外源性亚精胺对鼠卵巢生殖激素受体基因表达的影响
2017-05-19龙诗韵姜冬梅陈咨余管成易治鑫康波
龙诗韵,姜冬梅,陈咨余,管成,易治鑫,康波
(四川农业大学动物科技学院/畜禽遗传资源发掘与创新利用四川省重点实验室,成都611130)
外源性亚精胺对鼠卵巢生殖激素受体基因表达的影响
龙诗韵,姜冬梅,陈咨余,管成,易治鑫,康波*
(四川农业大学动物科技学院/畜禽遗传资源发掘与创新利用四川省重点实验室,成都611130)
为探讨外源性亚精胺对鼠卵巢生殖激素受体基因表达的影响,采用不同质量分数(0.05、0.10和0.15 mg/g)亚精胺腹腔注射6周龄昆明鼠,24 h后采集卵巢组织样品,应用实时荧光定量聚合酶链式反应检测卵巢组织中促卵泡激素受体、促黄体生成素受体、雌激素受体1、雌激素受体2和雄激素受体基因表达量。结果显示:与对照组相比,0.05 mg/g亚精胺处理组卵巢促黄体生成素受体表达量显著高于对照组(P<0.05),0.15 mg/g组促黄体生成素受体表达量显著低于对照组(P<0.05);随亚精胺处理量的增加,卵巢组织中促卵泡激素受体、雌激素受体1、雌激素受体2和雄激素受体表达量均呈逐渐增加的趋势,且均在0.15 mg/g处理组中的表达量显著高于对照组(P<0.05)。综上表明,亚精胺可通过介导鼠卵巢组织生殖激素受体基因表达来参与调控鼠卵巢功能。
亚精胺;卵巢;激素受体;基因表达
SummaryThe polyamines spermidine and spermine and their precursor putrescine are a class of compounds containing two or more amino groups and participate in the regulation of various physiological and pathological processes by interacting with negatively-charged molecules,such as DNA,RNA and proteins.Recently,increasing studies suggest that polyamines also play important roles in follicular development,ovulation and steroidogenesis in female animals.The spermidine exists ubiquitously in species ranging from yeast to mammals.Recently,studies from our and other laboratories indicated that spermidine is involved in regulating animal reproduction through mediating spermatogenesis,oogenesis,follicular development and ovulation.However, to the best of our knowledge,the effect of the spermidine on the expression levels of hormone receptor genes associated with reproduction is unknown.
To demonstrate the effect of the spermidine on the transcription of reproductive hormone receptor genes in mouse ovaries, Kunming mice were administrated with 0.05,0.10 and 0.15 mg/g(body mass)spermidine by intraperitoneal injection.Ovarian tissue samples were collected after 24 h of spermidine administration.The levels of follicle-stimulating hormone receptor (FSHR),luteinizing hormone receptor(LHR),estrogen receptor 1(ER1),ER2and androgen receptor(AR)mRNA expression in mouse ovaries were measured using quantitative real-time polymerase chain reaction.
The results showed that the level ofLHRmRNA expression in ovaries of mice administrated with 0.05 mg/g spermidine was significantly higher than the untreated control group(P<0.05).The amount ofLHRmRNA expression in ovaries of mice administrated with 0.15 mg/g spermidine was significantly lower than the untreated control group(P<0.05).With increasing spermidine administration,a gradually increasing trend was observed in the change ofFSHR,ER1,ER2andARexpression levels.The amount ofFSHR,ER1,ER2andARgenes in ovaries of mice administrated with 0.15 mg/g spermidine was significantly high,compared with the untreated control group,respectively(P<0.05).
In conclusion,our present results demonstrate that exogenous spermidine mediates the transcription of genes associated with reproductive hormone receptors in ovaries of mice.It is indicated that spermidine might play an important role in regulating the responsiveness of the ovary to reproductive hormones through an unknown mechanism.Further research is needed to elucidate the mechanism of action of spermidine mediating ovarian functions in animals.
亚精胺是广泛存在于动物、植物和微生物体内的一种多胺,可通过介导雄性动物精子发生,以及雌性动物卵泡发育、卵子发生和排卵活动,进而参与调控动物的繁殖过程[1-2]。多胺能通过与多种生殖激素建立复杂的反馈联系,进而参与调控动物繁殖[3]。多胺在维持卵巢类固醇激素水平和生成过程中具有重要作用[4]。雄性动物体内的多胺合成受到雄激素的调节[5]。FASHE等[6]研究发现,多胺合成受阻时,卵巢性激素含量显著降低。赵越超[7]研究发现,性激素可通过其受体途径影响鼠子宫内多胺代谢。大量研究证实,雄激素在治疗男性不育症上具有重要作用,亚精胺在不育男性精浆中的含量明显比正常男性低[8-10],推测亚精胺可能通过作用于雄激素及其受体来参与调控雄性动物的繁殖功能。而在雌性动物中,亚精胺是否与雄激素受体(androgen receptor,AR)存在直接或间接的作用还未有研究报道。
促卵泡激素(follicle-stimulating hormone,FSH)和促黄体激素(luteinizing hormone,LH)分别与促卵泡激素受体(follicle-stimulating hormone receptor, FSHR)和促黄体生成素受体(luteinizing hormone receptor,LHR)作用,共同参与调控动物卵泡发育和卵泡颗粒细胞增殖[11]。LH和FSH还可参与调控卵巢组织中雌激素的合成,而雌激素通过负反馈调节机制影响垂体LH和FSH的分泌[12-13]。雌激素受体(estrogen receptor,ER)包含ER1和ER2这2种亚型,双敲除ER1和ER2的雌性鼠不能排卵,说明ER对雌性动物排卵具有至关重要的调控作用[14]。研究表明,多胺可通过介导哺乳动物生殖激素合成与分泌来参与调控动物繁殖功能[4]。然而,目前有关亚精胺调控雌性动物卵巢功能的研究相对较少,而且尚未见有关亚精胺调控卵巢生殖激素受体基因表达影响的报道。因此,本试验研究了不同质量分数亚精胺对鼠卵巢组织中FSHR、LHR、ER1、ER2和AR基因表达的影响,以期为阐明多胺调控哺乳动物卵巢功能的作用机制提供理论依据。
1 材料与方法
1.1 试验动物及样品采集
选取雌性健康同胞昆明鼠(SPF级试验用昆明系鼠,购于成都达硕公司),常规分笼饲养,鼠自由采食;在试验过程中对动物处置符合动物伦理学标准。6周龄时,将鼠随机分为4组,每组8只,分别腹腔注射生理盐水和0.05、0.10和0.15 mg/g(体质量)亚精胺。注射24 h后颈部脱臼处死,迅速采集鼠卵巢组织,用生理盐水洗净,滤纸吸干后,置于-80℃冰箱保存,备用。
1.2 引物设计和实时荧光定量聚合酶链式反应
按照RNA提取试剂盒(RNAiso Plus kit,TaKaRa公司,大连)说明书提取鼠卵巢组织总RNA,然后参照反转录试剂盒说明书(TaKaRa公司,大连)将总RNA样品反转录成cDNA模板,置于-20℃冰箱中保存,备用。基于GenBank数据库,采用Primer Premier 5.0和Oligo 6.0软件设计特异性引物,并委托华大基因有限公司合成。引物序列信息见表1。
实时荧光定量反应体系(10 μL):实时荧光定量聚合酶链式反应试剂盒(iQTMSYBR Green Supermix kit,Bio-Rad公司,北京)5.0 μL,上、下游引物(10 μmol/L)各0.2 μL,cDNA模板0.5 μL,用无RNA酶水补足至10 μL。反应条件:95℃预变性3 min;95℃变性10 s,57~63℃退火30 s,72℃延伸30 s(采集荧光),40个循环;95℃保持10 s,绘制溶解曲线。用3-磷酸甘油醛脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GAPDH)基因作为内参基因。每个样品设3个重复。

表1 实时荧光定量聚合酶链式反应引物序列Table 1 Primer sequences used in quantitative real-time polymerase chain reaction(PCR)
1.3 数据处理及统计分析
采用2-ΔΔCT法处理数据[7],用GAPDH作为内参基因,以空白组基因的表达量作为对照组,计算不同质量分数亚精胺处理组卵巢组织FSHR、LHR、ER1、ER2、AR基因的相对表达量。应用SAS 9.2统计分析软件中的MEANS过程进行描述性统计分析,并进行方差分析和邓肯多重比较,结果用平均值±标准差表示,P<0.05表示差异有统计学意义。
2 结果
2.1 亚精胺对鼠LHR和FSHR基因表达的影响
由图1可知:腹腔注射0.05 mg/g亚精胺时,鼠卵巢组织中LHR相对表达量显著高于其他各组,是对照组的2.07倍(P<0.05);腹腔注射0.15 mg/g亚精胺组LHR表达量显著低于对照组,为对照组的9%(P<0.05)。腹腔注射0.15 mg/g亚精胺时,FSHR表达量显著高于对照组,是对照组的31.67倍(P<0.05);而腹腔注射0.05和0.10 mg/g亚精胺时,FSHR基因表达量与对照组相比差异无统计学意义(P>0.05)。

图1 亚精胺对鼠卵巢LHR和FSHR表达的影响Fig.1 Effect of spermidine onLHRandFSHRmRNA expression in mouse ovaries
2.2 亚精胺对ERER1 1和ERER2 2基因表达的影响
腹腔注射0.15 mg/g亚精胺处理组中,ER1和ER2表达量均显著高于对照组,分别为对照组的1.95倍和6.43倍(P<0.05);而注射0.05和0.10 mg/g组中,ER1和ER2与对照组间差异均无统计学意义(P>0.05)(图2)。

图2 亚精胺对鼠卵巢ER ER1 1和ER ER2 2表达的影响Fig.2 Effect of spermidine onER1andER2mRNA expression in mouse ovaries
2.3 亚精胺对AR基因表达的影响
如图3所示:当腹腔注射0.15 mg/g亚精胺时,卵巢组织中AR相对表达量显著高于对照组和其他处理组,是对照组的4.31倍(P<0.05);而注射0.05和0.10 mg/g组中,AR表达量与对照组间差异无统计学意义(P>0.05)。
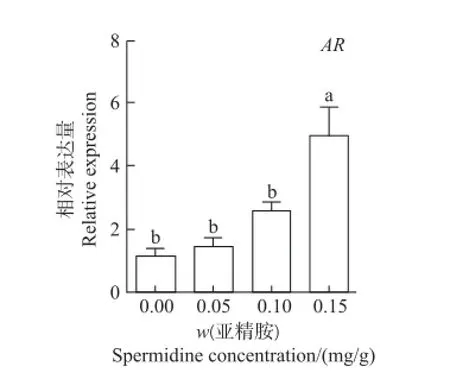
图3 亚精胺对鼠卵巢AR表达的影响Fig.3 Effect of spermidine onARmRNA expression in mouse ovaries
3 讨论
生殖激素及其特异性受体在调控动物繁殖过程中具有关键作用,而FSH和LH在调控动物繁殖过程中具有核心作用[15-16]。在卵巢颗粒细胞中LHR表达量降低会抑制排卵功能[17];而FSHR表达量升高能增强卵巢对外源性促性腺激素的应答能力。THYSSEN等[18]研究发现,外源性腐胺和亚精胺能抑制下丘脑和垂体组织中LH和FSH的分泌;用二氟甲基鸟氨酸(一种腐胺生物合成的不可逆抑制剂)处理后FSH分泌增加:表明多胺可参与调控垂体LH和FSH分泌。本研究发现,腹腔注射亚精胺显著提高了卵巢组织中FSHR和LHR表达量:说明亚精胺也可通过介导卵巢FSHR和LHR表达来发挥其对雌性动物繁殖功能的调控作用。表皮生长因子(epidermal growth factor,EGF)作为一种卵巢旁分泌因子,通过介导卵泡发育和卵母细胞生成来调节卵巢功能。研究表明,EGF可下调哺乳动物卵巢FSHR表达[19]。BLACHOWSKI等[20]发现,多胺能参与EGF信号转导通路,提示多胺与EGF参与的生殖调控具有相关性。本研究发现,注射0.15 mg/g亚精胺显著增加了卵巢组织中FSHR表达量,推测亚精胺促进FSHR表达的作用可能与EGF有关,有待进一步研究证实。此外,本研究还发现,低质量分数亚精胺(0.05 mg/g)能促进卵巢LHR表达,但对FSHR表达影响不显著;而高质量分数亚精胺(0.15 mg/g)抑制LHR表达,但促进了FSHR表达:表明亚精胺影响FSHR和LHR表达的效应具有剂量差异。
雌激素受体基因包括ER1和ER2,在鼠卵巢组织中均有表达,但其表达量和生理作用各不相同。在未成熟和成熟的啮齿类动物和人卵巢中,ER2表达量远高于ER1[21]。研究表明,在卵巢组织中ER2的表达和分布与鼠的生殖周期存在密切联系,尤其是在发情期的鼠卵巢组织中有高度表达,其表达规律与小鼠体内雌激素水平相适应[22]。本研究发现,0.15 mg/g亚精胺处理可显著增加鼠卵巢组织中ER1和ER2表达,提示高质量分数亚精胺可通过提高ER表达进而参与调控鼠卵巢功能。
AR在调节卵巢功能和维持女性生育能力方面具有重要作用[23]。KIMURA等[24]发现,在卵泡发育早期即有AR的表达,AR缺陷型鼠易出现卵泡发育障碍和卵巢早衰。AR和雄激素水平与哺乳动物在卵巢发育过程中FSHR表达密切相关[25]。此外,雄激素还参与调控卵巢癌的发生和发展过程,AR水平及其活性增强会使卵巢癌细胞对雄激素的敏感性增大,从而促进卵巢癌变[26]。本研究结果表明,0.15 mg/g亚精胺处理组的鼠卵巢组织中AR表达显著高于对照组:表明高质量分数亚精胺可通过促进AR表达进而参与调控鼠卵巢功能。
综上所述,在本试验中,腹腔注射不同质量分数亚精胺后,鼠卵巢组织中LHR、FSHR、ER1、ER2和AR表达量相较于对照组均发生了显著变化。低质量分数的亚精胺促进LHR表达;高质量分数的亚精胺促进FSHR、ER1、ER2和AR表达,同时抑制LHR的表达,且具有剂量依赖性。说明亚精胺可通过介导鼠卵巢组织生殖激素受体基因表达来参与调控鼠卵巢功能。
[1]向睿,何珲,康波.多胺调控动物繁殖的作用及其机制.动物营养学报,2014,26(11):3251-3255. XIANG R,HE H,KANG B.Effects of polyamine regulation on animal reproduction and its mechanism.Chinese Journal of Animal Nutrition,2014,26(11):3251-3255.(in Chinese with English abstract)
[2]PEGG A E,CASERO R A.Current status of the polyamine research field.Polyamines:Methods in Molecular Biology,2011, 720:3-35.
[3]VIJAYANATHAN V,THOMAS T J,NAIR S K,et al.Bending of the estrogen response element by polyamines and estrogen receptors alpha and beta:A fluorescence resonance energy transfer study.The International Journal of Biochemistry and Cell Biology,2006,38(7):1191-1206.
[4]LEFEVRE P L C,PALIN M F,MURPHY B D.Polyamines on the reproductive landscape.Endocrine Reviews,2011,32(5):694-712.
[5]GONZALEZ-MONTELONGO M C,MARIN R,PEREZ J A,et al.Polyamines transduce the nongenomic,androgen-induced calcium sensitization in intestinal smooth muscle.Molecular Endocrinology,2013,27(10):1603-1616.
[6]FASHE T M,KEINANEN T A,GRIGORENKO N A,et al.Cutaneous application of alpha-methylspermidine activates the growth of resting hair follicles in mice.Amino Acids,2010,38(2): 583-590.
[7]赵越超.小鼠围着床期子宫中多胺相关基因的表达、调节与功能.哈尔滨:东北农业大学,2008:61-67. ZHAOYC.Expression,regulation and function ofpolyamie-related genes in mouse uterus during peri-implantation period.Harbin: Northeast Agricultural University,2008:61-67.(in Chinese with Englishabstract)
[8]MILLER-FLEMING L,OLIN-SANDOVAL V,CAMPBELL K,et al.Remaining mysteries of molecular biology:The role of polyamines in the cell.Journal of Molecular Biology,2015,427 (21):3389-3406.
[9]CALANDRA R S,RULLI S B,FRUNGIERI M B,et al.Polyamines in the male reproductive system.Acta physiologica, pharmacologica et therapeutica latinoamericana:Órgano de la Asociación Latinoamericana de Ciencias Fisiológicas y[de]la Asociación Latinoamericana de Farmacología,1996,46(4):209-222.
[10]MELENDREZ C S,RUTTLE J L,HALLFORD D M,et al.Polyamines in ejaculated ram spermatozoa and their relationship with sperm motility.Journal of Andrology,1992,13(4):293-296.
[11]KANG B,GUO J R,YANG H M,et al.Differential expression profiling of ovarian genes in prelaying and laying geese.Poultry Science,2009,88(9):1975-1983.
[12]NATARAJA S G,YU H N,PALMER S S.Discovery and development of small molecule allosteric modulators of glycoprotein hormone receptors.Frontiers in Endocrinology,2015, 6:142.
[13]MEHL N S,KHALID M,SRISUWATANASAGUL S,et al.GnRH-agonist implantation of prepubertal male cats affects their reproductive performance and testicular LH receptor and FSH receptor expression.Theriogenology,2016,85(5):841-848.
[14]LAU K M,TO K F.Importance of estrogenic signaling and its mediated receptors in prostate cancer.International Journal of Molecular Sciences,2016,17(9):1434.
[15]AYOUB M A,YVINEC R,JEGOT G,et al.Profiling of FSHR negative allosteric modulators on LH/CGR reveals biased antagonism with implications in steroidogenesis.Molecular and Cellular Endocrinology,2016,436:10-22.
[16]LÓPEZ-DOVAL S,SALGADO R,LAFUENTE A.The expression of several reproductive hormone receptors can be modified by perfluorooctane sulfonate(PFOS)in adult male rats.Chemosphere,2016,155:488-497.
[17]SAMARDZIJA D,POGRMIC-MAJKIC K,FA S,et al.Atrazine blocks ovulation via suppression ofLhrandCyp19a1mRNA and estradiol secretion in immature gonadotropin-treated rats.Reproductive Toxicology,2016,61:10-18.
[18]THYSSEN S M,HOCKL P F,CHAMSON A,et al.Effects of polyamines on the release of gonadotropin-releasing hormone and gonadotropins in developing female rats.Experimental Biology and Medicine,2002,227(4):276-281.
[19]LIU K C,GE W.Differential regulation of gonadotropin receptors (fshrandlhcgr)by epidermal growth factor(EGF)in the zebrafish ovary.General and Comparative Endocrinology,2013,181:288-294.
[20]BLACHOWSKI S,MOTYL T,GRZELKOWSKA K,et al.Involvement of polyamines in epidermal growth factor(EGF), transforming growth factor(TGF)-alpha and-beta 1 action on culture of L6 and fetal bovine myoblasts.International Journal of Biochemistry,1994,26(7):891-897.
[21]SAUNDERS P T K,MILLARMR,WILLIAMS K,etal.Differential expression of estrogen receptor-alpha and-beta and androgen receptor in the ovaries of marmosets and humans.Biology of Reproduction,2000,63(4):1098-1105.
[22]郑可佳,杨彩荣,张岩,等.ERβ及p-ERβ在小鼠发情周期卵巢内的表达.东北农业大学学报,2009,40(7):85-89. ZHENG K J,YANG C R,ZHANG Y,et al.Study on the expression of ERβandp-ERβon mouse ovary during the oestrous cycle.Journal of Northeast Agricultural University,2009,40(7): 85-89.(in Chinese with English abstract)
[23]WALTERS K A.Role of androgens in normal and pathological ovarian function.Reproduction,2015,149(4):193-218.
[24]KIMURA S,MATSUMOTO T,MATSUYAMA R,et al.Androgen receptor function in folliculogenesis and its clinical implication in premature ovarian failure.Trends in Endocrinology and Metabolism,2007,18(5):183-189.
[25]DU X,LI Q Q,PAN Z X,et al.Androgen receptor and miRNA-126*axis controls follicle-stimulating hormone receptor expression in porcine ovarian granulosa cells.Reproduction,2016, 152(2):161-169.
[26]ZHU T Y,YUAN J,XIE Y D,et al.Association of androgen receptor CAG repeat polymorphism and risk of epithelial ovarian cancer.Gene,2016,575(2):743-746.
Effect of exogenous spermidine on expression profile of reproductive hormone receptors in mouse ovaries.
LONG Shiyun,JIANG Dongmei,CHEN Ziyu,GUAN Cheng,YI Zhixin,KANG Bo*(Farm Animal Genetic Resources Exploration and Innovation Key Laboratory of Sichuan Province/College of Animal Science and Technology,Sichuan Agricultural University, Chengdu 611130,China)
spermidine;ovary;hormone receptor;gene expression
Q 492
A
10.3785/j.issn.1008-9209.2016.09.191
Journal of Zhejiang University(Agric.&Life Sci.),2017,43(2):247-252
国家自然科学基金(31201798);高等学校博士学科点专项科研基金(20105103120003)。
康波(http://orcid.org/0000-0003-2811-0642),E-mail:bokang@sicau.edu.cn
(First author):龙诗韵(http://orcid.org/0000-0002-5735-2731),E-mail:longsy210@163.com
2016-09-19;接受日期(Accepted):2016-12-06
猜你喜欢
杂志排行
浙江大学学报(农业与生命科学版)的其它文章
- Evaluation on formation rate of Pleurotus eryngii primordium under different humidity conditions by computer vision
- 流域非点源污染的最佳管理措施成本效益分析研究进展
- Association analysis revealed importance of dominance effects on days to silk of maize nested association mapping(NAM)population
- 检测乙酰微小杆菌的双重实时荧光定量聚合酶链式反应方法的建立
- 基于竞争性杂交方法的猪-肠道微生物特异性互作靶点发掘
- 应用分子标记辅助选育甘蓝型油菜杂交种的可行性
