盐酸小檗碱对颅脑创伤模型小鼠双侧丘脑继发性损伤的神经保护作用
2017-05-15黄树宣朱飞奇裴中邓旭辉杨志朱瑾陈淳淳林伟丰
黄树宣 朱飞奇 裴中邓 旭辉杨 志朱瑾 陈淳淳 林伟丰
·基础研究·
盐酸小檗碱对颅脑创伤模型小鼠双侧丘脑继发性损伤的神经保护作用
黄树宣 朱飞奇 裴中邓 旭辉杨 志朱瑾 陈淳淳 林伟丰
目的探讨盐酸小檗碱对颅脑创伤(TBI)模型小鼠双侧丘脑继发性损伤(炎症反应、氧化损伤和神经元缺失)的神经保护作用。方法采用自由落体撞击法制备颅脑创伤模型,盐酸小檗碱组小鼠予以盐酸小檗碱50 mg/(kg·d)灌胃21 d,TBI组予等量生理盐水灌胃21 d,对照组不予自由落体撞击。免疫组织化学染色计数双侧丘脑诱导型一氧化氮合酶(iNOS)、环氧合酶⁃2(COX⁃2)、8⁃羟基脱氧鸟苷(8⁃OHdG)和神经元核抗原(Neu N)阳性神经元或胶质细胞数目,免疫荧光染色计数双侧丘脑胶质纤维酸性蛋白(GFAP)阳性星形胶质细胞和离子钙结合蛋白1(Iba1)阳性小胶质细胞数目。结果3组小鼠颅脑创伤同侧丘脑iNOS(P=0.015)、COX⁃2(P=0.022)、8⁃OHdG(P=0.000)和Neu N(P=0.000)阳性神经元或胶质细胞数目以及GFAP阳性星形胶质细胞数目(P=0.024)和Iba1阳性小胶质细胞数目(P= 0.000)差异均有统计学意义,其中,TBI组iNOS(P=0.005)、COX⁃2(P=0.011)和8⁃OHdG(P=0.000)阳性神经元或胶质细胞数目以及GFAP阳性星形胶质细胞数目(P=0.011)和Iba1阳性小胶质细胞数目(P= 0.000)均高于对照组,而Neu N阳性神经元数目低于对照组(P=0.000);盐酸小檗碱组iNOS(P=0.031)、COX⁃2(P=0.024)和8⁃OHd G(P=0.008)阳性神经元或胶质细胞数目以及GFAP阳性星形胶质细胞数目(P=0.031)和Iba1阳性小胶质细胞数目(P=0.012)均低于TBI组,仅8⁃OHdG阳性神经元数目(P= 0.014)和Iba1阳性小胶质细胞数目(P=0.024)仍高于对照组,而Neu N阳性神经元数目高于TBI组(P= 0.016)、仍低于对照组(P=0.027)。3组小鼠颅脑创伤对侧丘脑仅COX⁃2(P=0.029)和8⁃OHd G(P= 0.000)阳性神经元或胶质细胞数目差异有统计学意义,其中,TBI组COX⁃2(P=0.011)和8⁃OHd G(P= 0.000)阳性神经元或胶质细胞数目高于对照组,盐酸小檗碱组COX⁃2(P=0.047)和8⁃OHd G(P=0.010)阳性神经元或胶质细胞数目低于TBI组,仅8⁃OHd G阳性神经元数目仍高于对照组(P=0.004)。结论颅脑创伤可以引起双侧丘脑继发性损伤,尤以同侧丘脑显著,对侧丘脑仅出现炎症反应和氧化损伤;盐酸小檗碱通过抑制颅脑创伤后双侧丘脑炎症反应和氧化损伤而发挥神经保护作用。
小檗碱;颅脑损伤;丘脑;炎症;氧化性应激;神经元;疾病模型,动物
【Abstract】ObjectiveTo investigate the protective effect of berberine chloride on secondary damage(inflammation,oxidative damage and neuron loss)in bilateral thalami of traumatic brain injury(TBI) model mice.MethodsMice were randomly divided into 3 groups:control group(N=6),TBI group(N=6) and berberine group(N=6).TBI model was established by a free⁃falling hitting device.In control group, mice were not given free⁃falling hitting.Mice in berberine group were given a gavage of berberine chloride [50 mg/(kg·d)]for 21 d,while mice in TBI group were given the same dosage of normal saline for 21 d. Immunohistochemistry was used to count the number of neurons or gliocytes positive for inducible nitric oxide synthase(iNOS),cyclooxygenase⁃2(COX⁃2),8⁃hydroxy deoxyguanosine(8⁃OHdG)and neuronal nuclei (NeuN),the number of astrocytes positive for glial fibrillary acidic protein(GFAP)and the number of microglias positive for ionized calcium⁃binding adaptor molecule 1(Iba1).ResultsThe number of neurons or gliocytes positive for iNOS(P=0.015),COX⁃2(P=0.022),8⁃OHdG(P=0.000)and Neu N(P= 0.000),the number of astrocytes positive for GFAP(P=0.024)and microglias positive for Iba1(P=0.000) in TBI ipsilateral thalamus were significantly different among 3 groups.In TBI group,the number of neurons or gliocytes positive for iNOS(P=0.005),COX⁃2(P=0.011)and 8⁃OHd G(P=0.000),the number of astrocytes positive for GFAP(P=0.011)and microglias positive for Iba1(P=0.000)were significantly higher than those in control group,while the number of neurons positive for Neu N(P=0.000)was significantly lower than that in control group.In berberine group,the number of neurons or gliocytes positive for iNOS(P=0.031),COX⁃2(P=0.024)and 8⁃OHdG(P=0.008),the number of astrocytes positive for GFAP(P=0.031)and microglias positive for Iba1(P=0.012)were significantly lower than those in TBI group,while the number of neurons positive for 8⁃OHd G(P=0.014)and microglias positive for Iba1(P= 0.024)were significantly higher than those in control group.The number of neurons positive for Neu N in berberine group was significantly higher than that in TBI group(P=0.016),while lower than that in control group(P=0.027).Additionally,number of neurons or gliocytes positive for COX⁃2(P=0.029)and 8⁃OHdG (P=0.000)in TBI contralateral thalamus were significantly different among 3 groups.The number of neurons or gliocytes positive for COX⁃2(P=0.011)and 8⁃OHd G(P=0.000)in TBI group was significantly higher than that in control group,while the number of neurons or gliocytes positive for COX⁃2(P=0.047) and 8⁃OHd G(P=0.010)in berberine group was significantly lower than that in TBI group.The number of neurons positive for 8⁃OHd G in berberine group was significantly higher than that in control group(P= 0.004).ConclusionsTBI could cause secondary damage of bilateral thalami,especially in ipsilateral thalamus,but only cause inflammation and oxidative damage in contralateral thalamus.Berberine chloride might exert neuroprotective effect on bilateral thalami after TBI by significantly suppressing inflammation and oxidative damage.
This study was supported by Natural Science Foundation of Guangdong Province,China(No. S2013010015786)and Science and Technology Plan Project of Shaoguan,Guangdong Province,China(No. 2010-01).
颅脑创伤(TBI)导致的继发性脑损伤不仅可以发生在原发部位,还可以发生在远隔海马、基底节和丘脑等其他部位[1⁃2],是颅脑创伤高病残率和高病死率的重要原因,也是影响患者预后的关键。丘脑是大脑重要而复杂的中继站,与大脑皮质存在广泛纤维联系,不仅与感觉传导有关,而且与认知和行为密切相关,丘脑损伤可以导致感觉障碍、视觉障碍、睡眠障碍和认知功能障碍[3]。近年研究显示,小檗碱(又称黄连素)具有抗炎症反应、抗氧化应激、降低胆固醇等多种药理学作用,在中枢神经系统疾病中发挥重要神经保护作用[4⁃5]。本研究制备颅脑创伤小鼠模型,观察颅脑创伤后小鼠双侧丘脑炎症反应、氧化损伤和神经元缺失情况,并予以盐酸小檗碱治疗,探讨该药对颅脑创伤模型小鼠双侧丘脑继发性损伤(炎症反应、氧化损伤和神经元缺失)的影响,以为临床能够更有效治疗颅脑创伤提供理论依据。
材料与方法
一、实验材料
1.实验动物无特定病原体(SPF)雌性C57BL/ 6小鼠共18只,月龄6个月,体重25~31 g、平均(27.67±1.88)g,由中山大学实验动物中心提供[许可证号:SYXK(粤)2010-0107],于室温(22±2)℃、相对湿度(50±5)%、12 h昼-12 h夜循环照明环境中饲养,自由摄食、饮水。
2.试剂与仪器(1)主要试剂:盐酸小檗碱(规格:500 mg)由美国Sigma⁃Aldrich公司提供。Ⅰ抗工作液中兔抗小鼠诱导型一氧化氮合酶(iNOS)多克隆抗体(1∶200)为美国Proteintech公司产品,兔抗小鼠环氧合酶⁃2(COX⁃2)多克隆抗体(1∶200)由美国Bioworld公司提供,山羊抗小鼠8⁃羟基脱氧鸟苷(8⁃OHd G)多克隆抗体(1∶200)为美国Calbiochem公司产品,兔抗小鼠神经元核抗原(NeuN)单克隆抗体(1∶200)购自英国Abcam公司,兔抗小鼠胶质纤维酸性蛋白(GFAP)单克隆抗体(1∶500)为英国Abcam公司产品,兔抗小鼠离子钙结合蛋白1(Iba1)单克隆抗体(1∶500)由日本和光纯药工业株式会社提供。辣根过氧化物酶(HRP)标记的驴抗山羊IgGⅡ抗(1∶500)购自美国Life公司,辣根过氧化物酶标记的驴抗兔IgGⅡ抗(1∶500)由丹麦Dako公司提供,Alexa Fluor 555标记的山羊抗兔荧光IgGⅡ抗(1∶500)购自美国Life公司。(2)主要仪器:颅脑创伤撞击器为北京拜安吉科技有限公司产品,脑立体定位仪由深圳市瑞沃德生命科技有限公司提供,小鼠颅骨钻为韩国Saeshin公司产品,光学显微镜购自日本Nikon公司,荧光显微镜购自日本Olympus公司,CM1950型冰冻切片机由德国Leica公司提供。
二、研究方法
1.动物模型制备与分组(1)模型制备:采用自由落体撞击法制备颅脑创伤小鼠模型,在电热毯上以体积分数为4.2%水合氯醛(0.10 ml/10 g)腹腔注射全身麻醉,颅骨正中切开一长约1.50 cm头皮切口,矢状缝旁左侧、冠状缝和人字缝之间开一直径约5 mm骨窗,以20 g砝码自高度为20 cm处垂直自由下落,以预先消毒的撞击针头撞击骨窗。撞击后以骨蜡封闭骨窗,消毒后缝合头皮,将小鼠置于电热炉旁观察1 h,待苏醒后放回笼中饲养。(2)动物分组:采用随机数字表法随机分为对照组、颅脑创伤模型组(TBI组)和盐酸小檗碱治疗组(盐酸小檗碱组),每组各6只小鼠。盐酸小檗碱组于模型制备后予盐酸小檗碱50 mg/(kg·d)溶于生理盐水中灌胃(1次/d),连续21 d;TBI组于模型制备后予等量生理盐水灌胃(1次/d),连续21 d;对照组仅开骨窗,不予自由落体撞击。
2.脑组织灌注和冰冻切片制备模型制备后21 d,小鼠腹腔注射4.20%水合氯醛(0.10 ml/10 g)全身麻醉,仰卧位固定,剪开胸腔,显露心脏,直视下将针头插入左心室,剪开右心耳,先以4℃生理盐水50 ml快速心脏灌注,再以4℃质量分数为4%多聚甲醛溶液50~100 ml缓慢灌注。断头切取脑组织,置重新配置的4%多聚甲醛溶液中12 h,再依次置于4℃、体积分数为20%和30%的蔗糖溶液中脱水6 h至脑组织标本沉淀。取出脑组织标本,以光学相干断层扫描术(OCT)包埋剂包埋,于恒冷冰冻切片机上行冠状位连续切片,层厚10 μm,置⁃80℃冰箱中保存备用。
3.免疫组织化学染色取出脑组织冰冻切片,0.01 mol/L磷酸盐缓冲液(PBS)水化20 min,枸橼酸微波中火修复5 min,体积分数为0.3%Triton X⁃100破膜30 min,以0.01 mol/L磷酸盐缓冲液冲洗5 min(×3次),体积分数为3%过氧化氢灭活内源性过氧化物酶15 min,抗原封闭液封闭1 h,滴加Ⅰ抗工作液中兔抗小鼠iNOS多克隆抗体、兔抗小鼠COX⁃2多克隆抗体、山羊抗小鼠8⁃OHd G多克隆抗体和兔抗小鼠Neu N单克隆抗体,4℃过夜,0.01 mol/L磷酸盐缓冲液冲洗5 min(×3次),再滴加辣根过氧化物酶标记的驴抗山羊IgGⅡ抗或辣根过氧化物酶标记的驴抗兔IgGⅡ抗,常温孵育1 h,0.01 mol/L磷酸盐缓冲液冲洗5 min×(4次),二氨基联苯胺(DAB)显色1 min,苏木素复染3 min,中性树胶封片,于光学显微镜下观察。每只小鼠取3张大致相同层面脑组织切片,观察双侧丘脑iNOS、COX⁃2、8⁃Ohd G和Neu N阳性神经元或胶质细胞,随机选取5个视野(× 400),计数阳性细胞数目。
4.免疫荧光染色取出冰冻切片,0.01 mol/L磷酸盐缓冲液水化20 min,枸橼酸微波中火修复约5 min,0.3%Triton X⁃100破膜30 min,以0.01 mol/L磷酸盐缓冲液冲洗5 min(×3次),抗原封闭液封闭1 h,滴加Ⅰ抗工作液中兔抗小鼠GFAP单克隆抗体和兔抗小鼠Iba1单克隆抗体,4℃过夜,0.01 mol/L磷酸盐缓冲液冲洗5 min(×3次),再滴加Alexa Fluor 555标记的山羊抗兔荧光IgGⅡ抗,于37℃避光孵育1 h,以0.01 mol/L磷酸盐缓冲液冲洗5 min(×4次),含4’,6⁃二脒基⁃2⁃苯基吲哚(DAPI)封片剂封片,于荧光显微镜下观察。每只小鼠取3张大致相同层面脑组织切片,观察双侧丘脑GFAP阳性星形胶质细胞和Iba1阳性小胶质细胞,随机选取5个视野(×200),计数阳性细胞数目。
三、统计分析方法
采用SPSS 17.0统计软件进行数据处理与分析。计量资料以均数±标准差(x±s)表示,采用单因素方差分析,两两比较行LSD⁃t检验。以P≤0.05为差异具有统计学意义。
表1 3组小鼠颅脑创伤同侧丘脑炎症反应、氧化损伤和神经元缺失的比较Table 1.Comparison of inflammation,oxidative damage and neuron loss in TBI ipsilateral thalamus among 3 groups

表1 3组小鼠颅脑创伤同侧丘脑炎症反应、氧化损伤和神经元缺失的比较Table 1.Comparison of inflammation,oxidative damage and neuron loss in TBI ipsilateral thalamus among 3 groups
TBI,traumatic brain injury,颅脑创伤;iNOS,inducible nitric oxide synthase,诱导型一氧化氮合酶;COX⁃2,cyclooxygenase⁃2,环氧合酶⁃2;8⁃OHd G,8⁃hydroxy deoxyguanosine,8⁃羟基脱氧鸟苷;NeuN,neuronal nuclei,神经元核抗原;GFAP,glial fibrillary acidic protein,胶质纤维酸性蛋白;Iba1,ionized calcium⁃binding adaptor molecule 1,离子钙结合蛋白1。The same for table below
Group Control TBI Berberine F value P value N 6 6 6 iNOS 9.89±4.17 16.89±4.55 11.78±1.85 5.682 0.015 COX⁃2 8.04±4.92 16.88±6.88 9.23±3.48 4.945 0.022 8⁃OHdG 16.66±3.70 33.32±7.30 24.63±2.50 17.058 0.000 NeuN 22.56±3.00 16.52±1.39 19.69±1.16 13.369 0.000 GFAP 16.67±8.06 29.33±9.39 19.00±4.06 4.816 0.024 Iba1 11.30±3.69 28.92±6.76 19.53±6.11 14.469 0.000
结果
一、盐酸小檗碱对颅脑创伤模型小鼠同侧丘脑炎症反应、氧化损伤和神经元缺失的影响
3组小鼠颅脑创伤同侧丘脑iNOS(P=0.015)、COX⁃2(P=0.022)、8⁃OHd G(P=0.000)和Neu N(P= 0.000)阳性神经元或胶质细胞数目差异均有统计学意义(表1)。TBI组iNOS(P=0.005)、COX⁃2(P= 0.011)和8⁃OHd G(P=0.000)阳性神经元或胶质细胞数目均高于对照组,Neu N阳性神经元数目低于对照组(P=0.000)。盐酸小檗碱组iNOS(P=0.031)、COX⁃2(P=0.024)和8⁃OHd G(P=0.008)阳性神经元或胶质细胞数目均低于TBI组,其中iNOS和COX⁃2阳性神经元或胶质细胞数目降至正常水平(均P> 0.05)、8⁃OHd G阳性神经元数目仍高于对照组(P= 0.014);而Neu N阳性神经元数目高于TBI组(P= 0.016),但仍低于对照组(P=0.027,图1)。3组小鼠颅脑创伤同侧丘脑GFAP阳性星形胶质细胞数目(P=0.024)和Iba1阳性小胶质细胞数目(P=0.000)差异亦有统计学意义(表1)。对照组星形胶质细胞和小胶质细胞数目较少,TBI组星形胶质细胞和小胶质细胞数目明显增加(P=0.011,0.000),盐酸小檗碱组星形胶质细胞和小胶质细胞增殖受到抑制,细胞数目少于TBI组(P=0.031,0.012),其中星形胶质细胞数目降至正常水平(P>0.05)、小胶质细胞数目仍高于对照组(P=0.024,图2)。
二、盐酸小檗碱对颅脑创伤模型小鼠对侧丘脑炎症反应、氧化损伤和神经元缺失的影响
3组小鼠颅脑创伤对侧丘脑COX⁃2(P=0.029)和8⁃OHd G(P=0.000)阳性神经元或胶质细胞数目差异均有统计学意义,而iNOS和Neu N阳性神经元或胶质细胞数目以及GFAP阳性星形胶质细胞和Iba1阳性小胶质细胞数目差异均无统计学意义(P> 0.05,表2)。TBI组COX⁃2(P=0.011)和8⁃OHd G(P=0.000)阳性神经元或胶质细胞数目均高于对照组;盐酸小檗碱组COX⁃2(P=0.047)和8⁃OHd G(P= 0.010)阳性神经元或胶质细胞数目均低于TBI组,其中COX⁃2阳性神经元或胶质细胞数目降至正常水平(P>0.05)、8⁃OHd G阳性神经元数目仍高于对照组(P=0.004,图3)。
讨论
颅脑创伤可在原发部位形成病灶,原发部位的继发性脑损伤亦是当前颅脑创伤的研究热点,因此,大量关于颅脑创伤的研究易忽略远隔部位的继发性改变。在本研究中,我们发现颅脑创伤第21天小鼠双侧丘脑均存在炎症反应和氧化损伤的继发性改变,同侧丘脑甚至出现神经元缺失,与McAllister[6]报告的颅脑创伤可以导致远隔部位继发性损伤的结果相一致。我们观察到TBI组小鼠颅脑创伤同侧丘脑星形胶质细胞和小胶质细胞增殖活化、iNOS和COX⁃2表达、氧化损伤、神经元缺失均较对照组明显,且TBI组神经细胞形态也发生改变,表现为细胞轮廓不清、轴突难辨、胞体变小、胞核固缩,推测这种神经细胞形态改变是炎症反应和氧化损伤加重所致。小胶质细胞和星形胶质细胞是中枢神经系统炎症反应的主要参与者[7⁃8],前者可以释放氧自由基、一氧化氮(NO)、炎性因子等加重神经损伤[9⁃10];后者不仅可以释放炎性因子,还可以在损伤区域形成胶质瘢痕,对轴索修复和再生产生机械性阻碍屏障[11]。iNOS和COX⁃2是炎症反应的关键酶,在炎症反应中起重要作用,减少iNOS和COX⁃2过表达可以发挥神经保护作用[12⁃13];氧化损伤在颅脑创伤中也可以通过氧自由基释放、脂质过氧化或刺激炎症反应以对神经元产生损害作用[14⁃15]。在本研究中,我们还发现TBI组小鼠颅脑创伤对侧丘脑COX⁃2和8⁃OHd G表达水平较对照组明显增加,而星形胶质细胞和小胶质细胞增殖活化、iNOS表达水平、神经元缺失数目与对照组差异无统计学意义,这可能是由于颅脑创伤模型在原发部位的损伤较轻,也可能是由于损伤时间较长,对侧丘脑继发性炎症反应逐渐减轻,出现神经修复和再生。
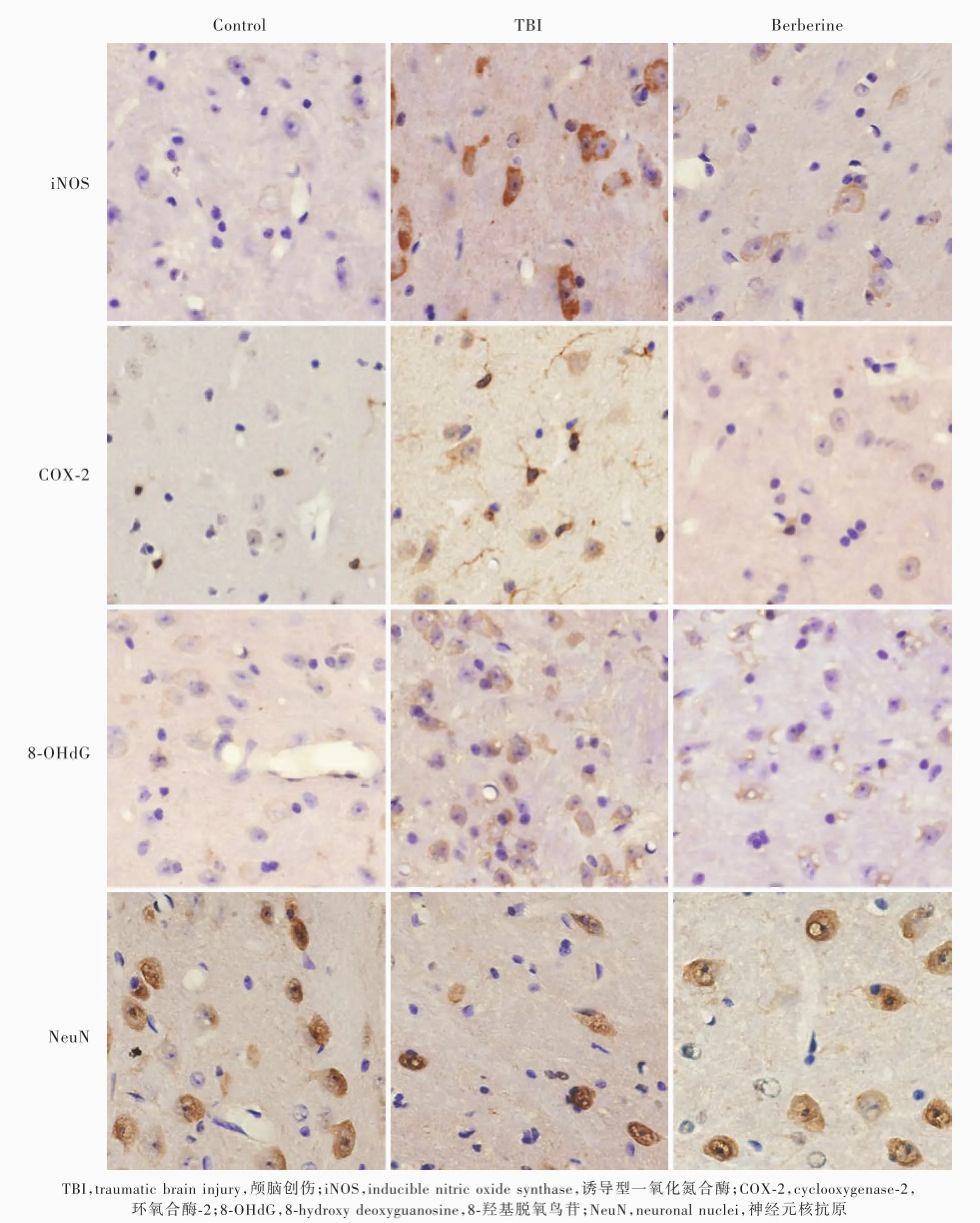
图1 光学显微镜观察,对照组颅脑创伤同侧丘脑iNOS、COX⁃2和8⁃OHd G阳性神经元或胶质细胞数目较少、神经元数目较多且形态完整,TBI组iNOS、COX⁃2和8⁃OHdG阳性神经元或胶质细胞数目增加、神经元数目减少,盐酸小檗碱组iNOS、COX⁃2和8⁃OHdG阳性神经元或胶质细胞数目明显减少、神经元数目增加且形态完整免疫组织化学染色(EnVision二步法)×400Figure 1Optical microscopy findings In control group,number of neurons or gliocytes positive for iNOS,COX⁃2 and 8⁃OHd G was small in TBI ipsilateral thalamus,the number of neurons was large,and the neurons were intact in morphology.In TBI group, the number of neurons or gliocytes was significantly increased,the number of neurons was decreased.In berberine group,the number of neurons or gliocytes was significantly reduced,the number of intact neurons was increased.Immunohistochemical staining(En Vision)×400
在本研究中,我们还观察到盐酸小檗碱对颅脑创伤模型小鼠双侧丘脑炎症反应和氧化损伤均有抑制作用,并增加同侧丘脑神经元存活数目。既往大量研究显示,小檗碱在细胞水平或其他动物模型上具有抗炎症反应、抗氧化应激等作用:Chen等[16]通过腹腔注射盐酸小檗碱以抑制神经炎症反应,从而发挥神经保护作用;Ye等[17]发现,在脂肪组织和脂肪细胞中,小檗碱可以抑制巨噬细胞激活、减少肿瘤坏死因子⁃α(TNF⁃α)和白细胞介素⁃1(IL⁃1)释放,从而改善胰岛素抵抗;有文献报道,小檗碱通过腺苷酸活化蛋白激酶(AMPK)通路抑制小胶质细胞活化,下调炎性因子iNOS和COX⁃2表达,从而发挥抑制神经炎症反应的作用[18];亦有研究显示,小檗碱可以减弱酒精性肝病小鼠模型氧化应激和脂质过氧化[19];在6⁃羟基多巴胺(6⁃OHDA)诱导的帕金森病动物模型中,小檗碱通过抗氧化反应减少多巴胺能神经元缺失,并改善运动功能[20]。但在颅脑创伤模型中,小檗碱对双侧丘脑炎症反应、氧化损伤的影响尚未见诸报道,本研究证实盐酸小檗碱能够通过抑制炎症反应和氧化损伤来对双侧丘脑产生神经保护作用。在本研究中,TBI组小鼠双侧丘脑均出现较严重的氧化损伤,且同侧丘脑炎症反应较对侧丘脑更严重,甚至出现神经元缺失,因此我们推测,丘脑神经损害作用可能是以炎症反应介导为主,而盐酸小檗碱对颅脑创伤后丘脑的神经保护作用可能是同时通过抗炎症反应和抗氧化损伤的作用来介导的。

图2 荧光显微镜观察显示,对照组颅脑创伤同侧丘脑GFAP阳性星形胶质细胞和Iba1阳性小胶质细胞数目较少,TBI组GFAP阳性星形胶质细胞和Iba1阳性小胶质细胞数目明显增加,盐酸小檗碱组GFAP阳性星形胶质细胞和Iba1阳性小胶质细胞数目明显减少免疫荧光染色×200Figure 2Fluorescent microscopy findings The number of astrocytes positive for GFAP and microglias positive for Iba1 in TBI ipsilateral thalamus was small in control group,but was significantly increased in TBI group and significantly reduced in berberine group.Immunofluorescent staining×200
综上所述,颅脑创伤后双侧丘脑均出现炎症反应、氧化损伤等继发性脑损伤,甚至同侧丘脑出现神经元缺失,盐酸小檗碱可以显著抑制颅脑创伤后双侧丘脑炎症反应和氧化损伤,并增加同侧丘脑神经元存活数目,可能为未来更有效治疗颅脑创伤提供新的方向。
表2 3组小鼠颅脑创伤对侧丘脑炎症反应、氧化损伤和神经元缺失的比较Table 2. Comparison of inflammation,oxidative damage and neuron loss in TBI contralateral thalamus among 3 groups

表2 3组小鼠颅脑创伤对侧丘脑炎症反应、氧化损伤和神经元缺失的比较Table 2. Comparison of inflammation,oxidative damage and neuron loss in TBI contralateral thalamus among 3 groups
Group Control TBI Berberine F value P value N 6 6 6 iNOS 11.70±1.28 12.89±2.45 10.55±2.09 2.057 0.162 COX⁃2 9.34±3.63 15.19±4.60 10.79±1.67 4.508 0.029 8⁃OHdG 18.13±6.09 29.17±2.50 24.05±2.57 19.854 0.000 NeuN 23.29±2.95 22.42±1.80 20.00±2.35 2.982 0.081 GFAP 15.93±3.38 18.21±4.40 16.27±3.88 0.595 0.564 Iba1 12.80±2.63 14.97±2.11 15.67±2.37 2.378 0.127

图3 光学显微镜观察显示,对照组颅脑创伤对侧丘脑COX⁃2和8⁃OHd G阳性神经元或胶质细胞数目较为少见,TBI组COX⁃2和8⁃OHd G阳性神经元或胶质细胞数目明显增加,盐酸小檗碱组COX⁃2和8⁃OHd G阳性神经元或胶质细胞数目明显减少免疫组织化学染色(EnVision二步法)×400Figure 3Optical microscopy findings The number of neurons or gliocytes positive for COX⁃2 and 8⁃OHdG in TBI contralateral thalamus was small in control group,but was significantly increased in TBI group and was significantly reduced in berberine group.Immunohistochemical staining(EnVision)×400
[1]Hermans L,Beeckmans K,Michiels K,Lafosse C,Sunaert S, Coxon JP,Swinnen SP,Leunissen I.Proactive response inhibition and subcortical gray matter integrity in traumatic brain injury.Neurorehabil Neural Repair,2017,31:228⁃239.
[2]Leh SE,Schroeder C,Chen JK,Chakravarty MM,Park MT, Cheung B,Huntgeburth SC,Gosselin N,Hock C,Ptito A, Petrides M.Microstructural integrity of hippocampal subregions is impaired after mild traumatic brain injury.J Neurotrauma, 2016[.Epub ahead of print]
[3]Mitchell AS,Sherman SM,Sommer MA,Mair RG,Vertes RP, Chudasama Y.Advances in understanding mechanisms of thalamic relays in cognition and behavior.J Neurosci,2014,34: 15340⁃15346.
[4]Imenshahidi M,Hosseinzadeh H.Berberis vulgaris and berberine:an update review.Phytother Res,2016,30:1745⁃1764.
[5]Zuo R,Cao XB,Zhang WS.The progress of berberine's pharmacological effects.Huan Qiu Zhong Yi Yao,2014,7:568⁃572[.左茹,曹雪滨,张文生.黄连素药理作用研究进展.环球中医药,2014,7:568⁃572.]
[6]McAllister TW.Neurobiological consequences of traumatic brain injury.Dialogues Clin Neurosci,2011,13:287⁃300.
[7]Carta AR,Mulas G,Bortolanza M,Duarte T,Pillai E,Fisone G, Vozari RR,Del⁃Bel E.l⁃DOPA⁃induced dyskinesia and neuroinflammation:do microglia and astrocytes play a role.Eur J Neurosci,2017,45:73⁃91.
[8]Sochocka M,Diniz BS,Leszek J.Inflammatory response in the CNS:friend or foe.Mol Neurobiol,2016[.Epub ahead of print]
[9]Rodriguez MJ,Mahy N.Neuron⁃microglia interactions in motor neuron degeneration:the inflammatory hypothesis in amyotrophic lateral sclerosis revisited.Curr Med Chem,2016.[Epub ahead of print]
[10]Wolf SA,Boddeke HW,Kettenmann H.Microglia in physiology and disease.Annu Rev Physiol,2017,79:619⁃643.
[11]Kang W,Balordi F,Su N,Chen L,Fishell G,Hebert JM. Astrocyte activation is suppressed in both normal and injured brain by FGF signaling.Proc Natl Acad Sci USA,2014,111: E2987⁃2995.
[12]Lin C,Lin HY,Chen JH,Tseng WP,Ko PY,Liu YS,Yeh WL, Lu DY.Effects of paeonol on anti⁃neuroinflammatory responses in microglial cells.Int J Mol Sci,2015,16:8844⁃8860.
[13]Zhu L,Nang C,Luo F,Pan H,Zhang K,Liu J,Zhou R,Gao J, Chang X,He H,Qiu Y,Wang J,Long H,Liu Y,Yan T. Esculetin attenuates lipopolysaccharide(LPS)⁃induced neuroinflammatory processes and depressive⁃like behavior in mice.Physiol Behav,2016,163:184⁃192.
[14]Halstrom A,MacDonald E,Neil C,Arendts G,Fatovich D, Fitzgerald M.Elevation of oxidative stress indicators in a pilot study of plasma following traumatic brain injury.J Clin Neurosci,2017,35:104⁃108.
[15]Zhang G,Zhang F,Zhang T,Gu J,Li C,Sun Y,Yu P,Zhang Z, Wang Y.Tetramethylpyrazine nitrone improves neurobehavioral functions and confers neuroprotection on rats with traumatic brain injury.Neurochem Res,2016,41:2948⁃2957.
[16]Chen CC,Hung TH,Lee CY,Wang LF,Wu CH,Ke CH,Chen SF.Berberine protects against neuronal damage via suppression of glia⁃mediated inflammation in traumatic brain injury.PLoS One,2014,9:E115694.
[17]Ye L,Liang S,Guo C,Yu X,Zhao J,Zhang H,Shang W. Inhibition of M1 macrophage activation in adipose tissue by berberine improves insulin resistance.Life Sci,2016,166:82⁃91.
[18]Lu DY,Tang CH,Chen YH,Wei IH.Berberine suppresses neuroinflammatory responses through AMP⁃activated protein kinase activation in BV⁃2 microglia.J Cell Biochem,2010,110: 697⁃705.
[19]Zhang P,Ma D,Wang Y,Zhang M,Qiang X,Liao M,Liu X, Wu H,Zhang Y.Berberine protects liver from ethanol⁃induced oxidative stress and steatosis in mice.Food Chem Toxicol, 2014,74:225⁃232.
[20]Zhang C,Li C,Chen S,Li Z,Jia X,Wang K,Bao J,Liang Y, Wang X,Chen M,Li P,Su H,Wan JB,Lee SM,Liu K,He C. Berberine protects against 6⁃OHDA⁃induced neurotoxicity in PC12 cells and zebrafish through hormetic mechanisms involving PI3K/AKT/Bcl⁃2 and Nrf2/HO⁃1 pathways.Redox Biol,2016,11:1⁃11.
Protective effect of berberine chloride on secondary damage of bilateral thalami in traumatic brain injury model mice
HUANG Shu⁃xuan1,ZHU Fei⁃qi1,PEI Zhong2,DENG Xu⁃hui1,YANG Zhi1,ZHU Jin⁃hua1,CHEN Chun⁃chun1,LIN Wei⁃feng1
1Department of Neurology,Yuebei People's Hospital Affiliated to Shantou University Medical College, Shaoguan 512026,Guangdong,China
2Department of Neurology,the First Affiliated Hospital,Sun Yat⁃sen University,Guangzhou 510080, Guangdong,China
Berberine;Craniocerebral trauma;Thalamus;Inflammation;Oxidative stress; Neurons;Disease models,animal
ZHU Fei⁃qi(Email:zfqzsu2004@aliyun.com)
2017⁃02⁃27)
10.3969/j.issn.1672⁃6731.2017.04.009
广东省自然科学基金资助项目(项目编号:S2013010015786);广东省韶关市科技计划项目[项目编号:韶科(卫)2010-01]
512026韶关,汕头大学医学院附属粤北人民医院神经内科[黄树宣(现在广州医科大学附属第一医院神经内科,邮政骗码:510080),朱飞奇(现在广东省深圳市罗湖区人民医院神经内科,邮政编码:518001),邓旭辉,杨志,朱瑾华,陈淳淳,林伟丰];510080广州,中山大学附属第一医院神经科(裴中)
朱飞奇(Email:zfqzsu2004@aliyun.com)
