溶剂热后处理对石墨相氮化碳光化学固氮产氨性能的影响
2017-05-10白金陈鑫奚兆毅王翔李强胡绍争
白金 陈鑫 奚兆毅 王翔 李强 胡绍争
(辽宁石油化工大学化学化工与环境学部,辽宁抚顺113001)
溶剂热后处理对石墨相氮化碳光化学固氮产氨性能的影响
白金 陈鑫 奚兆毅 王翔 李强 胡绍争*
(辽宁石油化工大学化学化工与环境学部,辽宁抚顺113001)
采用离子液体[Bm im]Br为溶剂,溶剂热后处理法制备了具有大比表面积和氮空穴的石墨相氮化碳催化剂。采用X射线衍射(XRD)光谱、扫描电镜(SEM)、氮气吸附、紫外-可见(UV-Vis)光谱、X射线光电子能谱(XPS)、荧光光谱(PL)、电子顺磁共振谱(EPR)、程序升温脱附(TPD)等分析手段对制备的催化剂进行了表征。结果表明经过溶剂热后处理的催化剂形貌由无规则的层状结构变为尺寸为30-40 nm的纳米颗粒,导致比表面积从8.6m2·g-1增加到37.9m2·g-1。从N2-TPD、荧光光谱及密度泛函理论(DFT)模拟计算的结果得出,氮空穴不仅能捕获光生电子促进电子空穴的有效分离,还能吸附并活化反应物氮气分子。溶剂热处理后,增加的比表面积导致更多的氮空穴作为反应活性位暴露在催化剂表面,是固氮活性显著提高。本文还探讨了可能的反应机理。
石墨相氮化碳;离子液体;[Bm im]Br;光催化固氮
1 Introduc tion
Nitrogen is a necessary element of human,animal and plant grow th.Although constituting~78%of the atmosphere,nitrogen, inmolecular form,isunusable tomostorganisms because of its strong nonpolar N≡N covalent triple bond.Thus,artificial nitrogen fixation is carried out through the Haber-Bosch process,in which hydrogen gas reactswith nitrogen gas to yield ammonia in the presence of catalysts under high pressure and temperature. Both theenergy consumption and raw material costs arehigh for this process.Therefore,artificialnitrogen fixation underm ilder conditionshasbeen a chem ical issueof greatsignificance,since the reduction in the inputenergy during the fixation processand no useof hydrogen from anaturalgasmay be preferred from the viewpoints of costand environmental preservation.This significance has directed many chemists tofind chem ical1,2,electrochem ical3,4and photochem ical routes5,6tofix nitrogen undermild conditions.
Nitrogen photofixation technology is considered to be a prom isingmethod to replace the traditional Haber-Bosch process. In 1977,Schrauzer etal.7first reported thatN2can be reduced to NH3over Fe doped TiO2under UV light.Since then,many Tibasedmetaloxidesand composite catalystwere reported8-12.Ranjit etal.8investigated thenitrogen photofixation activity of precious metalsmodified TiO2,and found thatRumodified TiO2exhibited thebestphotofixation performance.A linear relationship observed between the concentration of NH4+and the strength ofmetal-H bond.Rusina etal.9investigated the N2photofixation performance using Fe2Ti2O7as catalyst and ethanolas the hole-trapping agent. Hoshino et al.10,11prepared conducting polymer/TiO2hybrid material for nitrogen photofixation under white light.The main product is NH4ClO4.Zhao et al.12prepared Fe-doped TiO2nanoparticles with highly exposed(101)facets by two-step hydrothermalmethod.They found the quantum yields of nitrogen photofixation depend on the partial pressure of nitrogen in the reaction.However,because of the poor visible light absorption caused by thewideband gap energy,thenitrogen fixation ability of the Ti-basedmetal oxides and composite catalyst isstill low under visible light.Moreover,asmost photoexcited electrons tend to recombinewith their twinborn holes,rather than to be captured by the adsorbed N2,the interfacial charge transfer efficiency of these semiconductor photocatalysts is far from satisfactory13-15. Besides that,compared w ith the photocatalytic H2evolution and CO2reduction,photocatalytic N2fixation ismore challenging because the N2fixation is seriously hampered by thehigh-energy N2intermediates in the reduced or protonated form(N2-or N2H)16. These disadvantages lim it the developmentand practicaluse of photocatalytic N2fixation.Designing new photocatalysts is not only important but also a challenge in the promotion of the developmentof photocatalytic N2fixation.
Recently,graphitic carbon nitride(g-C3N4)has been w idely applied in a variety of fields,including photocatalysis17,18,fuel cells19,20,organic synthesis21,and gas storage22,23.The versatile application of g-C3N4is largely due to itsunique physicochemical properties,such asmoderate band gap energy,energy-storage capacity,special optical propertiesand gas-adsorption capacity. Ionic liquids(ILs),regarded asdesigner solvents,havebeen extensively investigated in recentyears.They show great prom ise in organic synthesis,catalysis,separation and polymerization24-26. Their favorable properties,such as thermal stability,negligible vapor pressure,high ionic conductivity andw ideelectrochemical w indow,make them attractive as reactionmedia and solvents.The combination of ionic liquidswith nanotechnology has led tomajor advances inmaterials science.Nanorods,nanospheres,nanotubes, and mesostructures of semiconductor materials have been synthesized using ILs assolvent,electrolyteand template27-30.More recently,ILshavealso been used forsynthesizing carbon nitridebased semiconductormaterials.Xu etal.31prepared graphite-like C3N4hybridizedα-Fe2O3(g-C3N4/α-Fe2O3)hollow microspheres. It is found that ionic liquid 1-butyl-3-methylimidazolium tetrachlorideferrate(III)[Bm im]FeCl4is supposed to have the triple rolesof reactant,dispersingmedia and template at the same time. Di etal.32prepared g-C3N4/BiOBr visible-light-driven photocatalystusing ionic liquid[C16m im]Br assolvent,reactant,template and dispersing agent at the same time.X iao et al.33reported an econom ical and facile hydrothermal approach to synthesize fluorescent carbon nitride dots(CNDs)derived from ionic liquids. The results suggest that the obtained CNDs are highly water soluble and exhibit a strong fluorescence.Li etal.34synthesized novelsphere-likeg-C3N4/BiOIcomposite photocatalystsby aonepot EG-assisted solvothermal process in the presence of reactable ionic liquid 1-butyl-3-methylimidazolium iodine([Bmim]I).The g-C3N4/BiOIcomposite displayed enhanced photocatalytic activity for degradation of Rhodamine B(RhB),methylblue(MB),methyl orange(MO),bisphenolA(BPA),and chlorophenol(4-CP).
Dong35and Li36etal.reported that the introduction of nitrogen vacancy into g-C3N4can chemisorb and activate N2molecules thus significantly improving thenitrogen photofixation ability.Hong etal.37found this nitrogen-deficientgraphitic carbon nitride(g-C3N4-x)can be prepared via hydrothermal treatment using ammonium thiosulfate asan oxidant.However,in their investigation, themore concentration of ammonium thiosulfate cannot introduce more nitrogen vacanciesas active sites37.In thiswork,based on the preparation method of Hong,we introduced ionic liquid [Bmim]Br into the solvothermalsystem.The resultsshow that the introduction of[Bmim]Br can producemorenitrogen vacancies in the g-C3N4lattice.Besides that,themorphology of the asprepared g-C3N4isalso changed,leading to themarkedly increased surface area.This increased surface area of as-prepared g-C3N4causes thatmore nitrogen vacancies,as the active sites,are exposed on the surface,leading to themarkedly promoted nitrogen photofixation ability.
2 Experim en tal
2.1 Preparation and characterization
All the chemicals used in thisexperimentwere reagentgrade and w ithout further treatment.[Bm im]Br is purchased fromJCNANO Tech Co.,Ltd.The pureg-C3N4wasprepared using urea as the precursor.10 g of ureawas calcined at550°C for 4 h w ith a ramp rate of 2°C·min-1.The productwasdenoted asG-CN.1 g of G-CN wasdispersed into 80mL ionic liquid[Bmim]Brunder vigorous stirring at50°C.10mL of ammonium thiosulfate solution(10 g·L-1)wasadded into above suspension under vigorous stirring.The formed suspension was transferred to a 100 m L Teflon-lined autoclave andmaintained at150°C for 20 h.The productwas washed w ith deionized water,dried at 80°C and denoted asATI-CN.For comparison,I-CNwasprepared following the same procedurementioned above in theabsenceof ammonium thiosulfate solution.When deionizedwaterwasused to replace the [Bmim]Br following the same procedureas synthesisof ATI-CN, the obtained productwas denoted as ATH-CN.H-CN w as prepared following the same procedureassynthesisof ATH-CN but in the absence of ammonium thiosulfate solution.
The XRD patterns of the prepared sampleswere recorded on a Rigaku D/max-2400 instrument(Shimadzu,Japan)using Cu-Kαradiation(λ=0.154 nm).The scan rate,step size,voltage and currentwere0.05(°)·min-1,0.01°,40 kV and 30mA,respectively. UV-Vis spectroscopywas carried outon a V-550model UV-Vis spectrophotometer(JASCO Japan)using BaSO4as the reflectance sample.Fourier transform infrared(FT-IR)spectrawere obtained on a FT-IR spectrometer(Nicolet20DXB,USA).Themorphologies of prepared catalystw ere observed by using a scanning electron m icroscope(SEM,JSM 5600LV,JEOL Ltd.,Japan). Nitrogen adsorptionwasmeasured at-196°C on aMicromeritics 2010 analyser(USA).All the sampleswere degassed at 393 K prior to themeasurement.The BET surface area(SBET)was calculated based on the adsorption isotherm.Electron paramagnetic resonance(EPR)spectrum wasmonitored using a digital X-band spectrometer(EMX-220,Bruker,USA)equipped w ith a Bruker ER 4121VT temperature controllerwithin the temperature range 113-273K.Inductively coupled plasma-massspectrometry(ICPMS)wasperformed on a Perkin-ElmerOptima3300DV apparatus (USA).The XPSmeasurementswere performed on a 250 XPS system w ith A l Kαradiation as the excitation source(Thermo Escalab,USA).The binding energies were calibrated by referencing the C 1s peak(284.6 eV)to reduce the sam ple charge effect.Temperature programmed desorption(TPD)studieswere performed using a CHEMBET-3000(Quantachrome,USA)instrument in the temperature range from 313 to 1073 K.The photoluminescence(PL)spectra were measured at room temperaturewith a fluorospectrophotometer(JASCO FP-6300,Japan) using a Xe lamp as theexcitation source.
Isotopic labeling experiments are carried out as follows.Labeled15N2gas was purchased from Sigma-A ldrich Chemical Company.In the experimentalprocess,Arwasused to eliminate air and the possible adsorbed ammonia in the reaction system. Then,15N2was passed through the reactionmixture for 30m in. After that,the reactorwas sealed.Other experiment conditions were the sameas those for14N2photofixation.Indophenolmethod was used to exam ine the produced15NH4+,ow ing to the low mass of15NH4+for liquid chromatograph-mass spectrometer(LC-MS) studies.The sample for LC-MSanalysiswas prepared as follows. 0.5m L of the reaction reacted with 0.1mL of 1%phenolic solution in 95%ethanol.Then,0.375mL of 1%NaClO solution and 0.5m L of 0.5%sodium nitroprusside solution w ere added into abovesolution.MSstudieswere carried on an Ultimate3000-TSQ (LCMS-ESI).
The DFT simulations were performed using the program package Dmol3.The substrate ismodelled by one layerof g-C3N4separated by a vacuum layer of 1.2 nm.A ll the atoms in the layer and the N2moleculeareallowed to relax.The Brillouin zonesof the supercellswere sampled by theGamma points.Based on the structures of g-C3N4,the g-C3N4surface with nitrogen atom vacancywasmodelled to study the N2adsorption properties.
2.2 Photocatalytic reaction
The nitrogen photofixation propertywas evaluated according to previous literature12.The nitrogen photofixation experiments were performed in a double-walled quartz reactor in air.For these experiments,0.2 g of photocatalystwasadded to a500mL 0.789 g·L-1ethanolasa hole scavenger12.The suspension was dispersed using an ultrasonicator for10min.During the photoreaction under visible light irradiation,the suspension was exposed to a250W high-pressure sodium lampwithmainemission in the rangeof 400 to 800 nm,and N2was bubbled at100mL·m in-1through the solution.The UV lightportion of the sodium lamp was filtered by a0.5mol·L-1NaNO2solution.All runswereconducted atambient pressureand 30°C.Atgiven time intervals,5m Laliquotsof the suspensionwere collected and immediately centrifuged to separate the liquid samples from the solid catalyst.The concentration of ammonia wasmeasured using the Nessler′s reagentspectrophotometrymethod(JB7478-87)with a UV-2450 spectrophotometer (Shimadzu,Japan)12,36.
3 Resu lts and discussion
The nitrogen photofixation performance over the as-prepared catalysts under visible light is shown in Fig.1(a).The control experiment results indicate thatno NH4+isgenerated in the absence of irradiation,N2or photocatalyst,indicating that nitrogen photofixation occurs via a photocatalytic process.G-CN shows theof 0.38mg·L-1·h-1·g-1.I-CN showsof 0.61mg·L-1·h-1·g-1,slighthigher than thatof GCN.W hen ammonium thiosulfate is added during the preparation process,theforATH-CN sharply promotes to6.4mg·L-1· h-1·g-1.TheorATI-CN further increases to10.4mg·L-1· h-1·g-1,with the turn overnumber(numberof productmolecules per catalystmolecule)of 0.96×10-2.This hints that the introduction of ionic liquid[Bm im]Br is beneficial to the nitrogen photofixation performanceof catalysts.The Fig.1(a)insert shows the photocatalytic stabilitiesof ATI-CN.No obvious decrease in nitrogen photofixation ability is observed after 20 h,hinting its good stability.
The N2photofixation ability of ANI-CN under15N isotope-labeled N2(purity>98%)was carried out tofurther investigate thenitrogen sourceof generatedreactswith phenolic and hypochlorite toform15N labeled indophenol,which wasanalyzed by LC-MS.A strong15N labeled indophenolanion mass spectroscopy signal presentsat199m/z in LC-MSstudies (Fig.1(b)).It isnoted that this signal intensity isobviously higher than the14N:15N naturalabundance ratio.This confirms thatN2is the nitrogen source of generated NH4+in this N2photofixation process.The change in the pH value of the ANI-CN suspension during thenitrogen photofixation process isanalyzed.Prior to the nitrogen photofixation process,the pH value of the suspension wasmeasured to be 6.2.However,Fig.1(c)shows that this pH value increases to 8.5 after24 h becauseof the consumption of H+during the nitrogen photofixation process,as shown in the following equations:
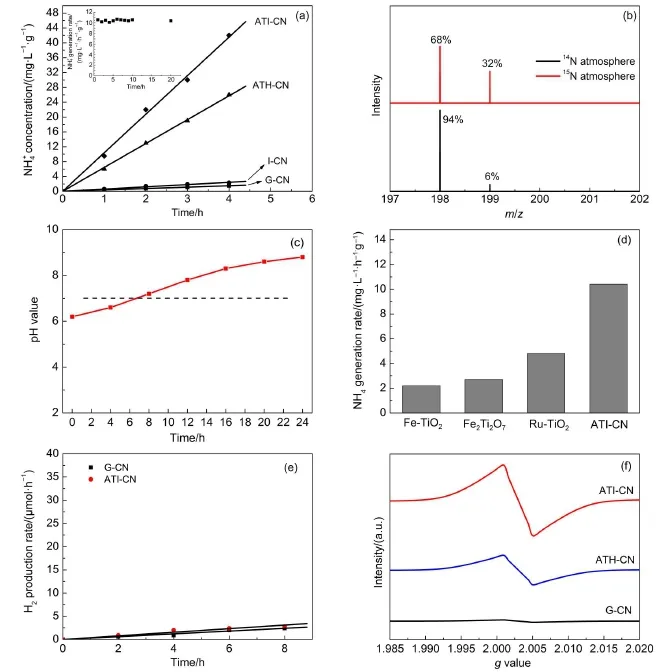
Fig.1production ability over as-prepared catalysts(a),m assspectra of the indophenol prepared from different atm osphere(b), pH value changeof ATI-CN suspension during thenitrogen photofixation process(c),com parison ofproduction rateof ATI-CN and Ti-based catalysts(d),H2production rateof G-CN and ATI-CN(e)and EPR spectra ofG-CN,ATH-CN and ATI-CN(f)


In order to compare thenitrogen photofixation abilitywith Tibased catalysts,the Fe-TiO2,Fe2Ti2O7and Ru-TiO2were prepared according to the previouswork8,9,12.The nitrogen photofixation abilitiesof prepared catalysts are shown in Fig.1(d).Obviously, Ru-TiO2showshighernitrogen photofixation ability than Fe-TiO2and Fe2Ti2O7,butmuch lower than ANI-CN.H2production is a possible com petitive reaction.Thus the photocatalytic H2production experiment is performed according to previouswork38. The result shown in Fig.1(e)indicates that the H2production abilitiesof as-prepared catalystsare very low(less than 1μmol· h-1).This isprobably due to theabsenceof a proper co-catalyst. EPR can provide direct information on monitoring various behaviors of native defects,such as oxygen and nitrogen vacancies39,40.Asshown in Fig.1(f),G-CN shows no peaks,suggestingthatno localized unpaired electronspresent in theG-CN.However, for ATH-CN and ATI-CN,a resonance signal at g=2.0031 is observed,which confirms the presenceof nitrogen vacancies.The stronger resonance signal for ATI-CN hints the higher nitrogen vacanciesconcentration compared w ith ATH-CN.
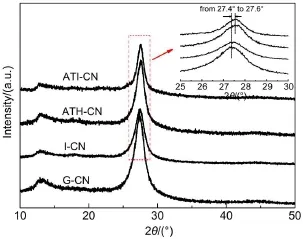
Fig.2 XRD patternsof as-prepared catalysts
The XRD patternsof as-prepared catalystsare shown in Fig.2. G-CN and I-CN show the typical characteristic peaksof g-C3N4located at13.1°and 27.5°.The peak at13.1°corresponds to the in-plane structuralpackingmotif of the tri-s-triazineunitsand is indexed as the(100)peak.The distance iscalculated to be d=0.67 nm.The peak at27.5°corresponds to the interlayer stacking of the aromatic segments,with a distanceof 0.326 nm,and is indexed as the(002)peak.It isnoted that,comparedw ith G-CN and I-CN, a0.2°shift to higher2θvalue isobserved forATH-CN and ATICN.This is probably due to the formation of some crystal lattice defects in g-C3N4when ammonium thiosulfatewasadded as an oxidant.The C/N ratios forboth G-CN and I-CN are0.73 obtained by elementalanalysis,close to the theoretical values.For ATHCN,the C/Nmolar ratio is 0.77.This value further increases to 0.82 forATI-CN.Combinew ith the XRD results,it is deduced that the crystal lattice defects in g-C3N4should be the nitrogen vacancies.Thehigher C/Nmolar ratioforATI-CN causes thehigher nitrogen vacancies concentration compared w ith ATH-CN,hinting that the introduction of[Bmim]Br into the solvothermalsystem is helpful for the formation of nitrogen vacancies in the g-C3N4lattice.The C/Nmolar ratiofor H-CN isalso 0.73,sameasG-CN, indicating H2O assolventcannot form the vacancy densities.
UV-Vis spectrum is used to investigate the light absorption property of as-prepared catalysts(Fig.3).g-C3N4shows typical semiconductor absorption,originating from charge transfer responseof g-C3N4from the valence band(VB)populated by N 2p orbital to the conduction band(CB)formed by C 2p orbital17.The obvious red shifts of absorption band are observed for ATH-CN and ATI-CN,indicating their band gap energies are decreased. Thishints that the presence of nitrogen vacancies could affect the electronic structure of g-C3N4,thus changes itsoptical property41. The band gap,estimated from themethod of Oregan42,decreases from 2.74 eV for G-CN and I-CN to 2.63 eV for ATH-CN and ATICN.

Fig.3 UV-Visspectra ofas-prepared catalysts
The FT-IR resultof[Bm im]Br,G-CN and ATI-CN are shown in Fig.4.ForG-CN,a series of peaks in the range from 1200 to 1600 cm-1are attributed to the typical stretchingmodes of CN heterocycles,while the sharp peak located at810 cm-1isassigned to thebending vibration of heptazine rings,which indicating the synthesized g-C3N4is composed of heptazine units.The broad absorption band around 3200 cm-1isoriginated from the stretching vibration of N―H bond,associated w ith uncondensed am inogroups43.ForATI-CN,all the characteristic vibrational peaksof g-C3N4areobserved,suggesting that the structure of g-C3N4isnot changed after post-treatment.No peak for[Bm im]Br isobserved in ATI-CN,indicating that[Bm im]Br is only used as solvent but notanchored on the surfaceof ATI-CN.
Themorphologiesof the representative sampleswereexamined by SEM analysis(Fig.5).The results in Fig.5(a)indicate thatGCN is composed of a largenumber of irregular particles.These particlesexhibita layered structure similar to thatof thegraphite analogue.In Fig.5(b),after hydrothermal treatment,the morphology changes from layered structure to bulk crystal.This morphological change is consistentw ith previouswork44.When [Bmim]Brwas introduced into the solvothermalsystem(Fig.5(c, d)),the morphology of I-CN and ATI-CN changes to the nanoparticleswith theuniform sizedistributionaround 30-40 nm. Thissmaller particle sizemay lead to the larger specific surface area.

Fig.4 FT-IR spectra of[Bm im]Br,G-CN and ATI-CN
To characterize the specific surfaceareaof as-prepared g-C3N4catalysts,thenitrogen adsorption and desorption isothermsweremeasured(Fig.6).The isotherm of ATI-CN isof classical type IV, suggesting the presence ofmesopores.The BET specific surface areas(SBET)of G-CN is 8.6m2·g-1,higher than thatof ATH-CN (7.2m2·g-1).ATI-CN and I-CN show much higher SBETthan that of G-CN,36.7 and 37.9m2·g-1.This is due to the decreased catalystparticle sizes,which is shown in SEM images.The large SBETcan not only providemore reactive sites but promote adsorption,desorption and diffusionof reactantsand products,which is favorable to the photocatalytic performance.The pore size distribution of ATI-CN is presented in Fig.6 insert.The pore distribution centered around 40-80 nm is observed in the BJH pore-size distribution curve,which should be formed by theaccumulation of secondary particles.

Fig.5 SEM imagesof G-CN(a),ATH-CN(b),I-CN(c)and ATI-CN(d)
XPSwas used to characterize the surface chem ical compositionsof theas-prepared g-C3N4-based catalysts.In Fig.7(a),two components located at284.6 and 287.8 eV for both catalysts.The sharp peak around 284.6 eV is attributed to the pure graphitic species in the CNmatrix.The peak with binding energy of 287.8 eV indicates the presenceof sp2C atomsbonded to aliphatic amine (―NH2or―NH―)in the aromatic rings45.In Fig.7(b)(N 1s region),the two contributionsof G-CN located at398.5 and 400.0 eV are assigned to the sp2-hybridized aromatic nitrogen atoms bonded to carbon atoms(C―N=C)and nitrogen atoms bonded to three carbonatoms(N―C3)in thearomatic rings46.ForATI-CN, no obvious difference in peak position isobserved.However,the peak area ratio of(N―C3)/(C―N=C)decreases from 0.327 for G-CN to 0.272 for ATI-CN,clearly indicating that nitrogen vacanciesare primarily located at the tertiary nitrogen lattice sites.
In order tofurther investigate the band structure of as-prepared catalysts,the VB XPspectrawereemployed(Fig.7(c)).The VB potentialsof G-CN,I-CN,ATH-CN and ATI-CN are calculated to be+1.71,+1.69,+1.67 and+1.73 eV,respectively.Combined with theUV-Vis results,theopticalCB potentialsof G-CN,I-CN,ATHCN and ATI-CN locate at-0.92,-0.94,-1.07 and-1.01 eV, respectively.This result indicates that the formation of nitrogenvacancies influences the band structureof as-prepared catalysts. It is reported that the standard redox potential for N2/NH3is-0.09 V(vs NHE)10.Themorenegative reduction potential causes the larger CB driving force.This CB driving force determ ines the m igration rate of photogenerated holesand electrons,causing the higher N2photofixation ability47.
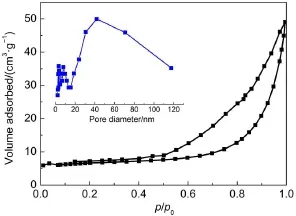
Fig.6 N2adsorption-desorption isotherm and poresize distribution of ATI-CN
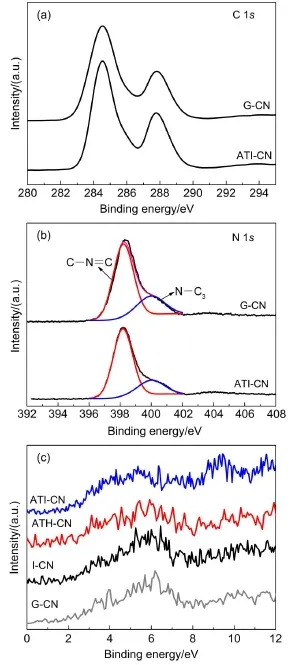
Fig.7 XPS spectra of G-CN and ATI-CN in the region ofC 1s(a), N 1s(b)and VBXPSof as-prepared catalysts(c)
Fig.8 shows the PL spectra of as-prepared catalysts under N2atmosphere.In general,ata lower PL intensity,the separation rate of the photogenerated electron-hole pairs ishigher.A broad PL band around 470 nm isobserved for all the catalysts.This isassigned to theband-band PL phenomenonwhich the lightenergy isequal to theband gap of g-C3N4.I-CN shows theslight lower PL intensity than G-CN.This is probably due to the decreased grain size of I-CN,causing a shortermigration distancewhich is beneficial to charge transfer from the bulk to the surface of the g-C3N4materialand leads toahigher separation rate.In the caseof ATHCN and ATI-CN,the PL intensities obviously decreased compared w ith G-CN and I-CN.This is due to the fact that the nitrogen vacancies formed by introducing ammonium thiosulfate could trap the photogenerated electrons,causing the increased separation rate.
Chemisorption is considered to be the essential step in heterogeneous catalysis because the chem ical adsorption sites are generally the reaction centers to activate reactantmolecules.N2-TPD was carried out to investigate the N2adsorption situation on the surface of G-CN,ATH-CN and ATI-CN(Fig.9).Obviously, two adsorbed N2species in ATH-CN and ATI-CN are observed. One peak at110-130°C isassigned to physicaladsorption.The otherpeak at320-360°C isattributed to the strong chemisorption species of N2molecule.In the case of G-CN,only physicaladsorbed N2species is observed.This result indicates thatnitrogen vacancies could actas chem ical adsorption sites to activate N2molecule for nitrogen photofixation.This is consistent with previous results35.It isnoted that,compared with ATH-CN,more N2species chem isorbson ATI-CN surface.This is due to themore nitrogen vacancies on ATH-CN which providesmore chemical adsorption sites.
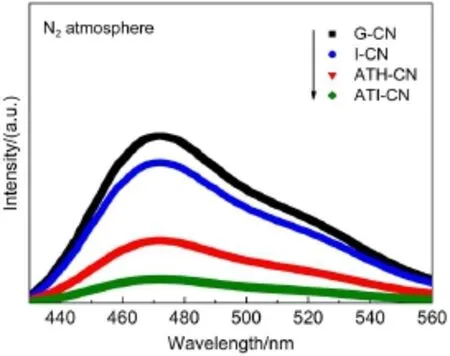
Fig.8 PL spectra ofas-prepared catalysts

Fig.9 N2-TPD of G-CN,ATH-CN and ATI-CN
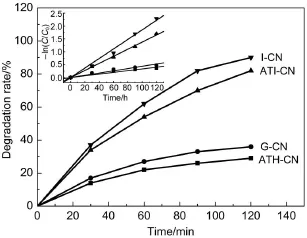
Fig.10 RhB degradation abilitiesover as-prepared catalysts under visible light
RhB degradation abilities over as-prepared catalysts under visible lightare shown in Fig.10.The reaction rate constant(k) wasobtained by assum ing that the reaction follow ed first-order kinetics(Fig.10 insert).The results indicate that the catalystwith higher surfacearea displayshigher RhB degradation rate.The rate constant forATI-CN is0.014min-1,which is3.3-fold greater than thatof G-CN.However,the NH4+generation rate forATI-CN increases 27.4-fold compared w ith G-CN,w hich ismuch higher than the increase of RhB degradation rate.It is deduced that the enhanced nitrogen photofixation ability is not only due to the increased SBETbutalso due to the formation ofnitrogen vacancies.
According to the XPS results,it is deduced that the nitrogen vacanciesare located at the three-coordinated nitrogen,as shown in Fig.11.Tofurther confirm thatN2isactivated by nitrogen vacancies,density functional theory(DFT)simulationswere employed to investigate the interaction between N2molecule and the g-C3N4with nitrogen vacancies(Fig.11).The calculation results show that theadsorption energy is-166.2 kJ·mol-1,confirm ing the chemisorption occurs.When the N2molecule adsorbs on the nitrogen vacancies,anσbond between the N2molecule and thenearestC atom is formed,causing the N≡N bond prolonged from 0.1107 to 0.1242 nm.This result confirms that the nitrogen vacancies can activate N2molecule.
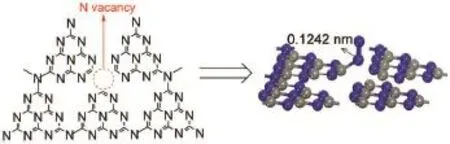
Fig.11 OptimalN2adsorptionmodelson nitrogen vacancies

Fig.12 Possiblenitrogen photofixation processover g-C3N4w ith nitrogen vacancies
In summary,the possiblenitrogen photofixation process over g-C3N4with nitrogen vacancies isshown in Fig.12.Firstof all,N2species chem isorbs on the nitrogen vacancies.Under visible light irradiation,photogenerated electron-hole pairsare formed(step 1). The photogenerated electrons are trapped by the nitrogen vacanciesand immediately transferred to theadsorbed N2molecule (step 2).Because thebonding orbitalsof N2moleculeareoccupied by four electrons,this photogenerated-electron has to occupy the anti-bonding orbitals,leading to thenitrogen activation(step 3). Theactivated N2molecule reactsw ith H+in water toform NH3, and then it finally forms
4 Conc lusions
By introducing ionic liquid[Bmim]Br as solvent into the solvothermal post-treatment,graphitic carbon nitride w ith larger surface area andmore nitrogen vacancies is synthesized in this work.Thesenitrogen vacanciesnotonly trap the photogenerated electrons to promote the separation rate,butserveasactive sites to adsorb and activate N2molecules.The adsorption energy is-166.2 kJ·mol-1when N2molecule interacts w ith nitrogen vacancy sites.The N≡N bond isprolonged from 0.1107 to 0.1242 nm,confirm ing thatnitrogen vacancy can activate N2molecule. Thisincreased surfaceareaof as-prepared g-C3N4causes thatmore nitrogen vacancies,as theactive sites,areexposed on the surface, leading to themarkedly promoted nitrogen photofixation ability. Under visible light,thegeneration rate of ANI-CN reaches 10.4mgwhich is27-fold higher than thatofG-CN.
(1)Vol′pin,M.E.;Shur,V.B.;Berkovich,E.G.Inorg.Chim.Acta 1998,280,264.doi:10.1016/S0020-1693(98)00206-0
(3)Tamelen,E.E.;Akermark,B.J.Am.Chem.Soc.1968,90,4492. doi:10.1021/ja01018a074
(4)Dickson,C.R.;Nozik,A.J.J.Am.Chem.Soc.1978,100,8007. doi:10.1021/ja00493a039
(5)Hu,S.Z.;Li,Y.M.;Li,F.Y.;Fan,Z.P.;Ma,H.F.;Li,W.; Kang,X.X.ACS.Sus.Chem.Eng.2016,4,2269.doi:10.1021/ acssuschemeng.5b01742
(6)Ranjit,K.T.;Viswanathan,B.Indian J.Chem.Sect.A 1996,35, 443.
(7)Schrauzer,G.N.;Guth,T.D.J.Am.Chem.Soc.1977,99,7189. doi:10.1021/ja00464a015
(8)Ranjit,K.T.;Varadarajan,T.K.;Viswanathan,B.J.Photochem. Photobiol.A:Chem.1996,96,181.doi:10.1016/1010-6030(95) 04290-3
(9)Rusina,O.;Linnik,O.;Eremenko,A.;Kisch,H.Chem.Eur.J. 2003,9,561.doi:10.1002/chem.200390059
(10)Hoshino,K.Chem.Eur.J.2001,7,2727.doi:10.1002/1521-3765(20010702)7:13<2727::AID-CHEM 2727>3.0.CO;2-4
(11)Hoshino,K.;Inui,M.;Kitamura,T.;Kokado,H.Angew.Chem. Int.Ed.2000,39,2509.doi:10.1002/1521-3773(20000717)39: 14<2509::AID-ANIE2509>3.0.CO;2-I
(12)Zhao,W.R.;Zhang,J.;Zhu,X.;Zhang,M.;Tang,J.;Tan,M.; Wang,Y.Appl.Catal.B:Environ.2014,144,468.doi:10.1016/j. apcatb.2013.07.047
(13)Liang,Y.T.;Vijayan,B.K.;Gray,K.A.;Hersam,M.C.Nano Lett.2011,11,2865.doi:10.1021/nl2012906
(14)Walter,M.G.;Warren,E.L.;M cKone,J.R.;Boettcher,S.W.; M i,Q.;Santori,E.A.;Lew is,N.S.Chem.Rev.2010,110,6446. doi:10.1021/cr1002326
(15)Britto,P.J.;Santhanam,K.S.V.;Rubio,A.;Alonso,J.A.; A jayan,P.M.Adv.Mater.1999,11,154.doi:10.1002/(SICI) 1521-4095(199902)11:2<154::AID-ADMA154>3.0.CO;2-B
(16)Zhu,D.;Zhang,L.;Ruther,R.E.;Ruther,R.J.Nat.Mater. 2013,12,836.doi:10.1038/nmat3696
(17)Wang,X.C.;Maeda,K.;Thomas,A.;Takanabe,K.;Xin,G.; Carlsson,J.M.;Domen,K.;Antonietti,M.Nat.Mater.2009,8, 76.doi:10.1038/nmat2317
(18)Zhao,Z.W.;Sun,Y.J.;Dong,F.Nanoscale 2015,7,15. doi:10.1039/c4nr03008g
(19)Zheng,Y.;Liu,J.;Liang,J.;Jaroniec,M.;Qiao,S.Energy Environ.Sci.2012,5,6717.doi:10.1039/c2ee03479d
(20)Zheng,Y.;Jiao,Y.;Chen,J.;Liu,J.;Liang,J.;Du,A.;Zhang, W.;Zhu,Z.;Jaroniec,M.;Sm ith,S.C.;Lu,G.;Qiao,S.J.Am. Chem.Soc.2011,133,20116.doi:10.1021/ja209206c
(21)Xu,J.;Wu,H.T.;Wang,X.;Xue,B.;Li,Y.X.;Cao,Y.Phys.Chem.Chem.Phys.2013,15,4510.doi:10.1039/c3cp44402c
(22)Li,Q.;Yang,J.;Feng,D.;W u,Z.;Wu,Q.;Park,S.S.;Ha,C.S.; Zhao,D.Nano Res.2010,3,632.doi:10.1007/s12274-010-0023-7
(23)Park,S.S.;Chu,S.W.;Xue,C.;Zhao,D.;Ha,C.S.Mater.J. Chem.2011,21,10801.doi:10.1039/c1jm10849b
(24)Welton,T.Chem.Rev.1999,99,2071.doi:10.1021/cr980032t
(25)Keim,W.Angew.Chem.Int.Ed.2000,39,3772.doi:10.1002/ 1521-3773(20001103)39:21<3772::AID-ANIE3772>3.0.CO;2-5
(26)Kubisa,P.Prog.Polym.Sci.2004,29,3.doi:10.1016/j. progpolymsci.2003.10.002
(27)Paramasivam,I.;Macak,J.M.;Selvam,T.;Schmuki,P. Electrochim.Acta 2008,54,643.doi:10.1016/j. electacta.2008.07.031
(28)Yoo,K.S.;Lee,T.G.;Kim,J.MicroporousMesoporousMat. 2005,84,211.doi:10.1016/j.m icromeso.2005.05.029
(29)Ding,K.L.;Miao,Z.J.;Liu,Z.M.;Zhang,Z.F.;Han,B.X.; An,G.M.;M iao,S.D.;Xie,Y.J.Am.Chem.Soc.2007,129, 6362.doi:10.1021/ja070809c
(30)Liu,Y.;Li,J.;Wang,M.J.;Li,Z.Y.;Liu,H.T.;He,P.;Yang,X. R.;Li,J.H.Cryst.Growth Des.2005,5,1643.doi:10.1021/ cg050017z
(31)Xu,L.;Xia,J.X.;Xu,H.;Yin,S.;Wang,K.;Huang,L.Y.; Wang,L.G.;Li,H.M.J.PowerSources2014,245,866. doi:10.1016/j.jpow sour.2013.07.014
(32)Di,J.;Xia,J.X.;Yin,S.;Xu,H.;Xu,L.;He,M.Q.;Li,H.M.; Xu,L.;Jiang,Y.P.RSCAdv.2013,3,19624.doi:10.1039/ c3ra42269k
(33)Xiao,D.L.;Li,S.Q.;Liu,S.B.;He,H.;Lu,J.R.New J.Chem. 2016,40,320.doi:10.1039/c5nj01717c
(34)Di,J.;Xia,J.X.;Yin,S.;Xu,H.;Xu,L.;Xu,Y.G.;He,M.Q.; Li,H.M.J.Mater.Chem.A.2014,2,5340.doi:10.1039/ c3ta14617k
(35)Dong,G.H.;Ho,W.K.;Wang,C.Y.J.Mater.Chem.A 2015,3, 23435.doi:10.1039/c5ta06540b
(36)Li,H.;Shang,J.;Ai,Z.H.;Zhang,L.Z.J.Am.Chem.Soc. 2015,137,6393.doi:10.1021/jacs.5b03105
(37)Hong,Z.H.;Shen,B.;Chen,Y.L.;Lin,B.Z.;Gao,B.F. J.Mater.Chem.A 2013,1,11754.doi:10.1039/c3ta12332d
(38)Hu,S.Z.;Li,F.Y.;Fan,Z.P.;Gui,J.Z.J.Power Sources 2014, 250,30.doi:10.1016/j.jpowsour.2013.10.132
(39)Yang,R.C.;Lu,X.J.;Huang,X.;Chen,Z.M.;Zhang,X.;Xu, M.D.;Song,Q.W.;Zhu,L.T.Appl.Catal.B:Environ.2015, 170-171,225.doi:10.1016/j.apcatb.2015.01.046
(40)Wang,Z.H.;M a,W.H.;Chen,C.C.;Ji,H.W.;Zhao,J.C. Chem.Eng.J.2011,170,353.doi:10.1016/j.cej.2010.12.002
(41)Wang,X.C.;Chen,X.F.;Thomas,A.;Fu,X.Z.;Antonietti,M. Adv.Mater.2009,21,1609.doi:10.1002/adma.200802627
(42)Oregan,B.;Gratzel,M.Nature1991,353,737.doi:10.1038/ 353737ao
(43)Yan,S.C.;Li,Z.S.;Zou,Z.G.Langmuir2009,25,10397. doi:10.1021/la900923z
(44)Hu,S.Z.;Ma,L.;Xie,Y.;Li,F.Y.;Fan,Z.P.;Wang,F.;Wang, Q.;Wang,Y.J.;Kang,X.X.;Wu,G.Dalton Trans.2015,44, 20889.doi:10.1039/c5dt04035c
(45)Lei,W.;Portehault,D.;Dimova,R.;Antoniettit,M.J.Am. Chem.Soc.2011,133,7121.doi:10.1021/ja200838c
(46)Zhang,Y.W.;Liu,J.H.;Wu,G.;Chen,W.Nanoscale 2012,4, 5300.doi:10.1039/c2nr30948c
(47)Hu,S.Z.;Ma,L.;Li,F.Y.;Fan,Z.P.;Wang,Q.;Bai,J.;Kang, X.X.;Wu,G.RSCAdv.2015,5,90750.doi:10.1039/ c5ra15611d
Influence of Solvothermal Post-Treatment on Photochemical Nitrogen Conversion to Ammonia with g-C3N4Catalyst
BAIJin CHEN Xin XIZhao-Yi WANG Xiang LIQiang HU Shao-Zheng*
(College ofChemistry,Chemical Engineering,and Environmental Engineering,Liaoning Shihua University, Fushun 113001,Liaoning Province,P.R.China)
In this work,g raphitic ca rbon nitride(g-C3N4)w ith la rge surface area and many nitrogen vacancies was synthesized by introducing ionic liquid[Bm im]Bras a solvent into the so lvotherma lpost-treatment.X-ray diffraction(XRD),N2adsorption,scanning electronm icroscopy(SEM),UV-Vis spectroscopy,X-ray photoelectron spectroscopy(XPS),electron paramagnetic resonance(EPR),tem perature-programmed desorption ofN2(N2-TPD),and photo lum inescence(PL)spectroscopy were used to characterize the p repared catalysts.The m orpho logy of the as-prepa red g-C3N4was m arked ly changed from an o rde rless laye red structure to nanoparticleswith a uniform size distribution ofaround 30-40 nm a fter the introduction of[Bm im]Br,leading an increase in surface area from 8.6 to 37.9m2·g-1.N2-TPD,photolum inescence spectra,and density functional theory(DFT)simulations indicated that the nitrogen vacancies notonly trapped the photogenerated e lectrons to enhance their separation rate,butalso served as active sites for the adsorption and activation ofN2molecules. The inc reased surface a rea of the as-prepared g-C3N4meant thatm ore nitrogen vacancies w ere exposed on the surface,leading to amarkedly promoted nitrogen photofixation ability.The possib le reactionmechanism is p roposed.
Graphitic carbon nitride;Ionic liquid;[Bm im]Br;Nitrogen photofixation
O643
eigh,G.J.Science1998,279,506.
10.1126/ science.279.5350.506
doi:10.3866/PKU.WHXB201611102
www.whxb.pku.edu.cn
Received:September 23,2016;Revised:November 10,2016;Published online:November 10,2016.
*Corresponding author.Email:hushaozhenglnpu@163.com;Tel:+86-13470570415.
Theprojectwas supported by theNationalNatural Science Foundation of China(41571464),Education Departmentof Liaoning Province,China (L2014145),and Natural Science Foundation of Liaoning Province,China(201602467).
国家自然科学基金(41571464),辽宁省教育厅项目(L2014145)及辽宁省自然科学基金(201602467)资助©Editorialofficeof Acta Physico-Chim ica Sinica
