戴云山自然保护区森林土壤氮转化特点研究①
2017-05-15李文周陈文伟张金波蔡祖聪
袁 磊,李文周,陈文伟,张金波,3,4,5*,蔡祖聪,3,4,5
(1 南京师范大学地理科学学院,南京 210023;2 戴云山国家级自然保护区管理局,福建德化 362500;3 江苏省地理环境演化国家重点实验室培育建设点,南京 210023;4 江苏省地理信息资源开发与利用协同创新中心,南京 210023;5 南京师范大学虚拟地理环境教育部重点实验室,南京 210023)
戴云山自然保护区森林土壤氮转化特点研究①
袁 磊1,李文周2,陈文伟2,张金波1,3,4,5*,蔡祖聪1,3,4,5
(1 南京师范大学地理科学学院,南京 210023;2 戴云山国家级自然保护区管理局,福建德化 362500;3 江苏省地理环境演化国家重点实验室培育建设点,南京 210023;4 江苏省地理信息资源开发与利用协同创新中心,南京 210023;5 南京师范大学虚拟地理环境教育部重点实验室,南京 210023)
利用15N稳定同位素成对标记法并结合MCMC数值模型,研究了戴云山国家级自然保护区天然毛竹林(BF)及其邻近黄山松–杉木林(NF)土壤氮素初级转化速率,以评估该地区森林生态系统土壤氮状态,并分析其保氮机制。结果表明:BF土壤-N的总产生速率(以N量计,13.16 μg/(g⋅d))是NF土壤的2倍(6.25 μg/(g⋅d)),其中黏土矿物对-N的解吸作用是BF产生-N的主要过程(55%),而NF主要以有机氮的矿化作用为主(56%)。BF土壤氮素初级矿化速率为 5.56 μg/(g⋅d),显著高于 NF的 3.40 μg/(g⋅d)。土壤氮素初级矿化速率与土壤全氮含量显著正相关( P<0.05),而与C/N比表现显著负相关(P<0.05)。BF与NF土壤-N总产生量的90% 均被土壤微生物的同化作用以及黏土矿物的吸附作用所消耗。两种土壤的硝化作用微弱,BF土壤总硝化速率(以N量计,0.23 μg/(g⋅d))与NF土壤(0.26 μg/(g⋅d))相差不大。两种林地土壤硝化作用均以有机氮的异养硝化为主,自养硝化过程可忽略不计。BF与NF土壤中-N消耗速率均超过了产生速率,表明BF与NF土壤均能有效降低-N的潜在淋失风险,其中BF土壤中-N的消耗以微生物的同化作用为主(58%),而NF土壤以-N异化还原为-N过程为主(68%)。戴云山国家级自然保护区两种亚热带森林土壤的氮转化过程均以-N转化为主,产生的绝大多数-N会迅速通过微生物对-N的同化作用以及黏土矿物对-N的吸附作用固持到有机氮库中;自养硝化过程微弱,使得无机氮主要以-N的形式保存于土壤中,同时酸性土壤环境有效削弱了-N的挥发损失。此外,相对较高的-N微生物同化速率以及异化还原为-N速率,进一步有效降低了-N的淋溶损失以及反硝化作用的气态损失风险,使该地区森林土壤能够在多雨的条件下有效保持氮素,满足植物的生长需求。
15N成对标记;MCMC数值优化模型;氮初级转化速率;保氮机制
氮素是植物生长所需的必要营养元素之一[1],同时也是控制生态系统物种组成及多样性、影响生态系统稳定性及其功能的关键因子[2],氮素的缺失将限制森林生态系统生产力[3]。地球表面的大部分地区,尤其是温带及北方森林,生态系统中的植被生长及微生物量的累积过程都是受氮素供应限制的[4]。
由于燃烧化石燃料、施用农业氮肥及种植豆科作物等人类活动的影响,大气中活性氮的沉降量日益增加[5]。短期、少量的氮沉降使生态系统中可利用氮素增加,有利于提高生态系统生产力及生物量的累积[4]。在受氮沉降影响较小的低污染地区,如瑞典中部及挪威等地,大气氮沉降绝大部分(超过95%)被植物林冠吸收,此时氮沉降作为一种养分来源而表现一定的施肥作用。研究表明,生态系统有限的保氮能力伴随着持续增加的大气氮沉降量将达到氮饱和状态[6–7]。此时,氮沉降的施肥作用逐渐消失、氮素的气态或淋溶损失风险增加,氮沉降可能造成空气质量下降[8]、水体酸化和富营养化[9]以及森林衰退[10]等危害,对生态系统的结构和功能造成严重影响。一般认为土壤保氮机制主要包括:①较低的有机氮矿化速率与-N自养硝化速率[11];②较高自养硝化速率结合较高的-N微生物同化速率[12];③较高的-N异化还原为-N作用[13–14]等。这些氮转化特点均能有效地将氮素固持于生态系统内,降低系统氮素的损失风险。因此,明确土壤氮转化的主要特点是评价土壤氮素保持能力的关键之一。土壤氮转化表征的是一种形态氮素向另一种形态氮素的转变过程[15]。土壤氮素的转化速率包括净转换速率和初级转化速率。净转化速率是指单位时间内某种特定形态氮素含量的变化速率,表征的是氮素的含量变化,不能指示该形态氮素含量变化的相关具体氮转化过程的实际转化速率。而初级转化速率是指单位时间内一种形态的氮素转变为另一种氮素形态的变化速率[16],是确定土壤氮素各转化过程真实速率的最佳指标。研究土壤氮素初级转化速率的基本方法是15N同位素稀释法[17]。随着分析技术的发展,15N同位素稀释法测定土壤氮素初级转化速率的原理与计算方法日趋完善。Müller等[18]在总结前人研究成果的基础上,建立了15N稳定同位素成对标记结合MCMC(Markov chain Monte Carlo)优化数值模型(图1),其能同时计算10个氮素转化过程的总转化速率,这是目前描述氮转化过程较为详细的15N概念模型之一。
戴云山国家级自然保护区属于典型的亚热带海洋性季风气候,夏季高温多雨。不同于温带森林生态系统受氮限制,一般认为亚热带森林土壤氮是相对富集、能够满足植物生长的需求。在温暖湿润且伴随强烈风化的环境条件下,亚热带森林土壤氮的潜在淋失风险较高,为什么氮素会表现出相对富集的状态?研究该地区土壤氮转化特点,明确其相关保氮机制,对于该地区活性氮的调控具有积极意义。毛竹林作为戴云山自然保护区主要植被群系之一,是福建省最主要的竹林(全省种植面积55.6万hm2),同时黄山松也是该地区代表性的植被。因此,本研究采集戴云山自然保护区内天然毛竹林土壤及其邻近黄山松-杉木林土壤样品,利用15N成对标记法并结合数值MCMC模型,测定土壤氮素初级转化速率,以期从土壤氮转化过程角度明确土壤保氮机制,研究结果对于更加准确地预测全球变化,如气候变化、氮沉降等条件下土壤氮动态具有一定的科学意义。
1 材料与方法
1.1 材料
戴云山国家级自然保护区位于福建省泉州市德化县内,属于典型的亚热带海洋性季风气候区。根据保护区内九仙山气象站资料记录,保护区年平均降雨量1 700 ~ 2 000 mm,80% 左右集中在3—9月;年平均气温15.6 ~ 19.5℃,其中最冷月1月均温6.5 ~10.5℃,最热月7月均温23 ~ 27.5℃;雾日年平均达220 d。
本研究土壤样品采自保护区内西溪村一片天然毛竹林及其临近的黄山松-杉木林,地理位置为118°05′ ~118°09′ E,25°38′ ~ 25°41′ N。毛竹林(BF)为纯林,林内其他乔木、灌木较少;黄山松-杉木(NF)林下植被以铁芒萁为主,地表枯枝落叶层约5 ~ 10 cm。在选定的样地内,随机划分出6个1 m × 1 m 的小区,每个小区距离50 m以上。每个小区内采用“S”型6点混合采样方法采集0 ~ 15 cm表层土壤样品,每个林型采集6个土壤样品。土壤样品带回实验室,挑出碎石及细根,过2 mm筛,储存于4℃冰柜,用于相关理化性质分析和培养实验。
1.2 方法
1.2.1 土壤理化性质测定 土壤样品相关理化性质的测定参照《土壤农业化学分析方法》[19]。土壤pH采用DMP-2 mV/pH计(Quark Ltd, Nanjing, China)测定;土壤有机碳和全氮分别采用重铬酸钾容量法以及半微量开氏法测量;土壤无机氮(-N和-N)含量由流动分析仪(Skalar, Breda, Netherlands)测定。图2为土壤样品具体的相关理化指标。
1.2.215N成对标记实验 分别称取相当于20 g干土重的鲜土土壤样品于250 ml锥形瓶中,每个土壤24瓶,在25℃恒温培养箱中预培养24 h。每个土壤样品分别设置15NH4NO3和NO3两个标记处理,每个处理12瓶。用移液管向预培养24 h后土壤样品中逐滴加入2 ml15NH4NO3(10.23% atom)或NO3(10.12% atom)标记液,使其均匀分布于土壤表面,-N和-N的加入量均为N 20 μg/g干土。加入蒸馏水调节土壤含水量至60%WHC,用保鲜膜封住锥形瓶口,将土样放入 25℃恒温培养箱中培养。在加入标记液后的0.5 h、2 d、4 d和6 d,每个标记处理随机取3个重复,按水土比为5∶1比例加入100 ml 2 mol/L KCl溶液浸提无机氮,在25℃ 250 r/min摇床振荡60 min,定性滤纸过滤。KCl浸提液中无机氮含量由氧化镁-代氏合金蒸馏法测定[20],具体操作为:先在 KCl浸提液中加氧化镁,蒸馏测定无机氮中-N;再加入代氏合金,将NO3–-N还原成-N,继续蒸馏测定-N。馏出液经过硼酸和混合指示剂(甲基红+溴甲酚绿)吸收液吸收,再由 0.02 mol/L H2SO4标准液滴定测定-N 和-N浓度。在蒸馏前,要先测定定氮仪-N 和-N的回收率,即用已知浓度的标准液(-N含量 1 g/L和-N含量 1 g/L)按上述操作蒸馏,结果表明-N和-N回收率分别为99% 和95%。经过H2SO4标准液滴定后的馏出液置于80℃烘箱中浓缩,烘干,由同位素质谱仪(IRMS 20-22, SerCon)测定-N和-N的15N丰度。
1.2.3 MCMC数值模型 Müller等[18]建立了马尔科夫链蒙特卡洛随机采样方法(Markov chain Monte Carlo, MCMC),该模型(图1)区分了5个氮库,其中将土壤有机氮库分为易矿化和难矿化氮库;能同时计算10个氮素转化过程的总转化速率,包括了难分解有机氮和易分解有机氮的矿化作用(MNrec和 MNlab)、-N被微生物固持于难分解有机氮库和易分解有机氮库(INH4_Nrec和INH4_Nlab)、黏土矿物对NH4+-N的吸附与解吸作用(ANH4和RNH4ads)-N自养硝化作用(ONH4)、有机氮异养硝化作用(ONrec)、-N的异化还原为-N (DNO3)以及微生物对-N的同化作用(INO3)。这是目前描述氮转化过程较为详细的15N概念模型之一。关于MCMC数值模型介绍、参数设置及具体操作在Müller等[18]、Rütting等[14]以及Zhang等[21]中有详细说明。

图115N示踪模型Fig. 115N tracing model
1.3 数据处理
采用 T检验来比较两类土壤样品基本理化性质以及土壤氮转化各过程的初级转化速率之间的差异,当P<0.05时,说明被检测的因素之间存在显著差异。利用Pearson相关系数对氮转化过程与土壤理化性质之间以及各氮转化过程之间的相关性进行统计分析,对于相关性显著的因子通过建立回归方程来分析其相互关系。所有统计分析在SPSS19.0软件上完成,图形制作使用Origin Pro 9.0软件。
2 结果分析
2.1 土壤理化性质
两种林地土壤样品的pH在4.31 ~ 4.80,其中毛竹林(BF)土壤样品pH均值为4.66(图2),显著高于黄山松–杉木林(NF)土壤 (pH 4.45) (P<0.01)。BF和NF土壤有机碳平均含量分别为53.19 g/kg和54.44 g/kg,全氮平均含量分别为3.71 g/kg和3.26 g/kg,但两种林地间差异均不显著。BF土壤C/N比为4.32,明显低于NF土壤(16.82)(P<0.01)。两种林地土壤中-N占无机氮总量比例均大于0.5,表明无机氮组成形式均以-N为主,两种土壤-N含量差别不大(BF为2.74 mg/kg,NF为2.64 mg/kg)。BF土壤-N含量(1.76 mg/kg)显著高于NF (0.43 mg/kg) (P<0.001)。

图2 BF与NF表层土壤理化性质Fig. 2 Physical and chemical properties in studied forest soils
2.2 土壤氮素初级转化速率
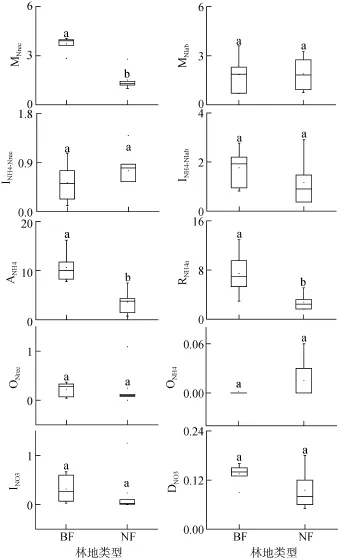
图3 BF与NF土壤氮素初级转化速率(μg/(g⋅d))Fig. 3 Gross N transformation rates in studied forest soils estimated by15N tracing model
INO3与DNO3作用是-N的两种主要利用途径。BF与NF土壤总-N的消耗速率(INO3+DNO3)分别为0.46 μg/(g⋅d)和0.32 μg/(g⋅d),但差异不显著。在BF与NF土壤中,-N消耗速率均超过了-N的总产生速率,其中INO3占BF土壤-N总消耗量的58%,而NF土壤以DNO3作用为主(68%)。BF与NF土壤INO3分别为0.32 μg/(g⋅d)和0.23 μg/(g⋅d),同时DNO3分别对应0.14 μg/(g⋅d)和0.09 μg/(g⋅d),但差异均不明显。BF与NF两种林地土壤,ONH4、ONre、INO3以及DNO3过程均与土壤理化性质如pH、有机碳、全氮、C/N比等无明显相关性。

图4 土壤氮素矿化速率(μg/(g⋅d))与土壤理化性质的相关性Fig. 4 Correlation between N mineralization rates and soil physical and chemical properties
3 讨论
3.1 NH4+-N动态
一般认为,森林土壤氮素总初级矿化速率低于10 μg/(g⋅d)[22]。BF土壤氮素初级矿化速率在4.33 ~7.59 μg/(g⋅d),其平均矿化速率为5.56 μg/(g⋅d),这与亚马逊东部原始低地森林砂质土的季节性平均矿化速率(6.0 μg/(g⋅d))[23]以及印度尼西亚受氮素限制的山地森林土壤矿化速率(>5.0 μg/(g⋅d))[24]相近。NF土壤氮素初级矿化速率在2.37 ~ 4.55 μg/(g⋅d),其平均矿化速率(3.40 μg/(g⋅d))与江西的次生常绿阔叶林(3.70 μg/(g⋅d))[21]以及 Zhang等[25]研究中国东部福建(2.72 μg/(g⋅d))以及广东(2.64 μg/(g⋅d))森林土壤相近。然而BF与NF两种林地土壤氮素总初级矿化速率,均低于亚马逊东部原始低地森林黏土的季节性平均矿化速率(13.5 μg/(g⋅d))[23]以及智利南部的原始森林土壤(14.7 μg/(g⋅d))[13]。
BF土壤氮素平均初级矿化速率显著高于NF土壤。土壤氮素初级矿化速率主要受土壤有机氮含量与质量(C/N)的影响[26]。土壤氮素初级矿化速率随土壤全氮含量的增加而增加,体现了基质在无机氮产生过程中的重要性。初级矿化速率与土壤 C/N比显著负相关,这与很多研究结果相一致[15,27],较低的 C/N比意味着土壤有机质可利用性较高[28],这也体现了基质质量在矿化过程中的重要性。BF与NF土壤都来自气候条件、土壤类型等相似的邻近区域,造成BF与 NF土壤氮素初级矿化速率不同的关键因素在于不同典型植被下土壤性质的差异。许多研究发现,植被种类的差异以及不同植被下凋落物质量的差异是控制森林土壤氮素矿化作用的重要因素[29]。植被类型的差异直接影响由植物残体及根系分泌物产生而来的土壤有机质的质量及其数量,进而导致土壤微生物群落的改变,而对土壤氮转化过程产生一定的影响[30]。此外,MNrec与土壤 pH显著正相关,可能是pH升高促使土壤有机质的可溶性增加,提高了土壤碳、氮的可利用性[31]。
已有的研究发现,硝化作用对土壤pH的变化很敏感[36],并且pH是控制ONH4和ONrec过程的重要因素之一[25]。在pH<5的土壤中,微生物将-N氧化为-N的过程微弱[37]。本研究中,BF与NF土壤pH在4.31 ~ 4.80,两种林地土壤总-N产生的90% 均来自 ONrec,ONH4相对 ONrec可忽略不计,这也证实了“即使-N含量充足时,自养硝化作用在一些酸性森林土壤中意义不大”的观点[38]。自养硝化作用受抑制,减少生态系统中氮素损失,可作为一种有效的保氮机制[11]。一般认为,异养硝化作用是由真菌起主导作用,并且广泛存在于拥有高度稳定微生物群落的原始森林生态系统中[39]。BF与NF土壤的硝化作用均以异养硝化为主,这与众多研究结果一致[34–35],表明异养硝化在土壤有机碳含量较高、pH较低的酸性森林土壤中有重要作用,是-N的主要产生途径。
BF与 NF土壤 DNO3作用的变化范围为 0.05 ~0.18 μg/(g⋅d),这与德国的温带草地土壤结果相近(0.090 μg/(g⋅d))[43],但低于热带高地森林土壤(0.23 ~0.6 μg/(g⋅d))[44]、亚马逊东部的原始低地森林(0.3 ~0.8 μg/(g⋅d))[23]以及智利南部的原始森林(0.45 μg/(g⋅d))[13]。DNO3是NF土壤N的一种重要的消耗方式。已有的研究结果表明,在土壤有机质含量较高且降雨丰富的(亚)热带湿润土壤中 DNO3广泛存在[45]。DNO3将N转化为更易被植物、微生物吸收利用且以不易移动的N形式,避免了更多氮素的淋溶或者气态损失,有效地将氮素保存于生态系统中[46]。Behrendt等[47]发现一些细菌能够同时进行 ONrec与DNO3,ONrec与DNO3的结合相对于矿化作用,能够提供另一种将土壤有机氮转化为N的途径。随着大气 CO2浓度的持续增加,可能加大植被对土壤氮素的需求,甚至造成缺氮的状况;而有效的保氮机制,如N微生物同化和DNO3作用,对于维持生态系统的生产力将尤为重要[48]。
4 结论
[1] Binkley D, Hart S C. The components of nitrogen availability assessments in forest soils[M]//Advances in soil science. New York: Springer, 1989: 57–112
[2] Bobbink R, Roelofs J G M. Nitrogen critical loads for natural and semi-natural ecosystems: The empirical approach[J]. Water, Air, and Soil Pollution, 1995, 85(4): 2413–2418
[3] Reich P B, Grigal D F, Aber J D, et al. Nitrogen mineralization and productivity in 50 hardwood and conifer stands on diverse soils[J]. Ecology, 1997, 78(2): 335–347
[4] Vitousek P M, Howarth R W. Nitrogen limitation on land and in the sea: how can it occur?[J]. Biogeochemistry, 1991, 13(2): 87–115
[5] Galloway J N, Hiram Levy I I, Kasibhatla P S. Year 2020: Consequences of population growth and development on deposition of oxidized nitrogen[J]. Ambio, 1994, 23(2): 120–123
[6] Aber J D. Nitrogen cycling and nitrogen saturation in temperate forest ecosystems[J]. Trends in Ecology & Evolution, 1992, 7(7): 220–224
[7] Aber J D, Nadelhoffer K J, Steudler P, et al. Nitrogen saturation in northern forest ecosystems[J]. BioScience, 1989, 39(6): 378–286
[8] Crutzen P J, Ehhalt D H. Effects of nitrogen fertilizers and combustion on the stratospheric ozone layer[J]. Ambio, 1977, 6(2/3): 112–117
[9] Boxman A W, van Dam D, van Dijk H F G, et al. Ecosystem responses to reduced nitrogen and sulphur inputs into two coniferous forest stands in the Netherlands[J]. Forest Ecology and Management, 1995, 71(1): 7–29
[10] Schulze E D. Air pollution and forest decline in a spruce (Picea abies) forest[J]. Science, 1989, 244(4906): 776–783
[11] Vitousek P M, Gosz J R, Grier C C, et al. Nitrate losses from disturbed ecosystems[J]. Science, 1979, 204(4392): 469–474
[12] Stark J M, Hart S C. High rates of nitrification and nitrate turnover in undisturbed coniferous forests[J]. Nature, 1997, 385(6611): 61–64
[13] Huygens D, Rütting T, Boeckx P, et al. Soil nitrogen conservation mechanisms in a pristine south Chilean Nothofagus forest ecosystem[J]. Soil Biology and Biochemistry, 2007, 39(10): 2448–2458
[14] Rütting T, Huygens D, Müller C, et al. Functional role of DNRA and nitrite reduction in a pristine south Chilean Nothofagus forest[J]. Biogeochemistry, 2008, 90(3): 243–258
[15] Hart S C, Nason G E, Myrold D D, et al. Dynamics of gross nitrogen transformations in an old-growth forest: The carbon connection[J]. Ecology, 1994, 75(4): 880–891
[16] Di H J, Cameron K C, McLaren R G. Isotopic dilution methods to determine the gross transformation rates of nitrogen, phosphorus, and sulfur in soil: A review of thetheory, methodologies, and limitations[J]. Soil Research, 2000, 38(1): 213–230
[17] Stark J M. Nutrient transformations[M]//Sala O E, Jackson R B, Mooney H A, et al. Methods in ecosystem science. New York: Springer, 2000: 215–234
[18] Müller C, Rütting T, Kattge J, et al. Estimation of parameters in complex15N tracing models by Monte Carlo sampling[J]. Soil Biology and Biochemistry, 2007, 39(3): 715–726
[19] 鲁如坤. 土壤农业化学分析方法[M]. 中国农业科技出版社, 2000
[20] Zhang J, Cai Z, Cheng Y, et al. Denitrification and total nitrogen gas production from forest soils of Eastern China[J]. Soil Biology and Biochemistry, 2009, 41(12): 2551–2557
[21] Zhang J, Müller C, Zhu T B, et al. Heterotrophic nitrification is the predominantproduction mechanism in coniferous but not broad-leaf acid forest soil in subtropical China[J]. Biology and Fertility of Soils, 2011, 47(5): 533–542
[22] Booth M S, Stark J M, Rastetter E. Controls on nitrogen cycling in terrestrial ecosystems: A synthetic analysis of literature data[J]. Ecological monographs, 2005, 75(2): 139–157
[23] Sotta E D, Corre M D, Veldkamp E. Differing N status and N retention processes of soils under old-growth lowland forest in Eastern Amazonia, Caxiuanã, Brazil[J]. Soil Biology and Biochemistry, 2008, 40(3): 740–750
[24] Corre M D, Dechert G, Veldkamp E. Soil nitrogen cycling following montane forest conversion in central Sulawesi, Indonesia[J]. Soil Science Society of America Journal, 2006, 70(2): 359–366
[25] Zhang J, Zhu T B, Cai Z C, et al. Nitrogen cycling in forest soils across climate gradients in Eastern China[J]. Plant and Soil, 2011b, 342(1/2): 419–432
[26] Ste-Marie C, Houle D. Forest floor gross and net nitrogen mineralization in three forest types in Quebec, Canada[J]. Soil Biology and Biochemistry, 2006, 38(8): 2135–2143
[27] Mack M C, D'Antonio C M. Exotic grasses alter controls over soil nitrogen dynamics in a Hawaii an woodland[J]. Ecological Applications, 2003, 13(1): 154–166
[28] Attiwill P M, Adams M A. Nutrient cycling in forests[J]. New Phytologist, 1993, 124(4): 561–582
[29] Lovett G M, Rueth H. Potential nitrogen mineralization and nitrification in American beech and sugar maple stands along a nitrogen deposition gradient in the northeastern US[J]. Ecological Applications, 1999, 9(1330): 44
[30] Patra A K, Abbadie L, Clays-Josserand A, et al. Effects of management regime and plant species on the enzyme activity and genetic structure of N-fixing, denitrifying and nitrifying bacterial communities in grassland soils[J]. Environmental Microbiology, 2006, 8(6): 1005–1016
[31] Curtin D, Campbell C A, Jalil A. Effects of acidity on mineralization: pH-dependence of organic matter mineralization in weakly acidic soils[J]. Soil Biology and Biochemistry, 1998, 30(1): 57–64
[32] Brady N C, Weil R R. The nature and properties of soils[M]. Prentice-Hall Inc., 1996
[33] Zhang J B, Zhu T B, Cai Z C, et al. Effects of long-term repeated mineral and organic fertilizer applications on soil nitrogen transformations[J]. European Journal of Soil Science, 2012, 63(1): 75–85
[34] Hall S J, Matson P A. Nutrient status of tropical rain forests influences soil N dynamics after N additions[J]. Ecological Monographs, 2003, 73(1): 107–129
[35] Corre M D, Brumme R, Veldkamp E, et al. Changes in nitrogen cycling and retention processes in soils under spruce forests along a nitrogen enrichment gradient in Germany[J]. Global Change Biology, 2007, 13(7): 1509–1527
[36] De Boer W, Kowalchuk G A. Nitrification in acid soils: Micro-organisms and mechanisms[J]. Soil Biology and Biochemistry, 2001, 33(7): 853–866
[37] Weber D F, Gainey P L. Relative sensitivity of nitrifying organisms to hydrogen ions in soils and in solution[J]. Soil Science, 1962, 94(3): 138–145
[38] Schimel J P, Firestone M K, Killham K S. Identification of heterotrophic nitrification in a Sierran forest soil[J]. Applied and Environmental Microbiology, 1984, 48(4): 802–806
[39] Wood P M. Autotrophic and heterotrophic mechanisms for ammonia oxidation[J]. Soil Use and Management, 1990, 6(2): 78–79
[40] Recous S, Machet J M, Mary B. The partitioning of fertilizer-N between soil and crop: Comparison of ammonium and nitrate applications[J]. Plant and Soil, 1992, 144(1): 101–111
[41] Recous S, Mary B, Faurie G. Microbial immobilization of ammonium and nitrate in cultivated soils[J]. Soil Biology and Biochemistry, 1990, 22(7): 913–922
[42] Magill A H, Aber J D, Hendricks J J, et al. Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition[J]. Ecological applications, 1997, 7(2): 402–415
[43] Rütting T, Müller C. Process-specific analysis of nitrite dynamics in a permanent grassland soil by using a Monte Carlo sampling technique[J]. European Journal of Soil Science, 2008, 59(2): 208–215
[44] Silver W L, Thompson A W, Reich A, et al. Nitrogen cycling in tropical plantation forests: potential controls on nitrogen retention[J]. Ecological Applications, 2005, 15(5): 1604–1614
[45] Templer P H, Silver W L, Pett-Ridge J, et al. Plant and microbial controls on nitrogen retention and loss in a humid tropical forest[J]. Ecology, 2008, 89(11): 3030–3040
[46] Tiedje J M. Ecology of denitrification and dissimilatory nitrate reduction to ammonium[J]. Biology of Anaerobic Microorganisms, 1988, 717: 179–244
[47] Behrendt U, Schumann P, Stieglmeier M, et al. Characterization of heterotrophic nitrifying bacteria with respiratory ammonification and denitrification activity-Description of Paenibacillus uliginis sp. nov., an inhabitant of fen peat soil and Paenibacillus purispatii sp. nov.,isolated from a spacecraft assembly clean room[J]. Systematic and Applied Microbiology, 2010, 33(6): 328–336
[48] Hungate B A, Dukes J S, Shaw M R, et al. Nitrogen and climate change[J]. Science, 2003, 302(5650): 1512–1513
Nitrogen Transformation of Different Subtropical Forest Soils in Daiyun Mountain National Nature Reserve
YUAN Lei1, LI Wenzhou2, CHEN Wenwei2, ZHANG Jinbo1,3,4,5*, CAI Zucong1,3,4,5
(1 School of Geography Science, Nanjing Normal University, Nanjing 210023, China; 2 Daiyun Mountain National Nature Reserve, Dehua, Fujian 362500, China; 3 State Key Laboratory Cultivation Base of Geographical Environment Evolution, Nanjing 210023, China; 4 Jiangsu Center for Collaborative Innovation in Geographical Information Resource Development and Application, Nanjing 210023, China; 5 Key Laboratory of Virtual Geographic Environment (Nanjing Normal University), Ministry of Education, Nanjing 210023, China)
A15N tracing study was carried out to identify the potential gross nitrogen transformations of natural Moso Bamboo forest (BF) soil and adjacent native forest of Huangshan pine (NF) soil in Daiyun Mountain National Nature Reserve of Fujian Province. The results showed that total-N production (N13.16 μg/(g⋅d)) was twice higher in BF soil than that in NF soil (6.25 μg/(g⋅d)) soil with amounted to 55% of totalN production came from release of adsorbedin BF soil while equal to 56% in NF soil was mineralization of soil labile and recalcitrant organic matter. Gross mineralization rate was significantly faster in BF soil (5.56 μg/(g⋅d)) compared to NF soil (3.40 μg/(g⋅d)) and gross mineralization rate was found positive correlated with TN and negative correlated with C/N ratio. Approximately 90% of totalN production was consumed by immobilization ofand adsorption ofon cation exchange sites in the two soils. TotalN production in BF soil (0.23 μg/(g⋅d)) was almost the same with NF soil (0.26 μg/(g⋅d)), of which approximately 90% were came from heterotrophic nitrification and oxidation ofwere negligible compared to ONrecin the two soils. TotalN consumption exceeded totalN production in both soils, which may reduce the risk of potential for N losses. INO3amounted to 58% of total-N consumption in BF soil while DNO3responsible for 68% of total consumption ofin NF soil. The N transformation processes in the two soils were dominant by-N dynamics and most of totalN production was immediately counterbalanced byimmobilization and adsorption ofon cation exchange sites. Soil inorganic nitrogen was mainly in form of-N as oxidation of-N was insignificant in combination with acidic soil environment inhibited ammonium volatilization. Moreover, higher INO3and DNO3can significantly reduce potential-N leaching or gaseous losses under this high temperature and rainfall condition and retain abundant available N in soil to maintain plant growth.
15N tracing; MCMC; Gross nitrogen transformation; Nitrogen retention mechanism
S158.3
A
10.13758/j.cnki.tr.2017.02.005
国家重大科学研究计划项目(2014CB953803)与江苏高校优势学科建设工程项目资助。
* 通讯作者(zhangjinbo@njnu.edu.cn)
袁磊(1990—),男,湖北黄冈人,硕士研究生,主要从事氮循环及其环境效应方面的研究。E-mail: 13776641831@163.com
