两种疏水型膦类离子液体的密度、动力粘度及电导率
2017-05-12郑其格刘青山
郑其格 刘 惠 夏 泉 刘青山,4,* 牟 林,*
两种疏水型膦类离子液体的密度、动力粘度及电导率
郑其格1刘 惠2,3夏 泉1刘青山1,4,*牟 林1,*
(1沈阳农业大学理学院,沈阳110866;2上海环境卫生工程设计院,上海200232;
3上海污染场地修复工程技术研究中心,上海200232;4沈阳农业大学土地与环境学院,沈阳110866)
通过传统的方法,制备了两种对水和空气稳定的四烷基膦类离子液体。离子液体是:己基三丁基膦四氟化硼和己基三丁基膦双三氟甲基磺酸亚胺。在T=283.15-353.15 K温度范围内,测定了两个离子液体的密度、动力粘度及电导率。讨论了温度、阴离子结构对离子液体的性质的影响。结合文献报道的其它离子液体,讨论了该类离子液体性质随阳离子结构的变化规律,并与咪唑类离子液体的性质进行了比较。通过经验方程,利用密度数据计算了两个离子液体的重要热力学性质参数,例如:分子体积、标准摩尔熵及晶格能等。并将估算性质与传统的咪唑、吡啶类离子液体进行了对比。通过密度和电导率确定了离子液体的摩尔电导率。讨论了Vogel-Fulcher-Tamman(VFT)方程和Arrhenius方程对于粘度和电导率拟合的可行性,并估算了电导活化能及流动活化能。通过Walden规则,描述了密度、粘度及电导率之间的联系。有关研究对新型离子液体的合成及其工业化的应用具有十分重要意义。
离子液体;密度;动力粘度;电导率;Walden规则
1 Introduction
The use of ionic liquids(ILs)as green solvents has received much attention because of their physico-chemical properties1-4.ILs commonly exhibit low melting temperatures,good solvation, negligible vapor pressure,high electrical conductivity,good thermal stability,and good designability,among other factors.
As a recent example,tetra(alkyl)phosphonium ionic liquids (TAPILs),with a stable cation,have been synthesized and systematically applied to different areas.These TAPILs commonly exhibit low density,low electrical conductivity,high dynamic viscosity,high thermal stability,and high electrochemical stability5,6.TAPILs with strong nucleophilic anions are significantly more stable than the analogous tetra-alkyl ammonium type ILs7. Because of the high dynamic viscosity,TAPILs are also used as the stationary phases for gas chromatography8,9.Many TAPILs exhibite a unique phase behavior with water by increasing or decreasing the temperature,and the mixing of water and TAPILs shows the lowers critical solution temperature(LCST)10-12.In addition to temperature,CO2and N2can also be used to reversibly change the phase from homogeneous to separated liquid-liquid13. TAPILs have also been used for the absorption of CO2and SO2, where CO2can be captured by tuning the basicity of the TAPIL14, and multiple-site absorption can be used for SO2capture in the anion of several azole-based TAPILs15.Although such TAPILs have exhibited outstanding properties,the basis for these properties is relatively unknown,which has prohibited the synthesis and application of new TAPILs.The properties of trihexyl (tetradecyl)phosphonium type ILs have also been studied as examples of common TAPILs16-18.However,such cation TAPILs exhibit greater molecular volumes and higher dynamic viscosities than do more common ILs19-29.
Recently,Tsunashima et al.6studied the physical and electrochemical properties of some quaternary phosphonium cation ILs with tetrafluoroborate and bis(trifluoromethylsulfonyl)imide anions included in this type of IL.These two types of anion IL include tributyl(octyl)phosphonium tetrafluoroborate ([P4448][BF4]),tributyl(dodecyl)phosphonium tetrafluoroborate ([P444(12)][BF4]),tributyl(octyl)phosphonium bis(trifluoromethylsulfonyl)imide([P4448][NTf2]),and tributyl(dodecyl)phosphonium bis(trifluoromethylsulfonyl)imide ([P444(12)][NTf2]). According to common experience,the influence of the alkyl chain length on the IL properties is clear.In this work,two TAPILs, [P4446][BF4]and[P4446][NTf2],were prepared for study by an ion exchange method to further understand this type of TAPIL. The two TAPILs have lower molecular volumes and lower dynamic viscosities relative to trihexyl(tetradecyl)phosphonium type ILs.The density,dynamic viscosity,and electrical conductivity were probed over the temperature range from(283.15±0.05)to (353.15±0.05)K.The influences of the anion and of methylene introduction on the properties are discussed.Other IL properties are also predicted based on empirical values from previous experiment.These predicted values include density,standard molar entropy,and lattice energy.The values are also compared with literature values6.The work provides information on the influence of the anion to the properties after probing of the basic properties of these systems.
2 Experimental
2.1 Materials
Ethyl acetate(Beijing Yili Chemical Reagent Co.,China), acetonitrile(Tianjin Tianhe Chemical Reagent Co.,China),sodium tetrafluoroborate(NaBF4)(Shanghai Zhuorui Chemical Co., China),bis(trifluoromethylsulfonyl)imide lithium salt(LiNTf2) (Rhodia Co.,China),1-bromohexane(Beijing Yili Chemical Reagent Co.,China),and tri-n-butylphosphine(Shandong Weitian Chemical Reagent Co.,China)were used in the synthesis process. All provenance and mass fraction purities of the used materials are listed in Table 1.
2.2 Preparation of TAPILs[P4446][BF4]and [P4446][NTf2]
TAPILs[P4446][BF4]and[P4446][NTf2]were synthesized by methods described elsewhere30,31.Tributyl(hexyl)phosphonium bromide([P4446][Br])was first synthesized.Aslight excess of 1-bromohexane was added dropwise into tributylphosphine with stirring at 353 K for 24 h.The white product,[P4446][Br],was recrystallized from an ethyl acetate and acetonitrile solution(the ratio of the volume being 1:1)several times.The white product was dried in high vacuum for 48 h at 353 K before the final product synthesis was carried out.
The hydrophobic TAPILs[P4446][BF4]and[P4446][NTf2] were synthesized by ion exchange in a distilled water/dichloromethane system.The white product[P4446][Br]was placed in aflask and dissolved with the distilled water/dichloromethane system.An equivalent amount of NaBF4or LiNTf2salt,for [P4446][BF4]or[P4446][NTf2],respectively,was added into the system with stirring.The TAPIL and dichloromethane phase was washed with distilled water until no Brwas present,where the presence was checked using an AgNO3/HNO3solution.The colorless liquid was obtained and dried(353 K for 48 h)under vacuum after washing.The final product TAPILs were characterized by1H NMR spectroscopy.The final mass fraction purities of the TAPILs were estimated from1H NMR spectra to be better than 99%(see Figs.S1 and S2 in the Supporting Information).The structures of TAPILs[P4446][BF4]and[P4446][NTf2]are presented in Scheme 1.
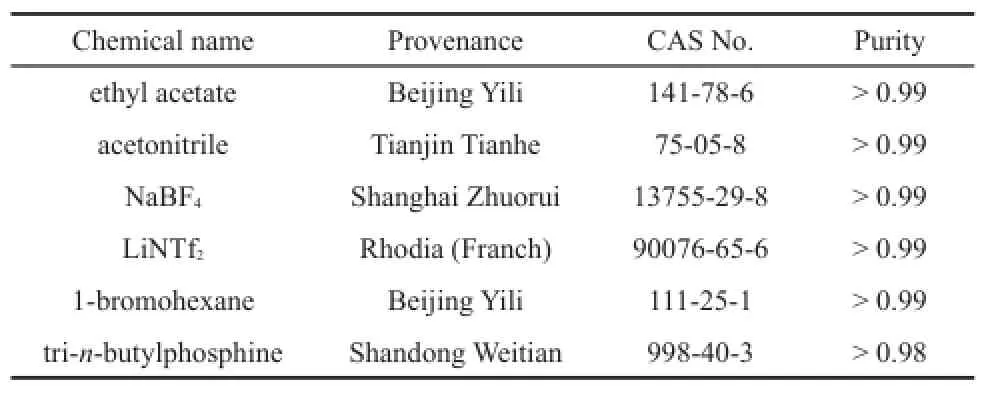
Table 1 Provenance and mass fraction purity of the used materials
2.3 Water content
Thewatercontentofthe TAPILs[P4446][BF4]and [P4446][NTf2]was determined by a Cou-Lo Aquamax Karl Fischer Moisture Meter(v.10.06).The water contents of the samples is less than 300×10-6(mass fraction)before and after determination of the system properties.
2.4 Density and dynamic viscosity
An Anton Paar SVM3000(Anton Paar Shanghai Trading Co. Ltd.)was used for determining the density and dynamic viscosity of the TAPILs[P4446][BF4]and[P4446][NTf2].The temperature range of the measurement is from(283.15±0.05)to(353.15± 0.05)K per 5 K,and thermal equilibrium was obtained in 30 min. The experimental error was estimated as±0.0002 g·cm-3for the density and the uncertainty was estimated to be±1%for the dynamic viscosity.
2.5 Electrical conductivity
The electrical conductivities of the TAPILs[P4446][BF4]and [P4446][NTf2]were recorded using a MP522 conductivity meter (San Xin Electronic Co.)with a 1 cm-1cell constant.The cell was calibrated using a standard aqueous KCl solution.The data were recorded every 5 K once thermal equilibrium was attained after 30 min from the temperature increase of(283.15±0.05)to (353.15±0.05)K.The uncertainty is estimated to be±1%.
The values of density,dynamic viscosity,and electrical conductivity are listed in Tables 2 and 3.
3 Results and discussion
From Tables 2 and 3,the density and dynamic viscosity can be seen to decrease with an increase temperature,and the electrical conductivity contrasts with the above two properties,where the data significantly increases in value with an increase temperature.
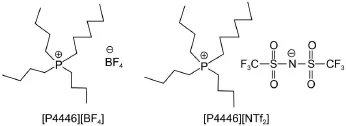
Scheme 1 Structures of TAPILs[P4446][BF4]and[P4446][NTf2]
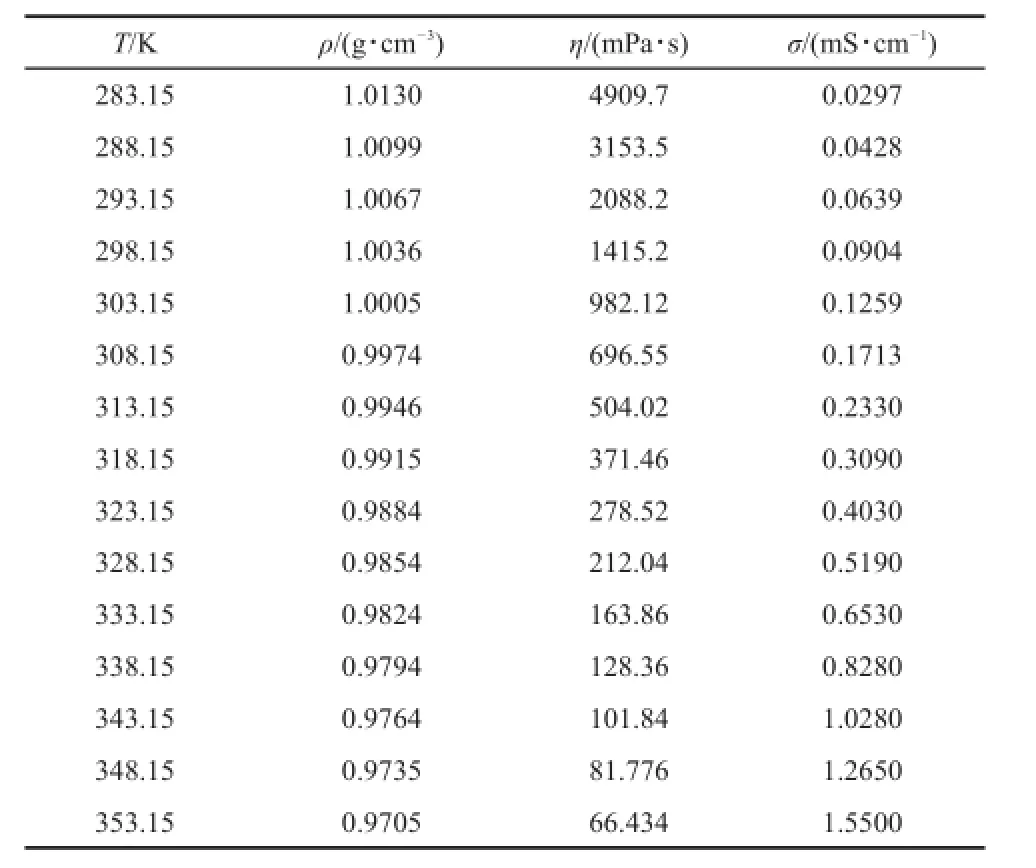
Table 2 Experimental values of density,ρ,dynamic viscosity,η, and electrical conductivity,σ,of[P4446][BF4]from 283.15 to 353.15 K at pressure p=0.1 MPa
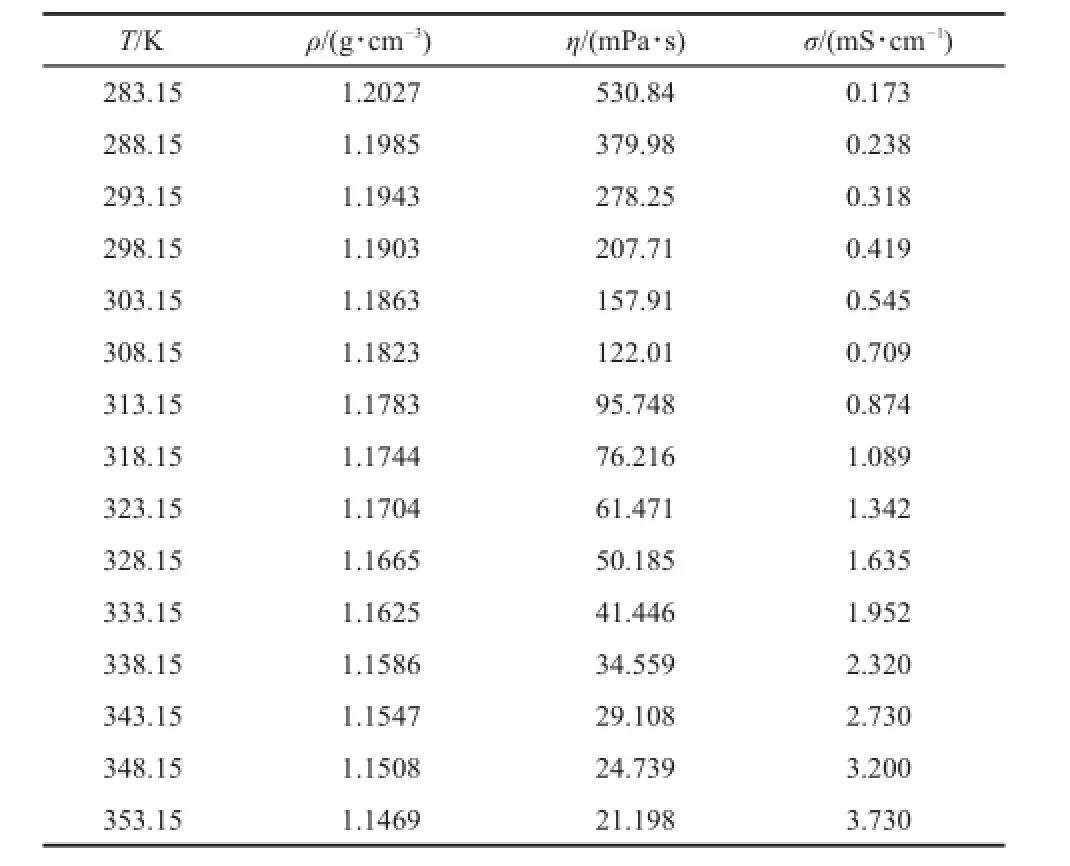
Table 3 Experimental values of density,ρ,dynamic viscosity,η, and electrical conductivity,σ,of[P4446][NTf2]from 283.15 to 353.15 K at p=0.1 MPa
At 293.15 K,the densities of[P4446][BF4]and[P4446][NTf2] are 1.0067 and 1.1943 g·cm-3,respectively.Although the value for[P4446][NTf2]is higher than[P4446][BF4],it is much lower than the common IL 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide[C4mim][NTf2](1.4378 g·cm-3)32.
At 298.15 K,the dynamic viscosity of the[P4446][BF4]and [P4446][NTf2]are 1415.2 and 207.71 mPa·s,respectively.These values are much higher than for the ILs[C4mim][BF4](75 mPa· s)and[C4mim][NTf2](40 mPa·s)33.The electrical conductivitiesof[P4446][BF4]and[P4446][NTf2]are 0.0904 and 0.419 mS· cm-1,respectively,and these values are much lower than those for ILs[C4mim][BF4](4.5 mS·cm-1)and[C4mim][NTf2](4.6 mS· cm-1)33.
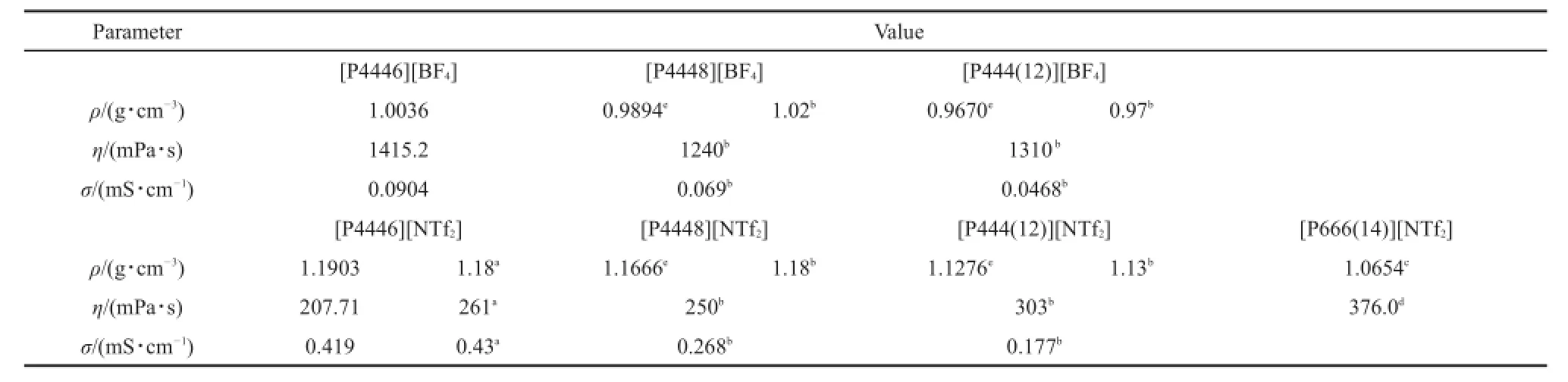
Table 4 Density,dynamic viscosity,and electrical conductivity of the some tetra-alkyl phosphonium type ionic liquids with literature at 298.15 K
To compare the density,dynamic viscosity,and electrical conductivity of the tetra(alkyl)phosphonium type ionic liquids with reported literature values6,7,19,22,the above property values of the TAPILs determined at 298.15 K are listed in Table 4.The TAPIL anions are[BF4-]and[NTf2-
].
From Table 3,the values at 298.15 K of density,dynamic viscosity,and electrical conductivity of[P4446][NTf2]are 1.1903 g·cm-3,207.71 mPa·s,and 0.419 mS·cm-1,respectively. At the same temperature,the values of the reference are 1.18 g· cm-3,261 mPa·s,and 0.43 mS·cm-1,respectively7.From the literature7,the density,dynamic viscosity,and electrical conductivity values were measured by a precalibrated pycnometer, slow-flow viscometer,and ThermoOrion conductivity meter, respectively.The primary reasons for discrepancies with the literature values are the water content,instrumentation used,and purity of the[P4446][NTf2].
3.1 Density
The temperature dependence on the density can be plotted(see Fig.1)and fitted over the temperature range from(283.15±0.05) to(353.15±0.05)K by the following equation:

where Y is the density;andA and B are adjustable parameters.The fitting equations are obtained from Y=1.1844-6.06×10-4T for [P4446][BF4]and Y=1.4273-7.95×10-4T for[P4446][NTf2]. The correlation coefficients of the two TAPILs are 0.9999,which indicates that the linear equation represents the density very well.
The temperature dependence on lnρ can be fitted by the following linear equation:

where b is an empirical constant and α is the thermal expansion coefficient.The fitting equations are lnρ=0.1860-6.11×10-4T for[P4446][BF4],and lnρ=0.3760-6.77×10-4T for[P4446] [NTf2].The correlation coefficients are higher than 0.9999 for both cases,thus indicating that the empirical linear equation represents the density very well.From Equation(2),the thermal expansion coefficients are 6.11×10-4for[P4446][BF4]and 6.77×10-4for [P4446][NTf2].These values are in good agreement with the values reported by Jacquemin et al.34that ranged between 5×10-4and 7×10-4K-1(at 293.15 K,[Bmim][NTf2]are 6.51×10-4and 6.90×10-4K-1at dried and saturated,respectively;like:[Bmim] [BF4]is 5.95×10-4at dried).The thermal expansion coefficients of the two TAPILs at 298.15 K are listed in Table 5.
At 298.15 K,the molecular volume,Vm,standard molar entropy, S0,and lattice energy,UPOT,were calculated for the TAPILs[P4446] [BF4]and[P4446][NTf2]by the traditional empirical equations from the density32:

where M is the molar mass,ρ is the density,and N is Avogadro′s constant.The obtained data from the above equations are listed in Table 5.
From Table 5,the lattice energy values are 379.0 kJ·mol-1for [P4446][BF4]and 357.4 kJ·mol-1for[P4446][NTf2].These values are much lower than for traditional organic melt salts.For example,the lowest lattice energy among the alkali halides is for fused CsI(613 kJ·mol-1)35.The lower lattice energy may be a reason why the two TAPILs have relatively low melting temperatures,such that[P4446][BF4]and[P4446][NTf2]can exist in the liquid state at room temperature.
From Table 5,the molecular volumes are 0.6195 nm3for [P4446][BF4]and 0.7922 nm3for[P4446][NTf2].These values
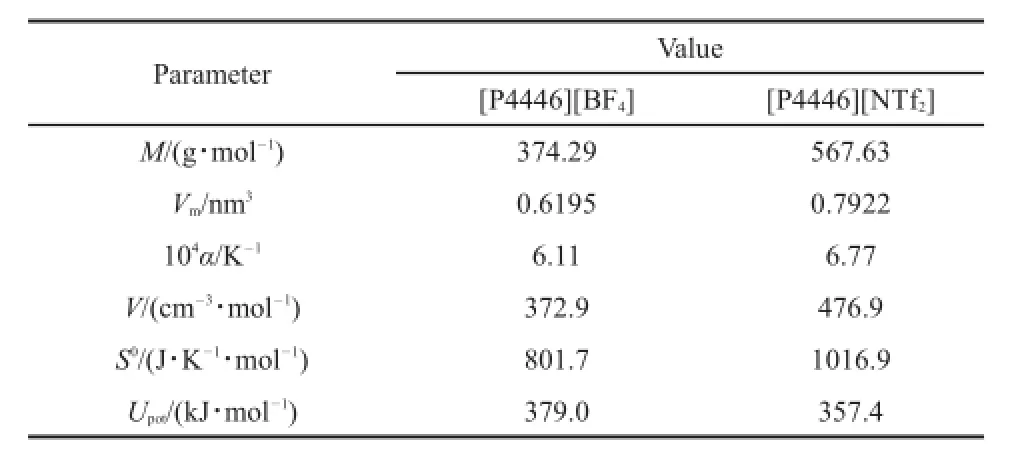
Table 5 Estimated values of physicochemical properties of two TAPILs[P4446][BF4]and[P4446][NTf2]at 298.15 K
M:molar mass;Vm:molecular volume;α:thermal expansion coefficients; V:molar volume;S0:standard molar entropy;Upot:lattice energy are higher than for more common ILs,e.g.,0.5320 nm3for [C6py][NTf2]31,0.5593 nm3for[C63mpy][NTf2]36,0.5633 nm3for [C64mpy][NTf2]36,0.5406 nm3for[C6Mim][NTf2]37,and0.5608 nm3for[C6DMim][NTf2]37.
According to previous reports,the mean contributions of the methylene group to the molecular volume are 0.0277 nm3for [Cn3mpy][NTf2]36,38,0.0289 nm3for[Cn4mpy][NTf2]38,39,0.0280 nm3for[Cnpy][NTf2]31,40,0.0282 nm3for[Cnmim][NTf2]32,0.0272 nm3for[Cnmim][BF4]32,and 0.0279 nm3for amino acid ionic liquids41-44.From the literature45,the basic properties of 1-alkyl-3-methylimizazolium tris(pentafluoroethyl)trifluorophosphate have been predicted in terms of the estimated molecular volume.In this work,we also predicted the properties of the series of TAPILs based on literature45methods.The mean value of the contribution can be calculated to be 0.0280 nm3.The density,standard entropy, and lattice energy were predicted according to Equations(3)-(5), and the values are listed in Table 6.
From Tables 4 and 6,the predicted density values are agreement with the literature6,except for TAPIL[P4448][BF4].The comparison,between the values predicted in this work versus the literature shows that the method employed herein is suitable for the prediction of TAPIL properties.
3.2 Electrical conductivity

The molar electrical conductivities of TAPIL[P4446][BF4]and [P4446][NTf2]were calculated by the following equation: where Λ is the molar conductivity,σ is the electrical conductivity, M is the molar mass and ρ is the density.The values of the molar conductivity are listed in Table 7.
The electrical conductivity of the TAPILs[P4446][BF4]and [P4446][NTf2]as a function of temperature is plotted in Fig.2. From Fig.2,it can be observed that for each TAPIL the electrical conductivity decreases significantly with an increase in temperature.

Vogel-Fulcher-Tammann(VFT)equations are commonly used for the fitting of temperature dependence to electrical conductivity for ILs.Herein,the temperature dependence of the electrical conductivity for the two TAPILs[P4446][BF4]and[P4446][NTf2] were also fitted according to the following VFT equation: where σ is the electrical conductivity;and σ0,B,and T0are fitting parameters.The fitted parameters of σ0,B,and T0,and the corresponding correlation coefficient,R,are listed in Table 8.From Table 8,as for other types of IL,the experimental electrical conductivity fits well aginst temperature by the VFT equation.
The Arrhenius equation is commonly used to fit the electrical conductivity against temperature.However,Vila et al.46have shown differing results for ILs,where the electrical conductivity does not always follow Arrhenius behavior with changes in temperature.
TheArrhenius equation is:

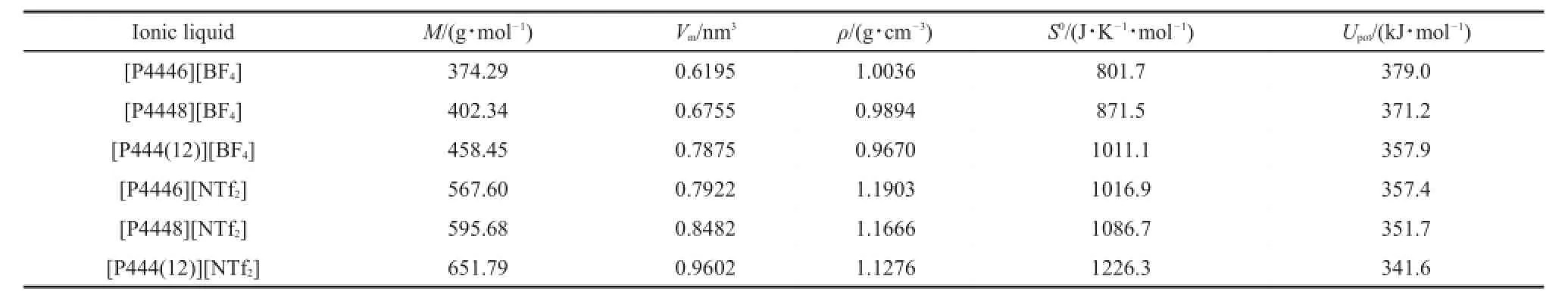
Table 6 Predicted values of the thermodynamic properties of the some tetra-alkyl phosphonium type ionic liquids at 298.15 K

Table 7 Molar electrical conductivity,Λ,of two TAPILs[P4446][BF4]and[P4446][NTf2]at the temperature from 283.15 to 353.15 K
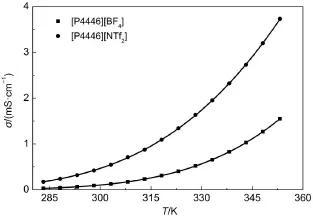
Fig.2 Plots of electrical conductivity vs temperature of two TAPILs[P4446][BF4]and[P4446][NTf2]from 283.15 to 353.15 K
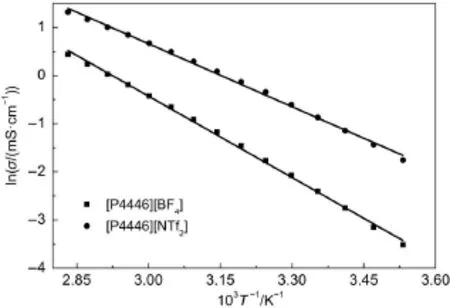
Fig.3 Plots of lnσ vs 1/T of two TAPILs[P4446][BF4]and [P4446][NTf2]from 283.15 to 353.15 K

where Eσis the activation energy,which indicates the energy needed for the ion to hop into a free hole,σ∞is the maximum electrical conductivity and kBis the Boltzmann constant.From Equation(9),a linear equation,the 1/T dependence on lnσ was plotted for the two TAPILs[P4446][BF4]and[P4446][NTf2](see Fig.3).
The plots should be on straight lines but the points clearly show that the experimental points are not on the straight lines(the solid straight lines were drawn to clarify the points).Therefore,the electrical conductivities of the TAPILs also do not follow Arrhenius behavior very well.Similar results were determined by us previously36.
Vila et al.46introduced the activation energy of the electrical conductivity into the VFT equation by establishing fitting parameters for the VFT equation via an Arrhenius equation:σ0=σ∞and B=Eσ/kB.The final version the VFT equation can therefore be expressed as follows:

The activation energies of electrical conductivities for TAPIL [P4446][BF4]and[P4446][NTf2]were calculated in this manner and are listed in Table 8.
3.3 Dynamic viscosity
The dynamic viscosities of the TAPILs[P4446][BF4]and [P4446][NTf2],as a function of temperature,are shown in Fig.4. From these values,it can be observed that the dynamic viscosity for each TAPIL decreases significantly with an increase in tem-perature.

Table 8 Fitted parameter values of σ0,B,T0,and correlation coefficient,R,by equation(8)and activation enerty,Eσ,for TAPILs[P4446][BF4]and[P4446][NTf2]by equation(10)
VFT equation is used for fitting the temperature dependence against the dynamic viscosity for ILs.Herein,the temperature dependence of the dynamic viscosity for each of the TAPILs [P4446][BF4]and[P4446][NTf2]were also fitted according to the following VFT equation:

where η is the dynamic viscosity;and η0,B,and T0are the fitting parameters.The best fitting parameters of η0,B,T0,and the corresponding correlation coefficient,R,are listed in Table 9.From Table 9,the obtained values of the correlation coefficient,R,are higher than 0.9999,which indicates that the VFT equation can be used for fitting the experimental dynamic viscosity.
As with the electrical conductivity,an Arrhenius equation was also used to fit the dynamic viscosity:

where Eais the activation energy for the dynamic viscosity,η∞is the maximum dynamic viscosity,and kBis the Boltzmann constant.The 1/T dependence of lnη was plotted for the TAPILs [P4446][BF4]and[P4446][NTf2](see Fig.5).From Fig.5,as with the electrical conductivity shown above,the points are also not following the straight lines(The solid straight lines were drawn in order to clarify the points).
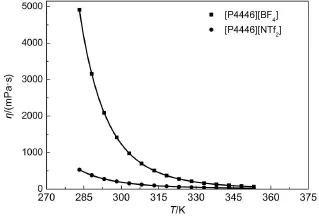
Fig.4 Plots of dynamic viscosity vs temperature of two TAPILs [P4446][BF4]and[P4446][NTf2]from 283.15 to 353.15 K
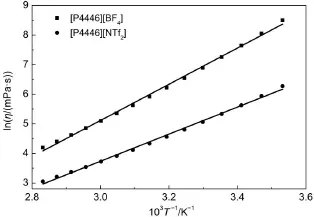
Fig.5 Plots of lnη vs 1/T of two TAPILs[P4446][BF4]and [P4446][NTf2]from 283.15 to 353.15 K
The activation energy of the dynamic viscosity was introduced into the VFT equation in a similar fashion as with the electrical conductivity above,and as discussed by Vila et al.46The final version of the VFT equation can be expressed as:

The activation energies of the dynamic viscosity for the TAPILs [P4446][BF4]and[P4446][NTf2]were calculated and are listed in Table 9.
3.4 Walden rule
The relationship between the molar conductivity and dynamic viscosity for TAPILs[P4446][BF4]and[P4446][NTf2]can be described by Walden's rule36,47-50:

where Λ is the molar conductivity,η is the dynamic viscosity,and k is a temperature dependent constant.The Walden product at 298.15 K(in[S·cm2·mol-1][mP·s])is 48 for[P4446][BF4]and 42 for[P4446][NTf2].
The lgΛ dependence on lgη-1from 283.15 to 353.15 K is plotted in Fig.6 for the two TAPILs[P4446][BF4]and[P4446][NTf2]. From Fig.6,the curves are approximately straight lines,which indicates that the ILs to some extent obey Walden′s rule.The slopes of the lines for the TAPILs[P4446][BF4]and[P4446][NTf2] are 0.931 and 0.967,respectively.These results indicate that the relationship between the conductivity and f l uidity is a constant for both cases.The position of the ideal line was established by using aqueous KCl solutions at high dilution,and the lines for the two TAPILs are close to the ideal KCl line.Most of the previously reported ILs36,47-50show the same tendency.The TAPILs[P4446][BF4]and[P4446][NTf2]can be called“subionic”51.
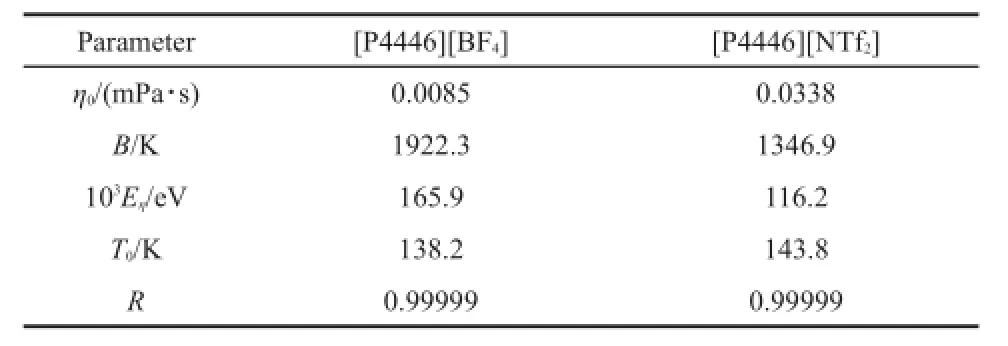
Table 9 Fitted parameter values of η0,B,T0,and correlation coefficient,R,by equation(11)and activation energy,Eη,for TAPILs[P4446][BF4]and[P4446][NTf2]by equation(13)
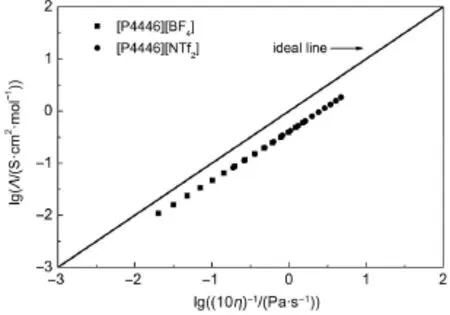
Fig.6 Walden plots for two TAPILs[P4446][BF4]and [P4446][NTf2]from 283.15 to 353.15 K
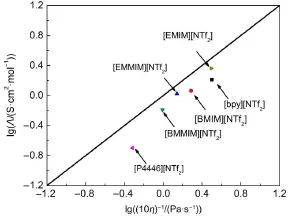
Fig.7 Walden plots for[P4446][NTf2]and literature values at 298.15 K
To compare Walden plots,the values for[P4446][NTf2]and from previous literature40,52,53are plotted in Fig.7.
4 Conclusions
The density,dynamic viscosity,and electrical conductivity of the air and water stable hydrophobic TAPILs[P4446][BF4]and [P4446][NTf2]were determined at atmospheric pressure over the temperature range from 283.15 to 353.15 K.The experimental density and electrical conductivity values for anion[NTf2]-are higher than for anion[BF4]-.However,the dynamic viscosity shows the opposite trend,and the two TAPILs show very low densities.The thermal expansion coeff i cient,molecular volume, standard molar entropy,and lattice energy of the TAPILs were estimated by empirical equations.The two TAPILs show lower lattice energies than those of more traditional salts(e.g.,CsI).The density and dynamic viscosity decrease,and the electrical conductivity increases,with an increase in temperature.The temperature dependence of the dynamic viscosity and electrical conductivity cannot be fitted by an Arrhenius equation(see Figs.3 and 5).However,the VFT equation can be used to fit the experimental values,and the TAPILs[P4446][BF4]and[P4446][NTf2] can be considered“subionic”according to Walden′s rule.
Supporting Information: The1H NMR spectra have been included.This information is available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1) Rantwijk,F.V.;Sheldon,R.A.Chem.Rev.2007,107,2757. doi:10.1021/cr050946x
(2)Greaves,T.L.;Drummond,C.J.Chem.Rev.2008,108,206. doi:10.1021/cr068040u
(3) Hapiot,P.;Lagrost,C.Chem.Rev.2008,108,2238. doi:10.1021/cr0680686
(4) Jessop,P.G.;Subramaniam,B.Chem.Rev.2007,107,2666. doi:10.1021/cr040199o
(5)Tsunashima,K.;Sugiya,M.Electrochem.Commun.2007,9, 2353.doi:10.1016/j.elecom.2007.07.003
(6) Tsunashima,K.;Sugiya,M.Electrochemistry 2007,75,734. doi:10.5796/electrochemistry.75.734
(7) Vega,J.A.;Zhou,J.;Kohl,P.A.J.Electrochem.Soc.2009,156, A253.doi:10.1149/1.3070657
(8) Pomaville,R.M.;Poole,S.K.;Davis,L.J.;Poole,C.F. J.Chramatogr.1988,438,1.doi:10.1016/S0021-9673(00) 90227-9
(9) Breitbach,Z.S.;Armstrong,D.W.Anal.Bioanal.chem.2008, 390,1605.doi:10.1007/s00216-008-1877-3
(10)Fukumoto,K.;Ohno,H.Angew.Chem.Int.Ed.2007,46,1852. doi:10.1002/anie.200604402
(11)Kohno,Y.;Deguchi,Y.Ohno,H.Chem.Commun.2012,48, 11883.doi:10.1039/c2cc36913c
(12) Kohno,Y.;Arai,H.;Saita,S.;Ohno,H.Aust.J.Chem.2011,64, 1560.doi:10.1071/CH11278
(13)Kohno,Y.;Arai,H.;Ohno,H.Chem.Commun.2011,47,4772. doi:10.1039/C1CC10613A
(14) Wang,C.;Luo,X.;Luo,H.;Jiang,D.;Li,H.;Dai,S.Angew. Chem.Int.Ed.2011,50,4918.doi:10.1002/anie.201008151
(15)Wang,C.;Cui,G.;Luo,X.;Xu,Y.;Li,H.;Dai,S.J.Am.Chem. Soc.2011,133,11916.doi:10.1021/ja204808h
(16) Blundell,R.K.;Licence,P.Phys.Chem.Chem.Phys.2014,16, 15278.doi:10.1039/C4CP01901F
(17) Chen,F.F.;Dong,Y.;Sang,X.Y.;Zhou,Y.;Tao,D.J.Acta Phys.-Chim.Sin.2016,32,605.[陈凤凤,董 艳,桑晓燕,周言,陶端健.物理化学学报,2016,32,605.]doi:10.3866/PKU. WHXB201512241
(18) Ferreira,A.F.;Simões,P.N.;Ferreira,A.G.M.J.Chem. Thermodynamics 2012,45,16.doi:10.1016/j.jct.2011.08.019
(19) Tariq,M.;Forte,P.A.S.;Gomes,M.F.C.;Lopes,J.N.C.; Rebelo,L.P.N.J.Chem.Thermodynamics 2009,41,790. doi:10.1016/j.jct.2009.01.012
(20) Diogo,J.C.F.;Caetano,F.J.P.;Fareleira,J.M.N.A.; Wakeham,W.A.J.Chem.Eng.Data 2012,57,1015. doi:10.1021/je200830j
(21) Goodrich,B.F.;Fuente,J.C.de la;Gurkan,B.E.;Lopez,Z.K.; Price,E.A.;Huang,Y.;Brennecke,J.F.J.Phys.Chem.B 2011, 115,9140.doi:10.1021/jp2015534
(22) Baldo,M.A.;Oliveri,P.;Simonetti,R.;Daniele,S. J.Electroanal.Chem.2014,731,43.doi:10.1016/j. jelechem.2014.08.001
(23) Li,A.;Tian,Z.;Yan,T.;Jiang,D.;Dai,S.J.Phys.Chem.B 2014,118,14880.doi:10.1021/jp5100236
(24) Tong,J.;Zhang,Q.G.;Hong,M.;Yang,J.Z.Acta Phys.-Chim. Sin.2006,22,71.[佟 静,张庆国,洪 梅,杨家振.物理化学学报,2006,22,71.]doi:10.3866/PKU.WHXB20060114
(25) Tong,J.;Chen,T.F.;Zhang,D.;Wang,L.F.;Tong,J.;Yang,J. Z.Acta Phys.-Chim.Sin.2016,32,1161.[佟 静,陈滕飞,张朵,王林富,佟 健,杨家振.物理化学学报,2016,32,1161.] doi:10.3866/PKU.WHXB201602232
(26)Bu,X.X.;Fan,B.H.;Wei,J.;Xing,N.N.;Ma,X.X.;Guan,W. Acta Phys.-Chim.Sin.2016,32,267.[卜晓雪,樊本汉,魏 杰,邢楠楠,马晓雪,关 伟.物理化学学报,2016,32,267.] doi:10.3866/PKU.WHXB201510303
(27) Hoogerstraete,T.V.;Binnemans,K.Green Chem.2014,16, 1594.doi:10.1039/C3GC41577E
(28) Ferreira,C.E.;Talavera-Pieto,N.M.C.;Fonseca,I.M.A.; Portugal,A.T.G.;Ferreira,A.G.M.J.Chem.Thermodynamics 2012,47,183.doi:10.1016/j.jct.2011.10.012
(29)Hayyan,M.;Mjalli,F.S.;Hashim,M.A.;AlNashef,I.M.;Tan, X.M.;Chooi,K.L.J.Appl.Sci.2010,10,1176.doi:10.3923/ jas.2010.1176.1180
(30) Tong,B.;Liu,Q.S.;Tan,Z.C.;Welz-Biermann,U.J.Phys. Chem.A 2010,114,3782.doi:10.1021/jp9047538
(31) Liu,Q.S.;Yang,M.;Li,P.P.;Sun,S.S.;Welz-Biermann,U.; Tan,Z.C.;Zhang,Q.G.J.Chem.Eng.Data 2011,56,4094. doi:10.1021/je200534b
(33)McEwen,A.B.;Ngo,H.L.;LeCompte,K.;Goldman,J.L. J.Electrochem.Soc.1999,146,1687.doi:10.1149/1.1391827
(34) Jacquemin,J.;Husson,P.;Padua,A.A.H.;Majer,V.Green Chem.2006,8,172.doi:10.1039/B513231B
(35) Lide,D.R.Handbook of Chemistry and Physics,82nd ed.;CRC Press:Boca Raton,FL,2001-2002.
(36) Liu,Q.S.;Li,P.P.;Welz-Biermann,U.;Chen,J.;Liu,X.X. J.Chem.Thermodynamics 2013,66,88.doi:10.1016/j. jct.2013.06.008
(37) Cheng,Z.;Lee,J.M.J.Phys.Chem.B 2014,118,2712. doi:10.1021/jp411904w
(38)Zhang,Q.G.;Wei,Y.;Sun,S.S.;Wang,C.;Yang,M.;Liu,Q. S.;Gao,Y.A.J.Chem.Eng.Data 2012,57,2185.doi:10.1021/ je300153f
(39) Liu,Q.S.;Li,P.P.;Welz-Biermann,U.;Liu,X.X.;Chen,J. J.Chem.Eng.Data 2012,57,2999.doi:10.1021/je3004645
(40)Liu,Q.S.;Yang,M.;Yan,P.F.;Liu,X.M.;Tan,Z.C.;Welz-Biermann,U.J.Chem.Eng.Data 2010,55,4928.doi:10.1021/je100507n
(41)Fang,D.W.;Tong,J.;Guan,W.;Wang,H.;Yang,J.Z.J.Phys. Chem.B 2010,114,13808.doi:10.1021/jp107452q
(42)Fang,D.W.;Guan,W.;Tong,J.;Wang,Z.W.;Yang,J.Z. J.Phys.Chem.B 2008,112,7499.doi:10.1021/jp801269u
(43)Tong,J.;Song,B.;Wang,C.X.;Li,L.;Guan,W.;Fang,D.W.; Yang,J.Z.Ind.Eng.Chem.Res.2011,50,2418.doi:10.1021/ ie101903t
(44)Xu,W.G.;Ma,X.X.;Li,L.;Tong,J.;Guan,W.Ind.Eng. Chem.Res.2012,51,4105.doi:10.1021/ie201530b
(45) Liu,Q.S.;Tong,J.;Tan,Z.C.;Welz-Biermann,U.;Yang,J.Z. J.Chem.Eng.Data 2010,55,2586.doi:10.1021/je901035d
(46) Vila,J.;Ginés,P.;Pico,J.M.;Franjo,C.;Jiménez,E.;Varela,L. M.;Cabeza,O.Fluid Phase Equilibria 2006,242,141. doi:10.1016/j.uid.2006.01.022
(47)Yoshizawa,M.;Xu,W.;Angell,C.A.J.Am.Chem.Soc.2003, 125,15411.doi:10.1021/ja035783d
(48)Angell,C.A.;Byrne,N.;Belieres,J.P.Acc.Chem.Res.2007, 40,1228.doi:10.1021/ar7001842
(49) Xu,W.;Cooper,E.I.;Angell,C.A.J.Phys.Chem.B 2003,107, 6170.doi:10.1021/jp0275894
(50) MacFarlane,D.R.;Forsyth,M.;Izgorodina,E.I.;Abbott,A.P.; Annat,G.;Fraser,K.Phys.Chem.Chem.Phys.2009,11,4962. doi:10.1039/B900201D
(51) Belieres,J.P.;Angell,C.A.J.Phys.Chem.B 2007,111,4926. doi:10.1021/jp067589u
(52) Cheng,Z.;Lee,J.M.J.Phys.Chem.B 2014,118,2712. doi:10.1021/jp411904w
(53) Liu,Q.S.;Yan,P.F.;Yang,M.;Tan,Z.C.;Li,C.P.;Welz-Biermann,U.Acta Phys.-Chim.Sin.2011,27,2762.[刘青山,颜佩芳,杨 淼,谭志诚,李长平,Welz-Biermann,Urs.物理化学学报,2011,27,2762.]doi:10.3866/PKU.WHXB20112762
Density,Dynamic Viscosity and Electrical Conductivity of Two Hydrophobic Phosphonium Ionic Liquids
ZHENG Qi-Ge1LIU Hui2,3XIAQuan1LIU Qing-Shan1,4,*MOU Lin1,*
(1School of Science,Shenyang Agricultural University,Shenyang 110866,P.R.China;2Shanghai Environmental Sanitation Engineering Design Institute,Shanghai 200232,P.R.China;3Shanghai Engineering Research Center of Contaminated Sites Remediation,Shanghai 200232,P.R.China;4College of Land and Environment,Shenyang Agricultural University,Shenyang 110866,P.R.China)
Two air and water stable hydrophobic phosphonium ionic liquids(ILs),tributyl-hexylphosphonium tetrafluoroborate([P4446][BF4])and tributyl-hexylphosphonium bis(trifluoromethylsulfonyl)imide([P4446][NTf2]), were prepared by the traditional method.Their basic physico-chemical properties of density,dynamic viscosity, and electrical conductivity were measured in the temperature range of 283.15-353.15 K.The effect of the temperature and structure of the anion on the thermodynamic properties were discussed.The properties are compared with the cation structures changing of the phosphonium type ILs.The most important thermodynamic properties for their practical application,such as molecular volume,standard molar entropy,and lattice energy, were calculated from their density using empirical equations.The calculated values were compared with those of imdazolium and pyridinium type ILs.Molar electrical conductivity was determined from density and electrical conductivity.The applicability of the Vogel-Fulcher-Tamman(VFT)andArrhenius equations to the fitting of the dynamic viscosity and electrical conductivity was validated.The activation of the electrical conductivity anddynamic viscosity were obtained from the final VFT equation.According to the Walden rule,the density,dynamic viscosity,and electrical conductivity were described by the Walden equation.The results are very important for academic studies as well as industrial applications of these ILs.
Ionic liquids;Density;Dynamic viscosity;Electrical conductivity;Walden rule
O642
Glasser,L.Thermochim.Acta 2004,421,87.
10.1021/ je200830j
doi:10.3866/PKU.WHXB201612293
Received:November 10,2016;Revised:December 29,2016;Published online:December 29,2016.
*Corresponding authors.LIU Qing-Shan,Email:liuqingshan@dicp.ac.cn;Tel:+86-13478787524.MOU Lin,Email:myname-mulin@tom.com; Tel:+86-13840537205.
The project was supported by the Program for Liaoning Excellent Talents in University,China(LJQ2015099).辽宁省高等学校优秀人才支持计划(LJQ2015099)资助项目
