石墨烯-层状双氢氧化物纳米复合材料在环境修复中的应用综述
2017-05-18林雅洁
林雅洁
(上海污染场地修复工程技术研究中心/上海环境卫生工程设计院有限公司;上海市环境工程设计科学研究院有限公司,上海 200232)
石墨烯-层状双氢氧化物纳米复合材料在环境修复中的应用综述
林雅洁
(上海污染场地修复工程技术研究中心/上海环境卫生工程设计院有限公司;上海市环境工程设计科学研究院有限公司,上海 200232)
选用石墨烯(G)及层状双氢氧化物(LDHs)两种新型高效纳米材料,合成结构多样、功能丰富的新型复合纳米材料L-G。结合二者各自优势,减少了各自的自聚积效应,提高分散性,对环境污染修复起协同促进作用。文章总结了目前研究中关于L-G复合材料对环境中温室气体CO2的捕获,以及染料及重金属的吸附去除进展,以期为复合材料的进一步研究开发提供参考。
石墨烯;层状双氢氧化物;重金属;环境修复
关于石墨烯(graphene)及层状双氢氧化物(LDHs,也称水滑石)的复合纳米材料的研究,最早的文献报道起于2011年[1-4],目前由SCI收录的相关文章约90篇。其中3篇综述[5-7],此外,研究内容包括以下三方面内容:①材料合成及结构研究[8-10];②纳米功能材料应用(超级电容器[11-13]、传感器[14-16]、催化材料[17-19]、析氧电催化[20-22],储能材料[23-25]、免疫分析检测[26-27]、阻燃剂[28-29]、锂电池[30-31]);③)环境修复(CO2吸收[32-33]、重金属及阴离子染料[34-39]吸附去除)。目前的研究尚少且新,主要集中在复合纳米材料的合成及其作为纳米功能材料在材料学中的应用,对于环境污染的修复研究应用较少,但石墨烯及LDHs都具有良好的吸附性能,能去除多种环境污染物,因此其复合物在环境修复中的应用仍具有较大的研究空间和应用前景。
1 石墨烯和水滑石
1.1 石墨烯
石墨烯[40],是指单层石墨层片,由sp2杂化的碳原子紧密排列而成蜂窝状晶体结构。晶格间以牢固的六边形状相连接,π键垂直于晶面,是石墨烯导电过程的重要载体。因此石墨烯可描述为由单层碳原子紧密堆积成的二维蜂窝状晶格结构,其间碳原子连接极其柔韧,结构稳定。石墨烯具有优异的光学、力学、热学等性能,导电性可控,化学稳定性强等,使其成为新兴的纳米材料,被广泛利用于复合材料领域中。
石墨烯及氧化石墨烯,因其高比表面积,片层二维结构等特性,具有很强的吸附能力,和循环利用能力,被广泛用于吸附环境中的污染重金属[41-46](如Pb、Cd、Hg、Cr、Ni、Co、As、Cu等)、染料[47-49](甲基蓝、甲基橙、番红、孔雀绿、结晶紫等)、油[50]、多环芳烃[51]、有机溶剂[50]等。可能的吸附机制包括石墨烯片层表面的含氧官能团和污染物间的络合作用[42,51,52,54]、离子交换作用[53,54]、静电吸附[54,55]、π键作用[51,55]、粒子内扩散作用[53,56]等。吸附过程受环境中pH、离子强度[57]等因素影响,一般为自发吸热反应。
1.2 LDHs
LDHs(Layered double hydroxides),层状双氢氧化物,由带正电荷的氢氧化物层及层间阴离子组成,是一类具有相同结构,不同理化性质的阴离子层状材料。因其板层金属离子可控性及层间的可塑性,在多领域被广泛应用。LDHs经剥离后为带正电的片层,具有分子水平的二维尺度,与不同物质重新组装后可构建不同的纳米功能材料,具有光学、电学、热稳定性等特征,近年来备受研究者们的亲睐[58-62]。
LDHs层板嵌含多种金属离子、含氧官能团,层间阴离子的灵活可变等特性,能通过静电作用、离子交换等方式,去除环境中的污染物[61-63]。其焙烧产物CLDHs,因层间阴离子及-OH的煅烧支除,具有很强的“记忆效应”,能通过吸收环境中的阴离子恢复其层状结构,因此可作为吸附剂及稳定剂,有效去除环境中的阴离子而被广泛应用于环境修复中[64-69]。Liang等人[70]总结了LDHs对金属阳离子的作用机制包括:表面沉淀作用(形成M(OH)2、MCO3)、表面络合作用(形成O-M-O)、同晶取代作用(取代层板上的Mg或Al)及层间阴离子的螯合作用(形成ML),可应用于解释LDHs对类金属As、Se等污染物的吸附作用机制,如图1所示。
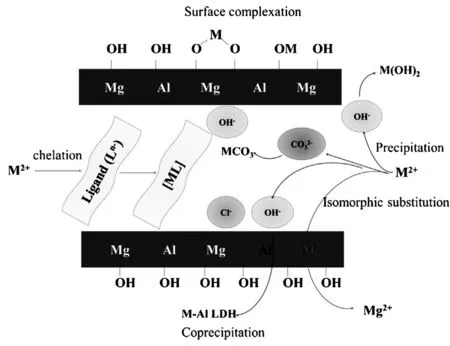
图1 LDHs对金属阳离子的吸附机制[70]
2 石墨烯-水滑石复合材料的合成
石墨烯-水滑石复合材料的合成方法有很多种[71-75],复合的形式也千变万化,研究者们通常根据实际需要,引入不同的官能团,改变组合方式,采用不同的合成方法,来获得目标的复合材料。本文主要介绍最常见的两种复合材料合成方法:自组装合成及以石墨烯为基底的合成方法。
2.1 自组装合成
自组装合成是以石墨烯及水滑石各自剥层为基础。其中,石墨烯可直接通过超声剥离。而LDHs由于层板的正电荷与层间阴离子间的静电作用太强,需要在层间引入大分子,扩大层间距,减弱阴离子与层板间的相互作用力,减弱其稳定性,才可剥离。通常用于LDHs剥离的溶剂包括甲酰胺、短链醇等。以甲酰胺为例,当LDHs置于甲酰胺溶液的环境中,大量的甲酰胺通过强氢键作用进入LDHs层空间,引起LDHs的膨胀,扩大
相邻金属层板间距,进而剥离分开。
剥离后的石墨烯和LDHs片层,分别带负电和正电,通过正负电荷相互吸引沉淀,形成石墨烯-水滑石复合材料,具体合成过程以CoAl-LDHs为例[71],如图2所示。

图2 (a)CoAl-LDH与GO复合材料的合成示意图;(b)实物图[71]
2.2 以石墨烯为基底合成
以石墨烯为基底合成L-G复合物,是以共沉淀法合成LDHs为基础的。一般选用共沉淀法中的尿素法,将尿素作为沉淀剂,加入到含有组成目标LDHs主体层板金属的盐溶液中,利用尿素受热分解得到碳酸氨,保持溶液碱性环境,从而提供LDHs合成所需的碱性环境及层间阴离子,再于一定条件下进行晶化。
L-G复合物合成时,选用剥离后的氧化石墨烯片层-尿素溶液作为阴离子溶液,加入到M2+及M3+的混合盐溶液中,通过搅拌、沉淀、陈化、润洗及干燥等步骤,合成相应的RGO-LDHs复合材料,如图3所示[73]。

图3 以石墨烯为基底合成L-G复合物流程示意图[73]
3 石墨烯-水滑石复合材料的表征
常用的表征方法,包括X射线粉末衍射(XRD)分析,用于表征复合材料的晶体结构,测量晶体参数;傅里叶红外光谱(FT-IR)分析,表征复合材料内的键结构,氢键的强弱及变化;采用热分析,判断复合材料的热稳定性,反应两种复合材料的结合情况;拉曼光谱(Raman)分析复合材料中石墨烯的特性;扫描及透射电子显微镜(SEM、TEM),表征复合材料的微观形态,膨胀水化程度。此外,还有电子顺磁光谱(ESR)、核磁共振(NMR)、X射线吸收近边结构光谱(XANES)等表征方法。
4 石墨烯-水滑石复合材料的环境应用
现有研究关于石墨烯-水滑石复合材料在环境中的应用,主要可归纳为三类[32-39]:1)用于环境中CO2的吸收,固碳贮存能量;2)用于阴离子染料的吸附去除;3)用于重金属离子的吸附去除。
4.1 用于CO2吸收,节能减排
L-G复合物用于CO2的吸附,主要基于LDHs对CO2特殊的强亲和力和吸附性,通过煅烧LDHs形成有效混合氧化物,对CO2进行催化吸收。虽然LDHs对CO2的吸附能力强,但是其吸附的稳定性及吸附能力还有待提高[32]。因此,选用质量轻、带电量充足的石墨烯纳米材料进行复合,以提高LDHs对CO2的吸附能力。研究结果表明,L-G复合物中石墨烯的含量仅需7%,其吸附能力与LDHs相比即有显著增加,吸附量增值高达60%[33]。
研究者们认为,此种吸附促进作用主要的原因包括两方面:其一,在L-G复合物中,LDHs起类似垫片作用,防止石墨烯片层的聚积;另一方面,对于LDHs,石墨烯的加入对LDHs起支撑作用,促进LDHs的扩散、分散,形成更具有活性的结构主体,因而提高了LDHs的吸附能力及循环吸附稳定性[33]。
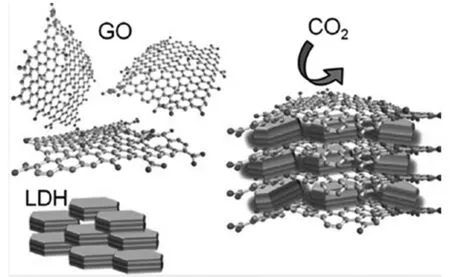
图4 L-G复合物吸收CO2示意图[33]
4.2 阴离子染料吸附
L-G复合物用于甲基蓝的去除,LDHs作为交联剂接入氧化石墨烯片层,通过电荷辅助氢键及晶格间的阳离子π键作用,形成3D网状结构,在溶液中具有很强的稳定性和较高的亲和力,克服了石墨烯易碎的特性[34]。L-G复合物减少了GO的自堆积,激活了更多的吸附位点,同时3D结构,易于分离和收集,提高了工作效率。
通过引入具有磁性的零价镍[35],用于甲基橙的吸附去除,石墨烯的加入,有效地阻止了LDHs的自积聚,同时带有磁性的镍提高了复合材料的回收循环利用性能。
4.3 重金属及其络合物的吸附去除
对重金属污染物的吸附去除研究较阴离子染料多,研究的内容包括复合物的合成方法、组成类型、环境因素(pH、温度)、接触时间、吸附剂浓度等因素对于L-G复合物吸附性能的影响,此外,还通过反应熵、焓的测定,研究吸附反应过程[36]。
研究结果表明,复合物中石墨烯的比例能影响复合物的吸附能力,石墨烯含量过高或过低,L-G复合物对As的吸附量都降低[37];吸附反应熵为负值,说明为自发吸附,温度升高,在一定程度上有利于吸附量的增加[36];环境pH值约为5.1时,吸附效率最高[36];在合成过程中加入尿素[38],能有效的减少氧化石墨烯的自聚积,促进 LDHs在石墨烯片层表面的原位成核,获得结构合理、分散性强的L-G复合物,进而提高吸附效率。
复合物对重金属的吸附机制,不同的研究者给出了不同的猜测,一般认为吸附过程以化学吸附为主,石墨烯表面吸附作用与煅烧后的LDHs的“记忆效应”间的协同作用共同促进吸附[38]。LDHs作为大孔径主体,为吸附过程提供了可靠的传输路径[39],石墨烯的加入大大提高了复合物的比表面积,暴露更多的吸附点位,极大促进吸附。
5 小 结
综上所述,通过不同方式合成不同形式的L-G复合物,是一种高效快速,稳定的吸附剂,可用于环境中多种污染物的修复。主要有以下几点优势:①利用两种材料的相互作用,减少两种材料各自的凝聚,增加接触面积;②对于带正电的污染物,利用LDHs对无机离子的亲和力,增加石墨烯对重金属的捕获和吸附量;③对于带负电的污染物,利用石墨烯对LDHs的分散,增加LDHs层空间,促进LDHs对络合物的吸附和结合。因此,研究L-G复合物在环境污染修复中推广应用,具有较大潜力。
[1]Chen D,Wang X,Liu T,et al.Electrically Conductive Poly(vinyl alcohol) Hybrid Films Containing Graphene and Layered Double Hydroxide Fabricated via Layer-by-Layer Self-Assembly[J].Acs Applied Materials & Interfaces,2010,2(7):2005-2011.
[2].Li M,Zhu J E,Zhang L,et al.Facile synthesis of NiAl-layered double hydroxide/graphene hybrid with enhanced electrochemical properties for detection of dopamine.[J].Nanoscale,2011,3(10):4240-6.
[3]Wu X L,Wang L,Chen C L,et al.Water-dispersible magnetite-graphene-LDH composites for efficient arsenate removal[J].Journal of Materials Chemistry,2011,21(43):17353-17359.
[4]Wang Y,Peng W,Liu L,et al.Enhanced conductivity of a glassy carbon electrode modified with a graphene-doped film of layered double hydroxides for selectively sensing of dopamine[J].Microchimica Acta,2011,174(1):41-46.
[5]Daud M,Kamal M S,Shehzad F,et al.Graphene/layered double hydroxides nanocomposites:A review of recent progress in synthesis and applications[J].Carbon,2016,104:241-252.
[6]Cao Y,Li G,Li X.Graphene/layered double hydroxide nanocomposite:Properties,synthesis,and applications[J].Chemical Engineering Journal,2016,292:207-223.
[7]Zhao M,Zhang Q,Huang J,et al.Hierarchical Nanocomposites Derived from Nanocarbons and Layered Double Hydroxides ‐ Properties,Synthesis,and Applications[J].Advanced Functional Materials,2012,22(4):675-694.
[8]Lonkar S P,Raquez J M,Dubois P.One-Pot Microwave-Assisted Synthesis of Graphene/Layered Double Hydroxide(LDH) Nanohybrids[J].Nano-Micro Letters,2015,7(4):332-340.
[9]Ma W,Wang L,Xue J,et al.Ultra-large scale synthesis of Co-Ni layered double hydroxides monolayer nanosheets by a solvent-free bottom-up strategy[J].Journal of Alloys & Compounds,2016,662:315-319.
[10]Chen S,Mao M,Liu X,et al.A high-rate cathode material hybridized by in-site grown Ni-Fe layered double hydroxide and carbon black nanoparticles[J].Journal of Materials Chemistry A,2016,4(13):4877-4881.
[11]Wu J,Liu W W,Wu Y X,et al.Three-dimensional hierarchical interwoven nitrogen-doped carbon nanotubes/Co x Ni 1-x -layered double hydroxides ultrathin nanosheets for high-performance supercapacitors[J].Electrochimica Acta,2016,203:21-29.
[12]Ma K Y,Cheng J P,Zhang J,et al.Dependence of Co/Fe ratios in Co-Fe layered double hydroxides on the structure and capacitive properties[J].Electrochimica Acta,2016,198:231-240.
[13]Wu X,Jiang L,Long C,et al.Energy Storage:Dual Support System Ensuring Porous Co-Al Hydroxide Nanosheets with Ultrahigh Rate Performance and High Energy Density for Supercapacitors(Adv.Funct.Mater.11/2015)[J].Advanced Functional Materials,2015,25(11):1648-1655.
[14]Wei Y,Chen S,Li F,et al.Highly Stable and Sensitive Paper-Based Bending Sensor Using Silver Nanowires/Layered Double Hydroxides Hybrids[J].Acs Applied Materials & Interfaces,2015,7(26).
[15]Wang Y,Wang Z,Rui Y,et al.Horseradish peroxidase immobilization on carbon nanodots/CoFe layered double hydroxides:direct electrochemistry and hydrogen peroxide sensing.[J].Biosensors & Bioelectronics,2015,64:57-62.
[16]A nonenzymatic reduced glutathione sensor based on Ni-Al LDHs/MWCNTs composites
[17]Zou Y,Wang X,Ai Y,et al.Coagulation Behavior of Graphene Oxide on Nanocrystallined Mg/Al Layered Double Hydroxides:Batch Experimental and Theoretical Calculation Study.[J].Environmental Science Technology,2016,50(7).
[18]Wang Y,Wang Z,Wu X,et al.Synergistic effect between strongly coupled CoAl layered double hydroxides and graphene for the electrocatalytic reduction of oxygen[J].Electrochimica Acta,2016,192:196-204.
[19]Carrasco J A,Romero J,Varela M,et al.Correction:Alkoxide-intercalated NiFe-layered double hydroxides magnetic nanosheets as efficient water oxidation electrocatalysts[J].Inorg.chem.front,2016,3(4):478-487.
[20]Qian L,Lu Z,Xu T,et al.Trinary Layered Double Hydroxides as High‐Performance Bifunctional Materials for Oxygen Electrocatalysis[J].Advanced Energy Materials,2015,5(13).
[21]Zhang G,Lin B,Yang W,et al.Highly efficient photocatalytic hydrogen generation by incorporating CdS into ZnCr-layered double hydroxide interlayer[J].Rsc Advances,2015,5(8):5823-5829.
[22]Huo R,Jiang W J,Xu S,et al.Co/CoO/CoFe2O4/G nanocomposites derived from layered double hydroxides towards mass production of efficient Pt-free electrocatalysts for oxygen reduction reaction.[J].Nanoscale,2013,6(1):203-6.
[23]Long X,Wang Z,Xiao S,et al.Transition metal based layered double hydroxides tailored for energy conversion and storage[J].Materials Today,2016,19(4):213-226.
[24]Ensafi A A,Jafari-Asl M,Nabiyan A,et al.Hydrogen storage in hybrid of layered double hydroxides/reduced graphene oxide using spillover mechanism[J].Energy,2016,99:103-114.[25]Du D,Yue W,Fan X,et al.Ultrathin NiO/NiFe2O4,Nanoplates Decorated Graphene Nanosheets with Enhanced Lithium Storage Properties[J].Electrochimica Acta,2016,194:17-25.
[26]Shen M,Zhang Z,Ding Y.Synthesizing NiAl-layered double hydroxide microspheres with hierarchical structure and electrochemical detection of hydroquinone and catechol[J].Microchemical Journal,2016,124:209-214.
[27]Li M,Zhu J E,Zhang L,et al.Facile synthesis of NiAl-layered double hydroxide/graphene hybrid with enhanced electrochemical properties for detection of dopamine.[J].Nanoscale,2011,3(10):4240-6.
[28]Liu Y J,Mao L,Fan S H.Preparation and study of intumescent flame retardant poly(butylene succinate) using MgAlZnFe-CO3layered double hydroxide as a synergistic agent[J].Journal of Applied Polymer Science,2014,131(17):8964-8973(10).
[29]Huang G,Chen S,Song P,et al.Combination effects of graphene and layered double hydroxides on intumescent flame-retardant poly(methyl methacrylate) nanocomposites[J].Applied Clay Science,2014,s 88-89(3):78-85.
[30]Du D,Yue W,Ren Y,et al.Fabrication of graphene-encapsulated CoO/CoFe2O4,composites derived from layered double hydroxides and their application as anode materials for lithium-ion batteries[J].Journal of Materials Science,2014,49(23):8031-8039.
[31]Latorre-Sanchez M,Atienzar P,Abellán G,et al.The synthesis of a hybrid graphene-nickel/manganese mixed oxide and its performance in lithium-ion batteries[J].Carbon,2012,50(2):518-525.
[32]Iruretagoyena D,Shaffer M S P,Chadwick D.Adsorption of carbon dioxide on graphene oxide supported layered double oxides[J].Adsorption,2014,20(2):321-330.
[33]Garciagallastegui A,Iruretagoyena D,Gouvea V,et al.Graphene Oxide as Support for Layered Double Hydroxides:Enhancing the CO2Adsorption Capacity[J].Chemistry of Materials,2013,24(23):4531-4539.
[34]Fang Q,Chen B.Self-assembly of graphene oxide aerogels by layered double hydroxides cross-linking and their application in water purification[J].Journal of Materials Chemistry,2014,2(23):8941-8951.
[35]Zhe Y,Ji S,Wei G,et al.Magnetic nanomaterial derived from graphene oxide/layered double hydroxide hybrid for efficient removal of methyl orange from aqueous solution[J].Journal of Colloid & Interface Science,2013,408(20):25-32.
[36]Nandi D,Basu T,Debnath S,et al.Mechanistic Insight for the Sorption of Cd(II) and Cu(II) from Aqueous Solution on Magnetic Mn-Doped Fe(III) Oxide Nanoparticle Implanted Graphene[J].Journal of Chemical & Engineering Data,2013,58(58):2809-2818.
[37]Wen T,Wu X,Tan X,et al.One-pot synthesis of water-swellable Mg-Al layered double hydroxides and graphene oxide nanocomposites for efficient removal of As(V) from aqueous solutions.[J].Acs Applied Materials & Interfaces,2013,5(8):3304-3311.
[38]Yuan X,Wang Y,Wang J,et al.Calcined graphene/MgAl-layered double hydroxides for enhanced Cr(VI) removal[J].Chemical Engineering Journal,2013,221(2):204-213.
[39]Wu X L,Wang L,Chen C L,et al.Water-dispersible magnetite-graphene-LDH composites for efficient arsenate removal[J].Journal of Materials Chemistry,2011,21(43):17353-17359.
[40]朱宏伟,徐志平,谢丹.石墨烯的结构、制备方法与性能表征[M].清华大学出版社,2011.
[41]Zhao G,Li J,Ren X,et al.Few-Layered Graphene Oxide Nanosheets As Superior Sorbents for Heavy Metal Ion Pollution Management[J].Environmental Science & Technology,2011,45(24):10454-62.
[42]Sitko R,Turek E,Zawisza B,et al.Adsorption of divalent metal ions from aqueous solutions using graphene oxide.[J].Dalton Transactions,2013,42(16):5682-9.[43]Dinda D,Gupta A,Saha S K.Removal of toxic Cr(VI) by UV-active functionalized graphene oxide for water purification[J].Journal of Materials Chemistry A,2013,1(37):11221-11228.
[44]Guo X,Du B,Qin W,et al.Synthesis of amino functionalized magnetic graphenes composite material and its application to remove Cr(VI),Pb(II),Hg(II),Cd(II) and Ni(II) from contaminated water[J].Journal of Hazardous Materials,2014,278:211-220.
[45]Hai T X,Jian H C,Xue S,et al.NH 2 -rich polymer/graphene oxide use as a novel adsorbent for removal of Cu(II) from aqueous solution[J].Chemical Engineering Journal,2015,263:280-289.
[46]Sitko R,Janik P,Zawisza B,et al.Green approach for ultratrace determination of divalent metal ions and arsenic species using total-reflection X-ray fluorescence spectrometry and mercapto-modified graphene oxide nanosheets as a novel adsorbent[J].Analytical Chemistry,2015,87(6):3535-42.
[47]Zhao F,Repo E,Yin D,et al.EDTA-Cross-Linked β-Cyclodextrin:An Environmentally Friendly Bifunctional Adsorbent for Simultaneous Adsorption of Metals and Cationic Dyes[J].Environmental Science & Technology,2015,49(17):10570-80.
[48]Shanshan,WANG,Yang,et al.β-Cyclodextrin functionalized graphene oxide:an efficient and recyclable adsorbent for the removal of dye pollutants[J].Frontiers of Chemical Science and Engineering,2015,9(1):77-83.
[49]Wang D,Liu L,Jiang X,et al.Adsorption and removal of malachite green from aqueous solution using magnetic β-cyclodextrin-graphene oxide nanocomposites as adsorbents[J].Colloids & Surfaces A Physicochemical & Engineering Aspects,2015,466(6):166-173.
[50]Xing L B,Hou S F,Zhou J,et al.Three dimensional nitrogen-doped graphene aerogels functionalized with melamine for multifunctional applications in supercapacitors and adsorption[J].Journal of Solid State Chemistry,2015,230:224-232.
[51]Shen Y,Chen B.Sulfonated graphene nanosheets as a superb adsorbent for various environmental pollutants in water.[J].Environmental Science & Technology,2015,49(12):7364-72.
[52]Moo J G,Khezri B,Webster R D,et al.Graphene oxides prepared by Hummers′,Hofmann′s,and Staudenmaier′s methods:dramatic influences on heavy-metal-ion adsorption.[J].ChemPhysChem,2014,15(14):2922-2929.
[53]Hur J,Shin J,Yoo J,et al.Competitive Adsorption of Metals onto Magnetic Graphene Oxide:Comparison with Other Carbonaceous Adsorbents[J].Scientific World Journal,2014,2015:1-11.
[54]Bian Y,Bian Z Y,Zhang J X,et al.Effect of the oxygen-containing functional group of graphene oxide on the aqueous cadmium ions removal[J].Applied Surface Science,2015,329:269-275.
[55]Liu J,Du H,Yuan S,et al.Alkaline deoxygenated graphene oxide as adsorbent for cadmium ions removal from aqueous solutions.[J].Water Science & Technology A Journal of the International Association on Water Pollution Research,2015,71(11):1611-9.
[56]Zhao F,Repo E,Sillanp M,et al.Green Synthesis of Magnetic EDTA- and/or DTPA-Cross-Linked Chitosan Adsorbents for Highly Efficient Removal of Metals[J].Industrial & Engineering Chemistry Research,2015,54(4):1271-1281.
[57]Hai T X,Jian H C,Xue S,et al.NH 2 -rich polymer/graphene oxide use as a novel adsorbent for removal of Cu(II) from aqueous solution[J].Chemical Engineering Journal,2015,263:280-289.
[58]Lin Y,Fang Q,Chen B.Perchlorate uptake and molecular mechanisms by magnesium/aluminum carbonate layered double hydroxides and the calcined layered double hydroxides[J].Chemical Engineering Journal,2014,237(2):38-46.
[59]Evans D G,Duan X.Preparation of layered double hydroxides and their applications as additives in polymers,as precursors to magnetic materials and in biology and medicine.[J].ChemInform,2006,37(15):485-96.
[60]Leroux F,Taviot-Guého C.Fine tuning between organic and inorganic host structure:New trends in layered double hydroxide hybrid assemblies[J].Journal of Materials Chemistry,2005,15(35-36):3628-3642.
[61]Goh K H,Lim T T,Dong Z.Application of layered double hydroxides for removal of oxyanions:a review.[J].Water Research,2008,42(6-7):1343-68.
[62]Ting Liu;Y,Kuang Wang,;M,T Y C,et al.Arsenate Sorption on Lithium/Aluminum Layered Double Hydroxide Intercalated by Chloride and on Gibbsite:Sorption Isotherms,Envelopes,and Spectroscopic Studies[J].Environmental Science & Technology,2007,40(24):7784-9.
[63]Newman S P,Jones W.Synthesis,characterization and application of layered double hydroxides containing organic guest[J].New Journal of Chemistry,1998,22(2):105-115.
[64]Williams G R,O′Hare D.Towards Understanding,Control and Application of Layered Double Hydroxide Chemistry[J].ChemInform,2006,37(45):3065-3074.
[65]Bish D L.Anion exchange in takovite:applications to other hydroxide minerals.[J].Bulletin De Mineralogie,1980,103(2):170-175.
[66]Wu X,Tan X,Yang S,et al.Coexistence of adsorption and coagulation processes of both arsenate and NOM from contaminated groundwater by nanocrystallined Mg/Al layered double hydroxides[J].Water Research,2013,47(12):4159-4168.
[67]Cheng X,Wang Y,Sun Z,et al.Pathways of phosphate uptake from aqueous solution by ZnAl layered double hydroxides.[J].Water Science & Technology A Journal of the International Association on Water Pollution Research,2013,67(8):1757-63.
[68]Chao Y F,Lee J J,Wang S L.Preferential adsorption of 2,4-dichlorophenoxyacetate from associated binary-solute aqueous systems by Mg/Al-NO3 layered double hydroxides with different nitrate orientations.[J].Journal of Hazardous Materials,2009,165(1-3):846-52.
[69]Morimoto K,Anraku S,Hoshino J,et al.Surface complexation reactions of inorganic anions on hydrotalcite-like compounds.[J].Journal of Colloid & Interface Science,2012,384(1):99-104.
[70]Liang X,Zang Y,Xu Y,et al.Sorption of metal cations on layered double hydroxides[J].Colloids & Surfaces A Physicochemical & Engineering Aspects,2013,433(35):122-131.
[71]Wang L,Wang D,Dong X Y,et al.Layered assembly of graphene oxide and Co-Al layered double hydroxide nanosheets as electrode materials for supercapacitors.[J].Chemical Communications,2011,47(12):3556-3558.
[72]Zhao M,Zhang Q,Huang J,et al.Hierarchical Nanocomposites Derived from Nanocarbons and Layered Double Hydroxides ‐ Properties,Synthesis,and Applications[J].Advanced Functional Materials,2012,22(4):675-694.
[73]Li H,Zhu G,Liu Z H,et al.Fabrication of a hybrid graphene/layered double hydroxide material[J].Carbon,2010,48(15):4391-4396.
[74]Wang Y,Li F,Dong S,et al.A facile approach for synthesizing Fe-based layered double hydroxides with high purity and its exfoliation.[J].Journal of Colloid & Interface Science,2016,467:28-34.
[75]Liang D,Yue W,Sun G,et al.Direct Synthesis of Unilamellar MgAl-LDH Nanosheets and Stacking in Aqueous Solution[J].Langmuir,2015.
Graphene/Layered Double Hydroxides Nanocomposites:A review of Recent Progress in Environmental Remediation
LIN Yajie
(Shanghai Engineering Research Center of Contaminated Sites Remediation/Shanghai Design Institute in EnvironmentalSanitary Engineering Co.,Ltd/ Shanghai Institute for Design and Research on Environmental Engineering Co.,Ltd,Shanghai 200232)
Applied grapheme and layered double hydroxides(LDHs),synthesis a diversified structure and functional nano-material. Making good use of their respective advantages,prevent the aggregation of LDHs and graphene,then received a synergistic contribution in environmental remediation. This paper summarized the current studies about L-G composite applied in carbon dioxides capture,dye and heavy metal adsorption removal,so that provide a reference for further study about the composite.
Graphene;LDHs;metal pollutants;environmental remediation
林雅洁,工程师、硕士,研究方向为污染场地修复技术研究
X21
A
1673-288X(2017)03-0095-05
项目资助:上海市国资委企业技术创新和能级提升项目(No. 2015016)
引用文献格式:林雅洁.石墨烯-层状双氢氧化物纳米复合材料在环境修复中的应用综述[J].环境与可持续发展,2017,42(3):95-99.
