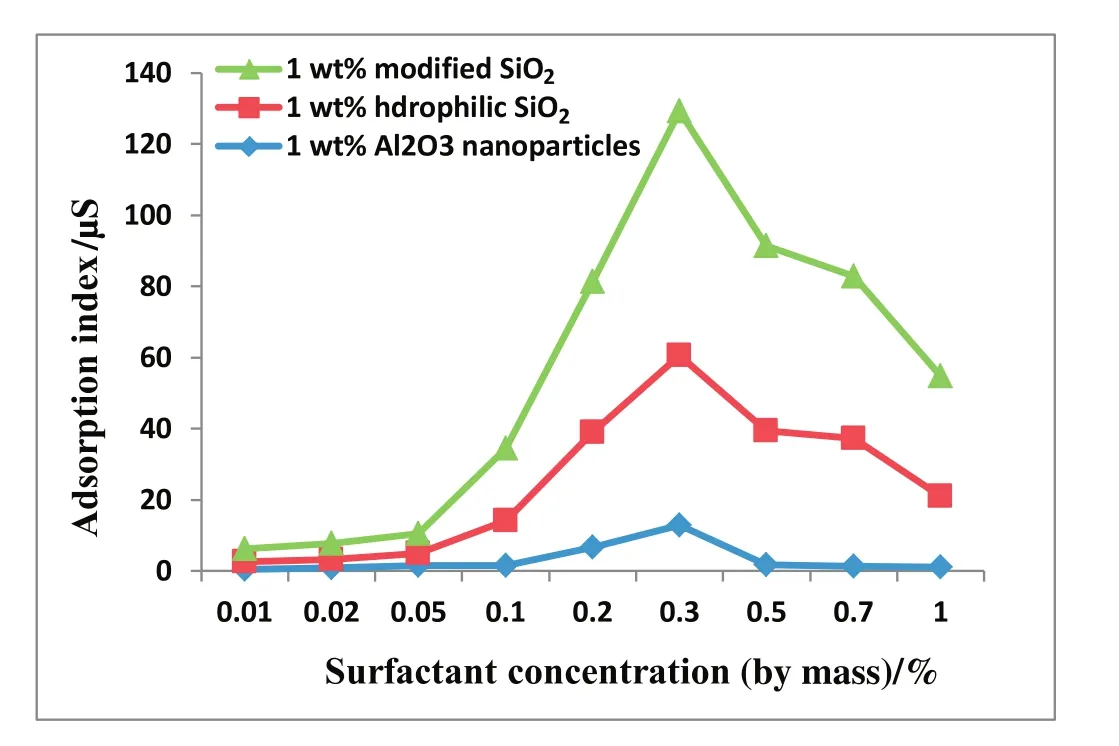
Fig.3.Adsorption index versus surfactant concentration for different nanoparticles.
Fig.3 shows the adsorption index for different nanoparticles as functions of surfactant concentration.The plotted graphs show that the rate of surfactant adsorption on nanoparticles was highest for modified SiO2nanoparticles and lowest for Al2O3nanoparticles.This further con firmed that the modified nanoparticles were more hydrophobic than the hydrophilic SiO2and Al2O3nanoparticles.The order of increase in adsorption index is as follows:modified SiO2nanoparticles>hydrophilic SiO2nanoparticles>Al2O3nanoparticles.The maximum adsorption for the three nanoparticles occurs at 3 wt%surfactant concentration close to the surfactant CMC obtained as 0.24 wt%in Fig.4.An increase in surfactant concentration increases the adsorption index until0.3 wt%.This can be attributed to frequent collisions between surfactant molecules and nanoparticles[22]due to the availability of more surfactant molecules at high surfactant concentration.Beyond this concentration,the adsorption index decreases with increasing surfactant concentration.The adsorption of surfactants on the nanoparticle surfaces continues until the nanoparticles reach the maximum hydrophobicity at 3 wt%surfactant concentration.Further increase in surfactant concentration beyond 3 wt%results in the adsorption of a second layer of surfactant molecules[22],this reduces particle hydrophobicity and the adsorption index.Hence,the 3 wt%surfactant concentration used in this study is appropriate for generation of stable nanoparticles/SDS foam.
3.2.In fl uence of nanoparticle concentration on foam generation
The effects of nanoparticle concentration on the foamability of CO2and air-foam are shown in Fig.5(a–c).The plotted graphs consistently show that the rate of foam generation decreases with increasing nanoparticle concentration.This result can be explained with the fact that the degree of surfactant adsorption on nanoparticles increases with increasing nanoparticle concentration.The adsorption index of different concentration of SDS increases as the SiO2nanoparticle concentration increases from 0.05 wt%to 1 wt%as shown in Fig.6.The nanoparticles absorb more surfactant molecules at high concentration to increase the surface tension of the foaming solution by reducing the free surfactant available in the bulk aqueous phase.Consequently foamgeneration reduces with increasing nanoparticle concentration[6].Increase in foam generation with increasing nanoparticle concentration can also be attributed to increasing viscosity of the foaming solution.Fig.7 shows that the viscosity of the foaming solution increased with increasing nanoparticle concentration.Since the gas flow rate for the foam generation was kept constant throughout the bulk stability experiment,the ease of foam generation will reduce with increasing foaming solution viscosity.Hence foamability reduces with increasing nanoparticle concentration.

Fig.4.Conductivity versus surfactant concentration.CMC is obtained from intersection of two straight lines.
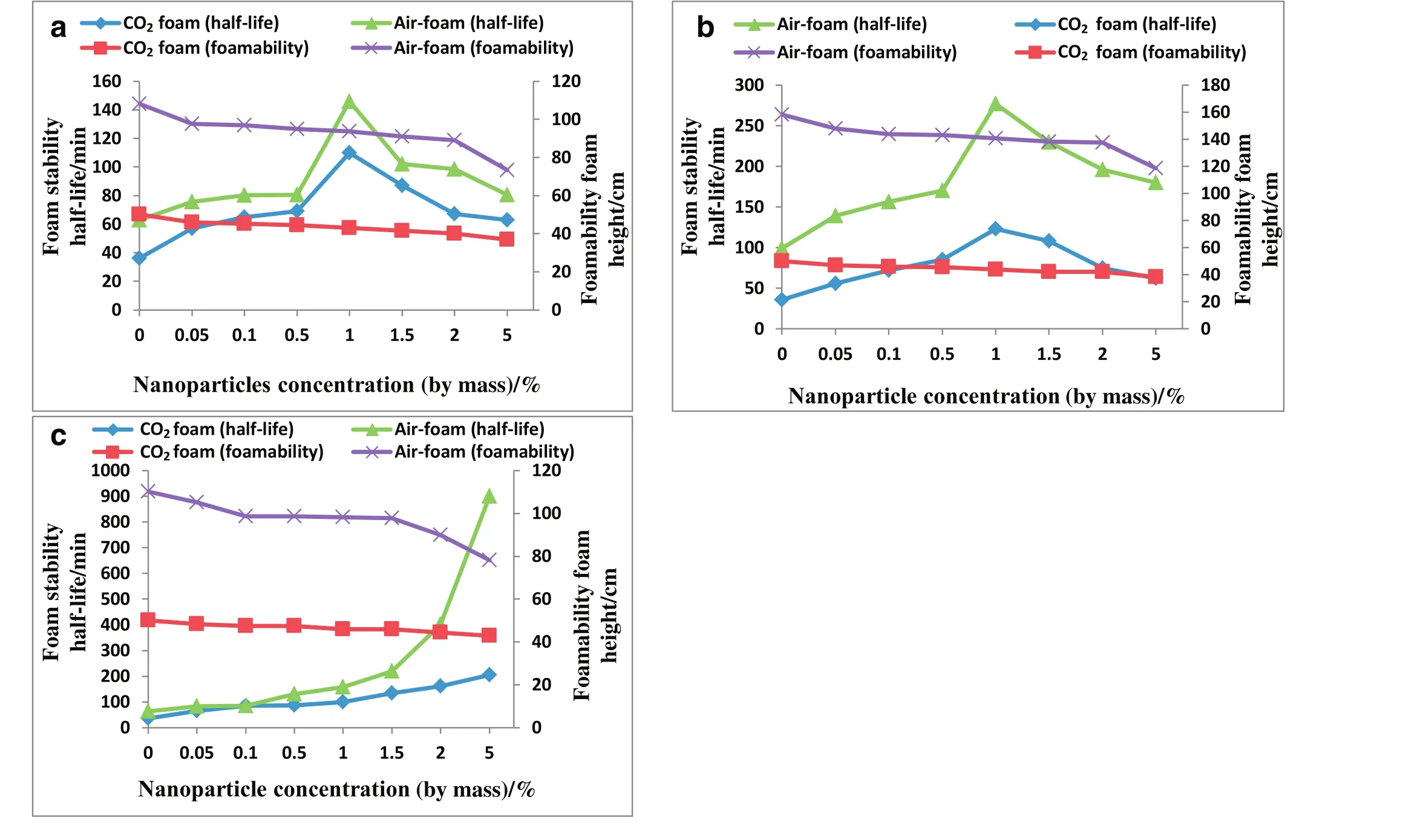
Fig.5.Effect of nanoparticle concentration on generation and stability of CO2 and air-foam stabilized with(a)Al2O3–SDS mixtures,(b)hydrophilic SiO2–SDS mixtures and(c)modified SiO2–SDS mixtures.
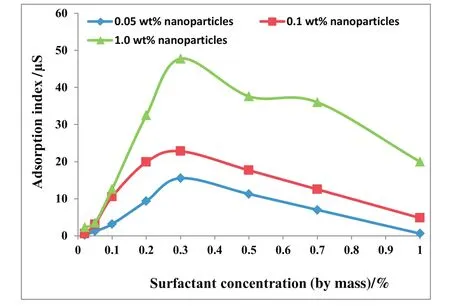
Fig.6.Adsorption index versus surfactant concentration for varying hydrophilic SiO2 concentration.
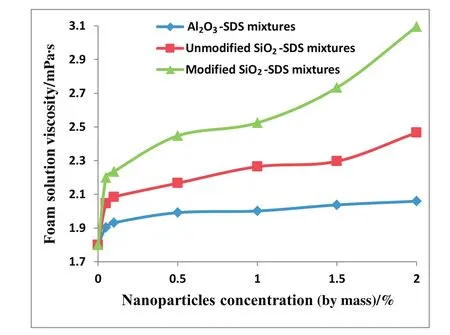
Fig.7.Effect of nanoparticle concentration on foam solution viscosity.
3.3.In fl uence of nanoparticle concentration on foam stability
Fig.5(a–c)shows the effects of nanoparticle concentration on the stability of CO2and air-foam.In the presence of hydrophilic SiO2and Al2O3nanoparticles(Fig.5a and b),foam stability(half-life)increased with increasing nanoparticle concentration from 0.05 wt%to 1 wt%.Beyond 1 wt%,foam stability started to decrease with the increasing nanoparticle concentration.This result was consistent for both CO2and air-foam.For the modified SiO2–SDS CO2and air-foam(Fig.5c),foamstability(half-life)increases with the increasing nanoparticle concentration,irrespective of the nanoparticle concentration.In this study,the optimum nanoparticle concentration for the maximum stability of the CO2and air-foam in the presence of hydrophilic SiO2and Al2O3nanoparticles was obtained at 1 wt%.Decrease in foam half-life with increasing nanoparticle concentration beyond 1 wt%can be attributed to the excessive agglomeration of the nanoparticles at the foam interface and bulk structure.
Although the nanoparticle concentration needs to exceed a certain critical value to improve and maintain foam stability[26],However only moderate and uniform accumulation of nanoparticles at the foam lamellae and the foam network can promote foam stability by reducing film thinning.Bigger particle sizes are formed from nanoparticles agglomeration at very high concentration[27].Thus,the bigger sized nanoparticles promote inter-bubble diffusion and increase the rate of liquid drainage from the foam lamellae by exerting gravity force on the generated bubbles[1,28,29].
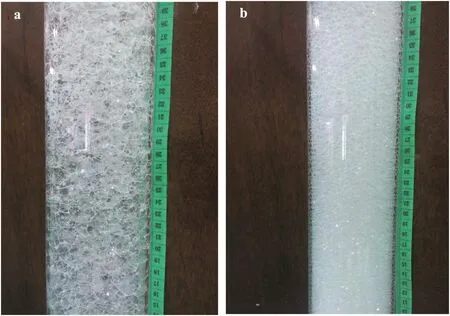
Fig.8.Images of Al2O3/SDS CO2 foam with(a)2 wt%Al2O3 concentration and(b)1 wt%Al2O3 concentration.
Fig.8a shows the foam image of Al2O3/SDS CO2foam with 2 wt%Al2O3concentration.Compared to foam generated with 1 wt%nanoparticle concentration(Fig.8b),the generated foam at 2 wt%became thickened and stained the foam column preventing further visual observation of the decreased in CO2foam height with respect to time.At2 wt%,it is likely that the Al2O3nanoparticles were not deposited uniformly at the gas–liquid interface but accumulated to create thick layers in the foam network.These accumulated nanoparticles increased the influence of surface forces on the interfacial films;hence,increases the rate of bubble coalescence and coarsening.This notion was ascertained from the bubble size distribution of hydrophilic SiO2/SDS and Al2O3/SDS mixtures air-foam at 5 wt%SiO2and Al2O3concentration obtained from the foam analyzer as shown in Fig.9.The bubble size distribution of these foams(Fig.9a and b)shows high rate of bubble coalescence and coarsening compared to the modified SiO2–SDS foam(Fig.9c).
As a result of nanoparticle agglomeration at high concentration,the total surface area of nanoparticles involved in adsorption process at foam air–water interface is reduced.This results in film thinning,faster liquid drainage and faster rate of bubble coalescence and coarsening observed in Fig.9(a-b).The concept of nanoparticles accumulation with increasing nanoparticle concentration was further investigated by determining the average particle sizes using zetasizer.Table 2 shows the average particle sizes distribution for Al2O3nanoparticles in Al2O3/SDS mixed solutions at different nanoparticle concentrations.The average particle sizes increased with increasing nanoparticle concentrations signifying the increasing particle aggregation at high nanoparticle concentration.The particle size distribution shown in Fig.10(a–c)shows that at 2 wt%,the nanoparticles agglomeration was higher for Al2O3nanoparticles in Al2O3/SDS solutions(Fig.10c)and hydrophilic SiO2nanoparticles in SiO2/SDS solutions(Fig.10b)compared to the modified SiO2/SDS system(Fig.10a).The average particle size ofAl2O3was 1209 nm(PDI0.474)while that of hydrophilic SiO2was obtained as917.5 nm(PDI0.223)compared to the modified SiO2nanoparticles with average size of 368.2 nm(PDI 0.146).At this concentration,the accumulated hydrophilic Al2O3and SiO2particles behaved large particle aggregates described by Fameau and Salonen[12].According to Fameau and Salonen[12],such large particles can be deposited faster than the flow of the liquid and can be trapped in the foam network.
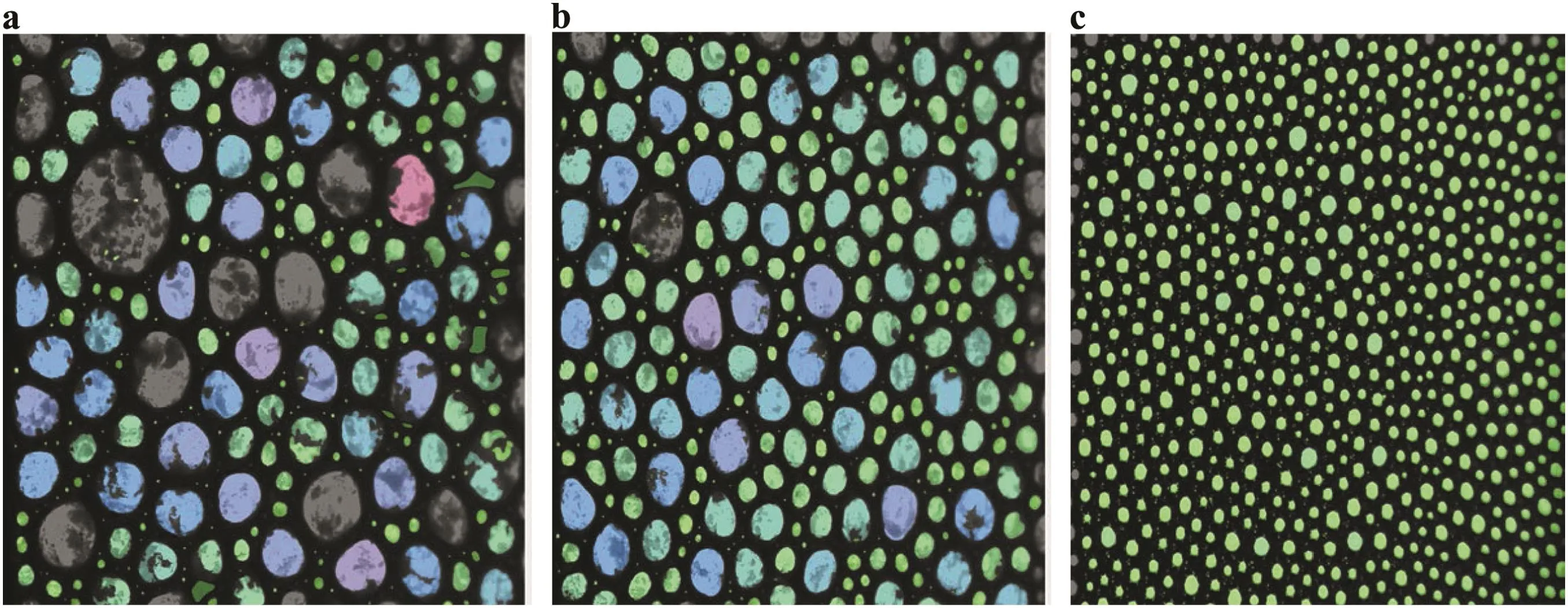
Fig.9.Bubble size distribution of foams of(a)Al2O3/SDS mixtures,(b)SiO2/SDS mixtures and(c)modified SiO2/SDS mixtures at 5 wt%nanoparticle concentration showing the effects of excessive particle accumulation on the bubble stability.

Table 2Average particle diameter for different concentrations of Al2O3 nanoparticles in Al2O/SDS dispersions
3.4.Foam stability improvement by nanoparticles from bulk and bubble scale-study
Fig.11 shows the improvement in foam stability in terms of the half decay times of CO2and air foams in the presence of different nanoparticles.In the presence of 1 wt%nanoparticle concentration,the half-life of the CO2foam increased from 36 min to 99 min(175%)in the presence of Al2O3nanoparticles.In the presence of the hydrophilic and modified SiO2nanoparticles,the foam half-life increased to 110(206%)and 123 min(242%)respectively.For the air-foam,the foam half-life increased from 63 min in the absence of nanoparticles to 139.808,153.95 and 165.25 min in the presence of Al2O3nanoparticles,hydrophilic and modified SiO2nanoparticles respectively.The improvement in stability of CO2and air-foam in the presence of nanoparticles is further analyzed from the bubble size distribution of the generated air foam shown in Fig.12.In the absence of nanoparticles(Fig.12a),the bubble sizes are bigger,the bubbles are fewer and span across several bins of the histogram indicating high rate of bubble coalescence and coarsening.However,with the addition of nanoparticles(1 wt%)to the foaming solutions,Fig.12(b–d)shows a remarkable improvement in bubble stability.The bubble sizes became smaller;the bubbles were many and concentrated within the first few bin sizes of the histogram.
The CO2foam bubble-scale stability was investigated in the transparent 2D Hele-Shaw cell described in Fig.2.Fig.13(a–d)shows the state of the SDS-foam and nanoparticles/SDS foams in the Hele-Shaw cell after 60 min.In the absence of nanoparticles Fig.13(a),the SDS-stabilized foam aged quickly.The effect of foam free drainage,coalescence and coarsening was more pronounced on the SDS-stabilized foam.The bubble size distribution in the presence of 1 wt%nanoparticles in Fig.13(b–d)shows that the foam demonstrated slower rate of liquid drainage,coalescence and coarsening.However the finest and most stable bubbles were obtained when the foam was generated in the presence of modified SiO2nanoparticles due to nanoparticles hydrophobicity.It was also generally observed that,majority of the smaller and finest bubbles of both the SDS foam and nanoparticles/SDS foams resides at the bottom and top of the cell while larger bubbles were found at the cell center.This phenomenon was also observed by Capset al.[30]in their studies and was attributed to falling fluid flow during foam drainage[30].
The general improvement in bulk and bubble-scale air and CO2foam stability in the presence of nanoparticles is due to the moderate aggregation of nanoparticles at the foam lamellas and plateau borders.This promotes the foam stability by delaying the rate of liquid drainage,film thinning,and bubble coalescence and coarsening[12].the accumulated nanoparticles in the foam network form an interfacial shield around the bubble surface[31]that prevents further film thinning and bubble coalescence.The order of improvement in foam stability in the presence of nanoparticles was obtained as follows:modified SiO2/SDS foam>hydrophilic SiO2/SDS foam>Al2O3-SDS foam>SDS foam.The most stable foam in terms of half-decay times and bubble size distributions was the modified SiO2foam.This can be attributed to the nanoparticles hydrophobicity.The sizes of nanoparticles-stabilized bubbles have been observed to decrease progressively with an increase in the hydrophobicity of the nanoparticles in previous studies[32–34].Generally,the stability of air-foam in the presence of nanoparticles was generally better than that of the CO2-foam.The low stability of the CO2-foam compared to air-foam can be attributed to the high solubility of CO2in water which reduces the amount of CO2present for foaming at any given conditions[35,36].
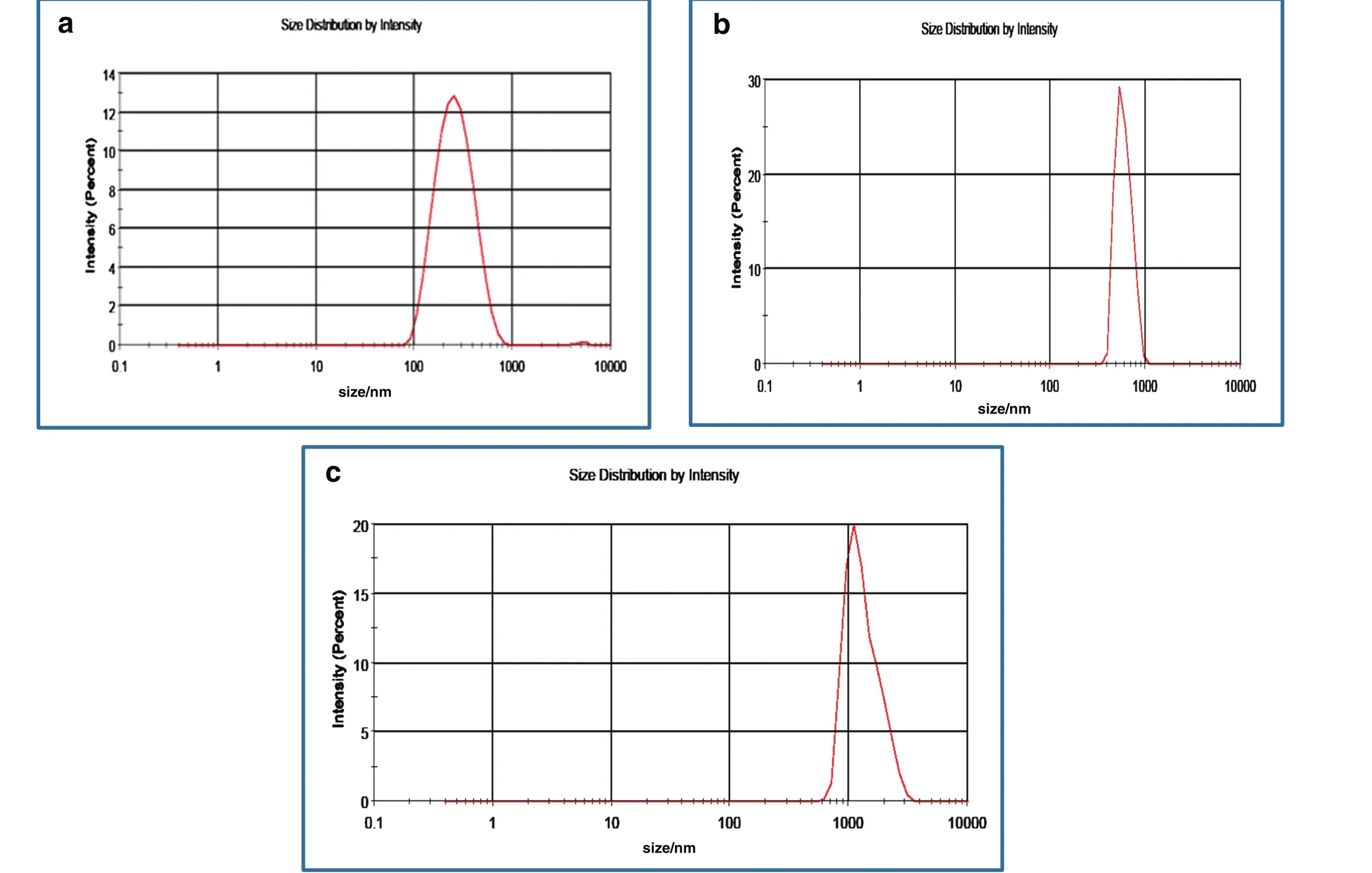
Fig.10.Average particle size showing nanoparticles agglomeration for(a)modified SiO2 nanoparticles in SiO2/SDS solution(b)hydrophilic SiO2 nanoparticles in SiO2/SDS solution and(c)Al2O3 nanoparticles in Al2O3/SDS solution at 2 wt%nanoparticle concentration.
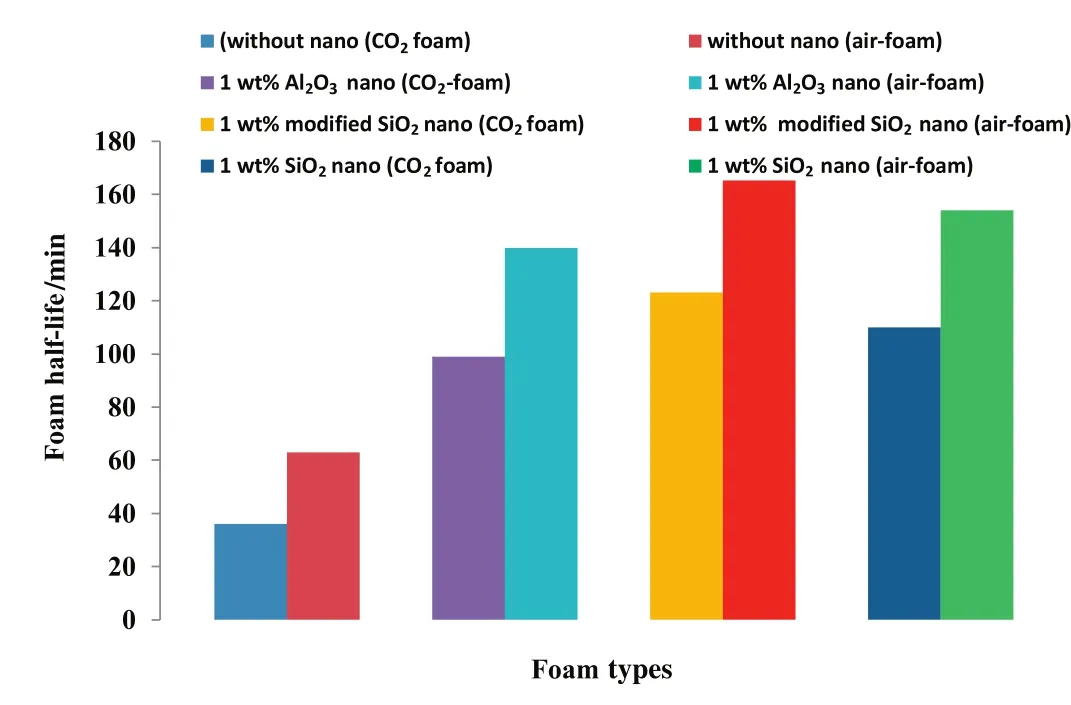
Fig.11.The half-decay times ofCO2 and air-foams in the absence and presence of different nanoparticles.
3.5.Mechanisms of foam stability improvement by nanoparticles from bubble morphology
The mechanism of foam stability improvement by nanoparticles was investigated by studying the bubbles'morphology, films strength and thickness and the rate of liquid drainage from the foam.Fig.14a shows the morphology of the CO2foam generated in the presence of 1.0 wt%Al2O3nanoparticles as revealed by Leica EZ4 HD stereomicroscope.Nanoparticles were located on the lamella between bubbles as observed from this figure.The nanoparticles/SDS foam bubbles are compact,smaller and finer with thicker films due to the presence of nanoparticle in the foam structure which improves the bubbles' stability.The foam lamellae show little or no sign of liquid drainage.The histogram of bubble size distribution(Fig.14b)shows that the bubble sizes are smaller(1 μm–380 μm)and located within the first few bin sizes of the histogram.In the absence of nanoparticles(Fig.15),the SDS-stabilized bubbles are bigger and showed signs of rapid liquid drainage from the foam lamellae.
The histogram of the bubble size distribution(Fig.15b)shows that the bubble sizes are bigger(61μm–700μm),the bubbles are fewer indicating high rate of bubble coalescence and coarsening.There is accumulation of nanoparticles at the lamellae of Al2O3/SDS foam compared to SDS foam.The nanoparticles are adsorbed at the gas–liquid interface of the foam with a very strong adhesion energy that is many times larger than that of surfactant molecules[18,33].The adsorbed nanoparticles on the lamellae of nanoparticle/SDS bubbles aggregated to create thick solid films that provide steric barrier to film thinning and inter-bubble diffusion[37].The moderate accumulation of nanoparticles slows down gravitational drainage,thus,increases the maximum capillary pressure,the foam lamellae can experience without rupturing[17].

Fig.12.Bubble size distribution and histograms of bubble size distribution of air-foam stabilized by(a)SDS,(b)hydrophilic SiO2/SDS,(c)modified SiO2/SDS and(d)Al2O3/SDS at 60 min.

Fig.13.State of CO2 foams of(a)SDS,(b)modified SiO2/SDS mixtures,(c)hydrophilic SiO2/SDS mixtures and(d)Al2O3/SDS mixtures in Hele-Shaw cell after 60 min.
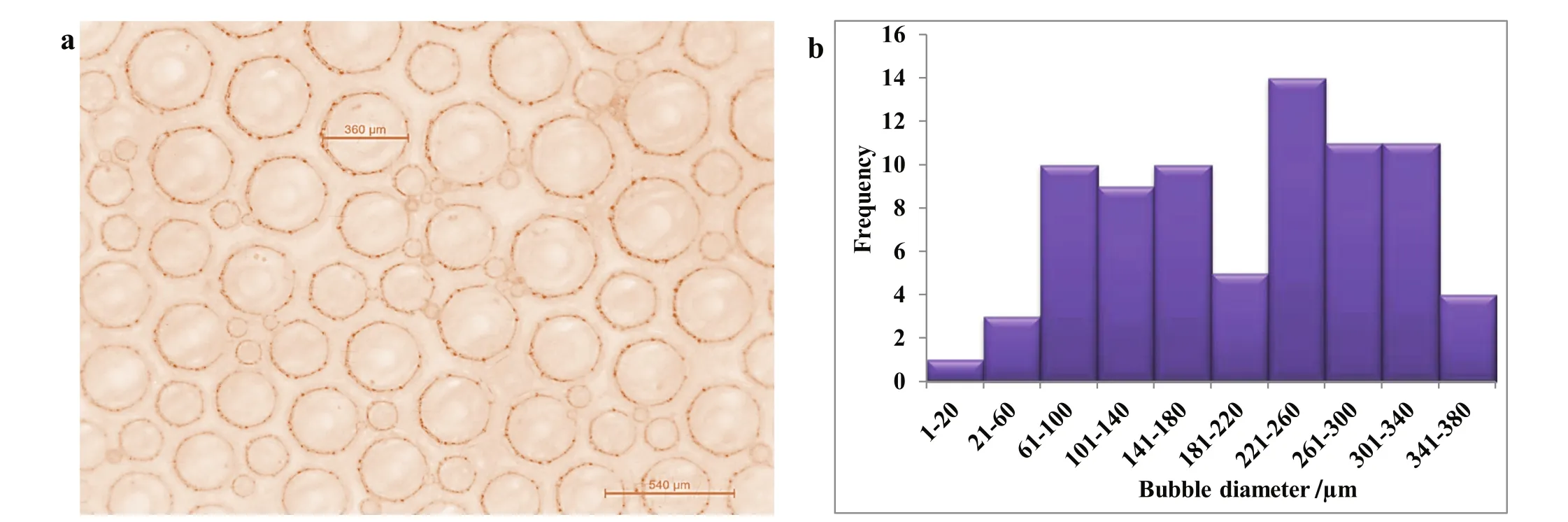
Fig.14.Bubble size distribution(a)and histogram of bubble size distribution(b)of Al2O3/SDS foam.
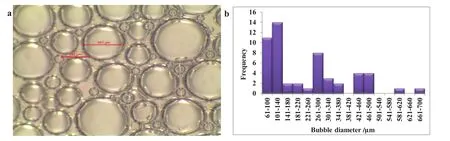
Fig.15.Bubble size distribution(a)and histogram of bubble size distribution(b)of SDS foam(without nanoparticles).
3.6.Mechanisms of foam stability improvement by nanoparticles from films thickness and liquid drainage
Foam stability is a function of the stability of the foam lamellae;hence,the mechanisms of foam stability improvement by nanoparticles will be studied from the film thickness and liquid drainage.Fig.16 shows the images of SDS bubbles while fig.17 shows that of hydrophilic SiO2/SDS airfoam(with 1 wt%SiO2concentration)after generation and after 60 min.The bubble sizes of the SDS foam seem the finest immediately after foam.This might be due to the low viscosity of SDS dispersion compared to SiO2/SDS dispersion.However the biggest film thickness for the SDS foam is 27.5 μm(Fig.16a).In the presence of 1 wt%SiO2nanoparticles(Fig.17a),the film thickness is 136 μm(394.55%more than that of SDS)despite the fact that the initial bubble sizes for SiO2/SDS foam were larger than that of SDS foam.The SDS bubbles collapse faster with time.After 60 min,the SDS bubble sizes became bigger with an irregular shape,the foam films and the bubbles become transparent.The film thickness of SDS bubbles reduced to 23.2 μm(Fig.16b).Conversely,there was no change in the bubble sizes and film thickness of SiO2/SDS foam after 60 min(Fig.17b).The bubbles' shape remains either spherical or ellipsoidal and the film thickness remains the same.
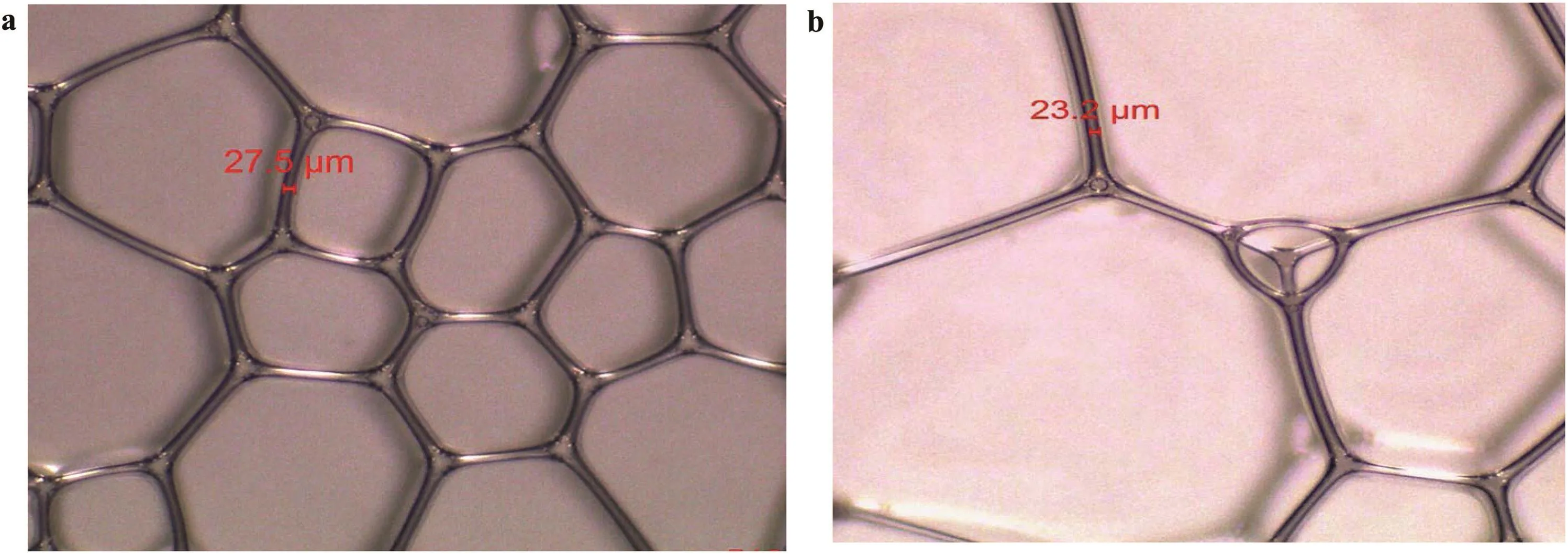
Fig.16.Foam images of SDS-stabilized foam(without nanoparticles)(a)immediately after generation and(b)60 min after generation.
This result can be attributed to the improved surface dilational viscoelasticity of the nanoparticles/SDS foam due to the nanoparticles adsorption and accumulation on the bubble surface and plateau border[16].As shown in Fig.18,the time taken for 25%,50%and 75%of the liquid to drain from the air foam was higher in the presence of modified SiO2nanoparticles compared to the SDS-stabilized foam.The time taken for liquid drainage increased with increasing nanoparticle concentrations.The faster liquid drainage from the SDS-foam stretches the foam film and results in film thinning.The film stretching also increases the surface tension gradient.The adsorbed surfactant on gas liquid interface of the foam migrates to a new surface due to the surface tension gradient[6].This reduces the film dilational viscoelasticity and the SDS-foam stability.For nanoparticles/SDS foams,the presence of nanoparticles on the bubbles' surface reduces foamdrainage and increases dilational viscoelasticity and the strength of the foam films during film stretching.The accumulated nanoparticles on the bubble surface prevent surfactant transfer from bulk solution to new surface[38].It also stabilized the foam against Ostwald ripening by reducing the surface area available for inter-bubble gas diffusion from small to large bubbles due to the pressure difference arising from Young–Laplace effect[39].
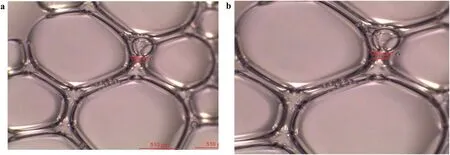
Fig.17.Foam images of SiO2/SDS-stabilized foam(without nanoparticles)(a)immediately after generation and(b)60 min after generation.
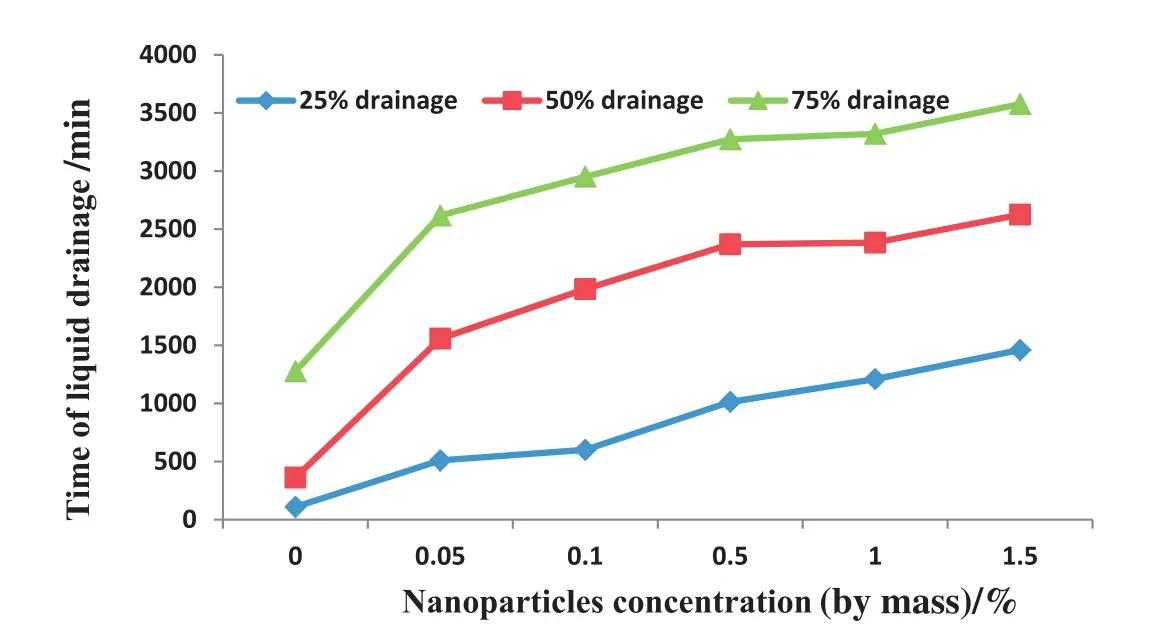
Fig.18.Influence of modified SiO2 nanoparticles on foam drainage.
3.7.Foam apparent viscosity determination in Hele-Shaw cell
The generaloutcome of the bulk and bubble levelfoam properties in this study shows that,nanoparticles do stabilize foam and can improve foam properties by their adsorption on gas–liquid interfaces of the foams.The apparent viscosity of the foam in Hele-Shaw cell was determined as described in Section 2.2.6 in order to know if the bulk and bubble level foam properties can translate intoin-situbehavior in porous media where interfaces are permanently created and destroyed,and particles can adsorb on rock walls.The foam apparent viscosity was determined at two foam qualities(50%and 75%)and varying flow rates ranging from0.1–0.5 ml·s−1.Fig.19 shows that the foam apparent viscosity increased in the presence of nanoparticles and with increasing nanoparticles hydrophobicity.The result further shows that foam apparent viscosity increases as foam quality increases and foam flow rate decreases.At the lowest flow rate used in this study(0.1 ml·s−1)and highest foam quality(75%),the apparent viscosity of the CO2foam increases from 20.34 mPa·s in the absence of nanoparticles to 44.91 mPa·s,56.73 cp and 84.84 mPa·s in the presence of hydrophilic Al2O3,hydrophilic SiO2and modified SiO2respectively.At the highest flow rate(0.5 ml·s−1),the apparent viscosity increases from 12.38 mPa·s to 25.48 mPa·s,26.06 mPa·s and 29.1 mPa·s with the addition of hydrophilic Al2O3,hydrophilic SiO2and modified SiO2respectively.This result shows that foam can controls gas mobility by increasing the apparent viscosity of displacing fluid in porous media[8,40].It also suggests that the SDS/nanoparticles bubble might be able to resists distortion in porous media surface due to the enhanced dilational surface viscoelasticity resulting from nanoparticles adsorption on their bubble surfaces.
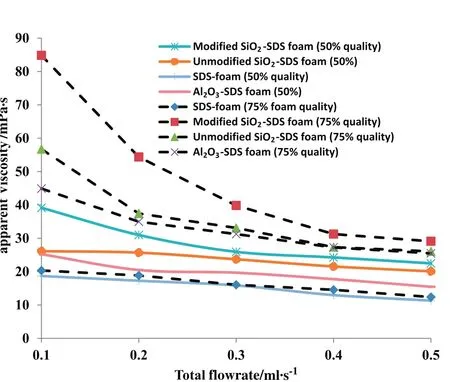
Fig.19.Foam apparent viscosity in the presence and absence of nanoparticles.
4.Conclusions
This study established that the addition of Al2O3and SiO2nanoparticles atappropriate concentrations and hydrophobicity into the surfactant solution significantly improves nanoparticles/surfactant mixed solution foams bulk-and bubble-scale stability.The sizes of nanoparticles/SDS bubbles were smaller;the bubbles were many and concentrated within the first bin size of the histogram.However,in the absence of nanoparticles,the bubble sizes were bigger and fewer and span across wider range of the histogram indicating high rate of bubble coalescence and coarsening.The nanoparticles/SDS foams exhibit longer foam halflife,stable bubbles and high apparent viscosity in Hele-Shaw cell due to the adsorption and accumulation of nanoparticles at the foam lamellae and plateau border.The accumulated nanoparticles in the foam network form an interfacial shield around the bubble surface to increase film thickness and elasticity,thus,improves foam static and dynamic stability by preventing liquid drainage, film thinning,and bubble coalescence and coarsening.
Acknowledgments
The authors would like to thank the Ministry of Higher Education(Vot no.Q.J130000.2542.08H61)and Universiti Teknologi(UTM)Malaysia,for supporting this research through research management grant.
[1]D.Wang,Q.Hou,Y.Luo,Y.Zhu,H.Fan,Stability comparison between particles stabilized foams and polymer-stabilized foams,J.Dispers.Sci.Technol.36(2)(2015)268–273.
[2]G.Bournival,Z.Du,S.Ata,G.Jameson,Foaming and gas dispersion properties of non-ionic surfactants in the presence of an inorganic electrolyte,Chem.Eng.Sci.116(2014)536–546.
[3]M.Firouzi,A.V.Nguyen,Effects of monovalent anions and cations on drainage and lifetime of foam films at different interface approach speeds,Adv.Powder Technol.25(4)(2014)1212–1219.
[4]B.Gardiner,B.Dlugogorski,G.Jameson,Rheology of fire- fighting foams,Fire Saf.J.31(1)(1998)61–75.
[5]E.A.Foegeding,P.Luck,J.Davis,Factors determining the physical properties of protein foams,Food Hydrocoll.20(2)(2006)284–292.
[6]Y.Zhang,Z.Chang,W.Luo,S.Gu,W.Li,J.An,Effect of starch particles on foam stability and dilational viscoelasticity of aqueous-foam,Chin.J.Chem.Eng.23(1)(2015)276–280.
[7]J.Kim,Y.Dong,W.R.Rossen,Steady-state flow behavior of CO2foam,SPE J.10(4)(2005)405–415.
[8]T.J.Zhu,D.Ogbe,S.Khataniar,Improving the foam performance for mobility control and improved sweep efficiency in gas flooding,Ind.Eng.Chem.Res.43(15)(2004)4413–4421.
[9]W.R.Rossen,Foams in enhanced oil recovery,Surf.Sci.Ser.(1996)413–464.
[10]D.Espinosa,F.Caldesas,K.Johnston,S.Bryant,C.Huh,Nanoparticle-stabilized presented supercritical CO2,foams for potential mobility control applications,Paper SPE 129925,atthe SPE Improved Oil Recovery Symposium,Tulsa,24–28 April,2010.
[11]S.S.Adkins,D.Gohil,J.L.Dickson,S.E.Webber,K.P.Johnston,Water-in-carbon dioxide emulsions stabilized with hydrophobic silica particles,Phys.Chem.Chem.Phys.9(48)(2007)6333–6343.
[12]A.-L.Fameau,A.Salonen,Effect of particles and aggregated structures on the foam stability and aging,C.R.Phys.15(8)(2014)748–760.
[13]V.Carrier,A.Colin,Coalescence in draining foams,Langmuir19(11)(2003)4535–4538.
[14]Z.G.Cui,Y.Z.Cui,C.F.Cui,Z.Chen,B.Binks,Aqueous foams stabilized by in situ surface activation of CaCO3nanoparticlesviaadsorption of anionic surfactant,Langmuir26(2010)12567–12574.
[15]T.Hunter,Behaviour of aqueous foam stabilised by nanosilica and non-ionic surfactant,Chemeca 2008:towards a sustainable Australasia,2008,pp.1694–1703.
[16]Q.Sun,Z.Li,S.Li,L.Jiang,J.Wang,P.Wang,Utilization of surfactant-stabilized foam for enhanced oil recovery by adding nanoparticles,Energy Fuel28(4)(2014)2384–2394.
[17]R.Singh,K.K.Mohanty,Synergy between nanoparticles and surfactants in stabilizing foams for oil recovery,Energy Fuel29(2)(2015)467–479.
[18]B.P.Binks,M.Kirkland,J.A.Rodrigues,Origin of stabilisation of aqueous foams in nanoparticle–surfactant mixtures,Soft Matter4(12)(2008)2373–2382.
[19]T.Zhang,M.Roberts,S.Bryant,C.Huh,Foams and emulsions stabilized with nanoparticles for potential conformance control applications,Paper SPE-121744-MS,SPE International Symposium on Oil field Chemistry,20–22 April,The Woodlands,Texas,2009.
[20]R.Farajzadeh,A.Andrianov,P.Zitha,Investigation of immiscible and miscible foam for enhancing oil recovery,Ind.Eng.Chem.Res.49(2009)1910–1919.
[21]K.Osei-Bonsu,N.Shokri,P.Grassia,Foam stability in the presence and absence of hydrocarbons:From bubble-to bulk-scale,Colloids Surf.A Physicochem.Eng.Asp.481(2015)514–526.
[22]H.Farhadi,S.Riahi,S.Ayatollahi,H.Ahmadi,Experimental study of nanoparticle surfactant-stabilized CO2foam:Stability and mobility control,Chem.Eng.Res.Des.(2016).
[23]M.Krzan,H.Caps,N.Vandewalle,High stability of the bovine serum albumine foams evidenced in Hele-Shawcell,ColloidsSurf.APhysicochem.Eng.Asp.438(2013)112–118.
[24]K.Osei-Bonsu,N.Shokri,P.Grassia,Fundamental investigation of foam flow in a liquid- filled Hele-Shaw cell,J.Colloid Interface Sci.462(2016)288–296.
[25]B.Binks,S.Lumsdon,Influence of particle wettability on the type and stability of surfactant-free emulsions,Langmuir16(23)(2000)8622–8631.
[26]I.Kim,A.Taghavy,D.DiCarlo,C.Huh,Aggregation of silica nanoparticles and its impact on particle mobility under high-salinity conditions,J.Pet.Sci.Eng.133(2015)376–383.
[27]H.Holthoff,S.U.Egelhaaf,M.Borkovec,P.Schurtenberger,H.Sticher,Coagulation rate measurements of colloidal particles by simultaneous static and dynamic light scattering,Langmuir12(1996)5541–5549.
[28]F.AttarHamed,M.Zoveidavianpoor,M.Jalilavi,The incorporation of silica nanoparticle and alpha olefin sulphonate in aqueous CO2foam:Investigation of foaming behavior and synergistic effect,Pet.Sci.Technol.32(21)(2014)2549–2558.
[29]S.Chen,Q.Hou,Y.Zhu,D.Wang,W.Li,On the origin of foam stability:Understanding from viscoelasticity of foaming solutions and liquid films,J.Dispers.Sci.Technol.35(2014)1214–1221.
[30]H.Caps,N.Vandewalle,G.Broze,Foaming dynamics in Hele-Shaw cells,Phys.Rev.E73(6)(2006)065301.
[31]U.T.Gonzenbach,A.R.Studart,E.Tervoort,L.J.Gauckler,Stabilization of foams with inorganic colloidal particles,Langmuir22(26)(2006)10983–10988.
[32]J.Yu,M.Khalil,N.Liu,R.Lee,Effect of particle hydrophobicity on CO2foam generation and foam flow behavior in porous media,Fuel126(2014)104–108.
[33]B.P.Binks,T.S.Horozov,Aqueous foams stabilized solely by silica nanoparticles,Angew.Chem.117(24)(2005)3788–3791.
[34]A.J.Worthen,H.G.Bagaria,Y.Chen,S.L.Bryant,C.Huh,K.P.Johnston,Nanoparticle stabilized carbon dioxide-in-water foams with fine texture,J.Colloid Interface Sci.391(2013)142–151.
[35]R.Farajzadeh,A.Andrianov,H.Bruining,P.L.Zitha,Comparative study of CO2and N2foams in porous media at low and high pressure–temperatures,Ind.Eng.Chem.Res.48(9)(2009)4542–4552.
[36]A.Andrianov,R.Farajzadeh,M.Mahmoodi Nick,M.Talanana,P.Zitha,Immiscible foam for enhancing oil recovery:Bulk and porous media experiments,Ind.Eng.Chem.Res.51(5)(2012)2214–2226.
[37]S.Zhang,D.Sun,X.Dong,C.Li,J.Xu,Aqueous foams stabilized with particles and nonionic surfactants,Colloids Surf.A Physicochem.Eng.Asp.324(1)(2008)1–8.
[38]Y.S.Lee,N.J.Wagner,Dynamic properties of shear thickening colloidal suspensions,Rheol.Acta42(2003)199–208.
[39]P.Stevenson,Inter-bubble gas diffusion in liquid foam,Curr.Opin.Colloid Interface Sci.15(2010)374–381.
[40]C.Huh,W.Rossen,Approximate pore-level modeling for apparent viscosity of polymer-enhanced foam in porous media,SPE J.13(2008)17–25.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- A novel green inhibitor for C-steel corrosion in 2.0 mol·L−1 hydrochloric acid solution
- An ecofriendly approach for corrosion control of 6061 Al-15%(v)SiC(P)composite and its base alloy
- Polymorphism of D-mannitol:Crystal structure and the crystal growth mechanism☆
- Simulation and optimization of an industrial gas condensate stabilization unit to modify LPGand NGL production with minimizing CO2 emission to the environment
- Efficient decolorization of dye-containing wastewater using mycelial pellets formed of marine-derived Aspergillus niger☆
- Characterization of pyrolytic lignins with different activities obtained from bio-oil☆
