傣药毛叶三条筋化学成分研究
2017-05-12姜明辉卯明霞陆应彩彭霞
姜明辉+卯明霞+陆应彩+彭霞
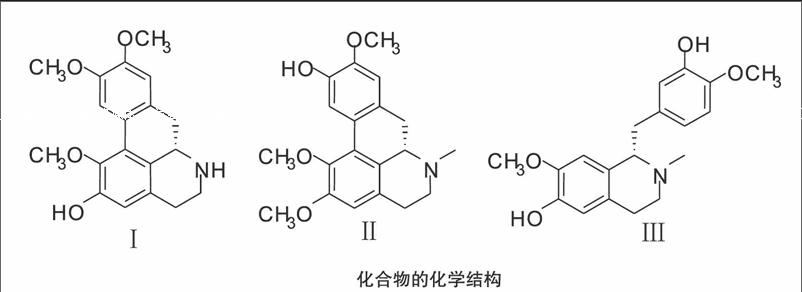
【摘要】目的:研究傣药毛叶三条筋中的化学成分。方法:利用各种色谱技术进行分离纯化,根据各种波谱数据和文献数据对照进行结构鉴定。结果:分离鉴定三个化合物,均为生物碱,分别为 Norpredicentrine(Ⅰ)、Lirioferine (Ⅱ)、Protosinomenine(Ⅲ)。结论:三个化合物均为首次从毛叶三条筋中分离得到。
【关键词】傣药毛叶三条筋;樟科;山胡椒属;异喹啉生物碱
【中图分类号】R2841【文献标志码】 A【文章编号】1007-8517(2017)08-0018-02
Abstract:Objective To study the chemical constituents from the Lindera caudata(Wall.)Bench. Method The compounds of the Lindera caudata(Wall.)Benchwere separated by multi-chromatography and their chemical structures were elucidated by chemical and spectral analysis. Result Three know compounds were isolated and identified as Norpredicentrine(Ⅰ)、Lirioferine (Ⅱ)、Protosinomenine(Ⅲ),respectevely.Conclusion All the compounds were isolated from the examined sample for the first time.
Keywords:Lindrera caudate;Lauracene;Isoquinoline Aldalieds
毛叶三条筋Lindera caudata(Wall)Bench为樟科(Lauraceae)山胡椒属(Lindera)植物,傣名为芽三英,为西双版纳州傣医常用的民族藥,具有除风通血,化瘀止痛,续筋接骨,止血生肌之攻效。用于骨折,跌打损伤,瘀血肿痛,外伤出血,风湿病肢体关节肿痛。研究对傣药毛叶三条筋进行化学成分的研究,从中分离到3异喹啉类生物碱个化合物:Norpredicentrine(Ⅰ)、Lirioferine (Ⅱ)、Protosinomenine(Ⅲ)。研究表明这类化合物在抗癌、抗疟、抗炎、治疗心血管疾病等方面具有明显的作用,是开发利用这类新药的重要资源[1]。
1仪器与材料
VG Autospec-3000Bruker 型质谱仪,AM-400、DRY-500 型核磁仪,柱色谱硅胶(100~200目,200~300目,300~400目),Sephadex LH-20。实验样品于2005年7月采自西双版纳州勐海县,经中国科学院西双版纳植物园陶国达鉴定为Lindera caudata(Wall)Bench的干燥叶。所用试剂均为分析纯。
2提取与分离
毛叶三条筋干燥品17 Kg,用90%乙醇提取三次,减压回收,得总提取物。用5%盐酸溶液调节pH至2,醋酸乙酯萃取,酸液用10%氢氧化钠溶液调节pH至10,用三氯甲烷萃取,回收萃取液。三氯甲烷部位300 g,硅胶柱色谱分离,以氯仿-甲醇梯度洗脱,合并斑点相同部位,分为A、B、C、D、E 5个部分,其中A部分减压浓缩后有难溶物存在,析出物反复重结晶,得到白色化合物Ⅰ(15mg), B、C、D、E部分经硅胶柱层析,制备薄层色谱反复纯化得到化合物Ⅰ(6 mg),化合物Ⅱ(10 mg),化合物Ⅲ(15 mg)。
3结构鉴定
化合物Ⅰ淡黄色粉末,EIMS:327[M]+,326(100%),312,296。1H-NMR(CDCl3):792(1H,s, 11-H),677(1H,s,8-H),667(1H,s,3-H,),390(6H,s,9,10-OCH3),361(3H,s,1-OCH3) ; 13C-NMR(CDCl3):1453(C-1),1285(C-1a),1267(C-1b),1520(C-2),1411(C-3),1292(C-3a),293(C-4),432(C-5),537(C-6a),368(C-7),1298(C-7a),1113(C-8),1442(C-9),1456(C-10),1109(C-11),1238(C-11a),559,560(9-OMe,10-OMe),601(C-OMe)。1H-NMR、13C-NMR谱数据与文献[3-4]报道的数据相符,鉴定该化合物为Norpredicentrine。
化合物Ⅱ灰色粉末,EIMS m/z(%):341(M+ ,81),326(49),310(25),267(28),298(20),283(21),154(24)。1H-NMR(CDCl3):804(1H,s,H-3),677(1H,s,H-3),656(1H,s,H-3),387(3H,s,OMe),385(3H,s,OMe),364(3H,s,OMe),251(3H,s,N-CH3);13C-NMR(CDCl3):1441(C-1),1270(C-1a),1272(C-16),1518(C-2),1141(C-3),1288(C-3a),291(C-4),532(C-5),625(C-6a),341(C-7),1300(C-7a),1101(C-8),1454(C-9),1451(C-10),1112(C-11),439(N-CH3),600(OMe,C-1),557,558(OMe)。1H-NMR、13C-NMR谱数据与文献[5]报道的数据相符,鉴定该化合物为Lirioferine。
化合物Ⅲ灰色粉末,EIMS:330[M+1]+。1H-NMR(CDCl3):674(1H,s,J=17),672(1H,d,J=82),656(1H,dd,J=82,17),653(1H,s ),628(2H,s),628(1H,s),382(3H,s,OMe),382(3H,s,OMe),249(3H,s,N-CH3)。13C-NMR(CDCl3):645(C-1),465(C-3),245(C-4),1290(C-4a),1158(C-5),1455(C-6),1436(C-7),1116(C-8),1244(C-8a),407(C-9),1324(C-1),1210(C-2),1138(C-3),1454(C-4),1452(C-5),558,558(OMe),249(N-CH3)。1H-NMR、13C-NMR谱数据与文献[6]报道的数据相符,鉴定该化合物为Protosinomenine。参考文献
[1]邵顺波.异喹啉类生物碱的生理活性及研究进展[J].安徽医药,2007,11(1):254 .
[2]Antonio G,Goniaiez,Josea,et al.4,5-dihydroblumenol A,A new nor-isoprenoid from perrottetia multiflora[J].Journal of Natural Products,1994,57(3):400-402.
[3]P.Rasoanaivo,S Ratsimamanga-Urverg,H.Rafatro,et al.Alkaloids of Hernandia voyronii:Chloropuine-Potentiating Activity and Structture Elucidation of Herveline D[J].Planta Medica,1998,64(1):58-62.
[4]Zhou Chan,Zhou Li- Xin,Ding Yi. To study the chemical constituents from the Lindera frlgrans[J].Chin Pharm J,2001,36 (3):185.
[5]Chen-Long Chen,Hou-min chang,ELLIS B.Cowling,et al.Aprophine alkaloids and Lignans formen in response to injury of sapwood is Lliriodendron tulipifer A[J].Phytochemistry,1976,15(6):1161-1167.
[6]CHOU Gui-Xin,NORIO Nakamura,MA Cao-Mei,et al.Isoquinoline alkaloids from Lindera aggregata[J].Chin J Nat Med,2005,3(5):272-275.
(收稿日期:2017-03-02编辑:梁志庆)
