Study on the Modifcation of Vacuum Residue by Ultrasonic Radiation
2017-05-09PelayoEnvoEsonoMayeYangJingyiYanTaoyanXuXinru
Pelayo Envo Esono Maye; Yang Jingyi; Yan Taoyan; Xu Xinru
(State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
Study on the Modifcation of Vacuum Residue by Ultrasonic Radiation
Pelayo Envo Esono Maye; Yang Jingyi; Yan Taoyan; Xu Xinru
(State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
The present study examined the effects of ultrasonic radiation on the properties, structural parameters and fuidized catalytic cracking (FCC) performance of vacuum residue. We found that ultrasonic radiation markedly decreased the viscosity, carbon residue and average molecular weight, but slightly affected the density of vacuum residue. Besides, chromatographic analyses of SARA fractions revealed that asphaltene, resin and aromatic components were reduced, while saturates increased after ultrasonic radiation. Furthermore, FT-IR,1H-NМR, elemental analysis and VPO analysis indicated that the structural unit number (n), unit structure weights (usw), carbon fraction in aromatic structure (fA), naphthenic structure (fN) and naphthenic rings (RN) were decreased while the carbon fraction in paraffnic structures (fP) was increased. FCC showed an increased conversion rate (by 2.7%) and gasoline yield (by 3.7%). In sum, the ultrasonic radiation may facilitate and improve the secondary processing of vacuum residue.
ultrasonic; vacuum residue; carbon residue; FCC
1 Introduction
Since the energy consumption demand has soared in recent years and conventional petroleum resource is limited, more concerns have been focusing on the processing of residue oil, which is a byproduct of heavy oil processing[1]. However, problems regarding carbon deposit, catalyst pores plugging, high content of carbon residue, metals in the residue oil and the asphaltenes deposition as well as catalyst deactivation remain unresolved[2-3].
In recent years, studies on the improvement of the properties of heavy oil by ultrasound waves have garnered growing attention[4-9]. Ultrasound radiation, which is characterized by strong directivity and penetrability, is a kind of sound wave with a frequency of above 20 kHz. Ultrasound radiation can affect liquid through the mechanics, thermotics and ultrasonic cavitation effects. The performance of mechanics modification can result in medium expansion and contraction while thermotics performance can increase the temperature by transforming vibrational energy into thermal energy during ultrasonic propagation[10-12].
Apart from these consequences, research on the ultrasonic radiation effect on crude oil has grown in signifcance[4-5]. Mohammadian[6]has adopted the demulsifying fow test and displacement experiments to study the mechanism of the extraction rate for enhancement by ultrasound.
Check[7]by applying ultrasonic waves to desalt and dewater crude oil had demonstrated that ultrasound can effectively remove water and soluble salt. Other researchers have undertaken micro-and thermodynamic studies on the influence of ultrasonic wave on the rheological properties of crude oil[8-11]. They have found that ultrasound can reduce the size of asphaltene aggregates and the viscosity of crude oil. Research done by Mousavi[13]has proven that the change of rheological properties of crude oil is caused by ultrasound. Subsequently, researchers have further elaborated on the fact that ultrasonic treatment can change the surface and size of the particles, and may increase the stability of the dispersion system[14-16].
Мoreover, it has been reported that the frequency and power of ultrasound under different controlled temperatureconditions could potentially decrease oil viscosity and this ultrasound method can reduce the asphaltene content through dehydrogenation and cracking[17-18]. Residue oils are highly complex systems[19-20], and those with high carbon residue will exert serious infuence on the catalyst activity resulting in catalyst deactivation[21]. Although the application of ultrasonic radiation on crude oil has been widely studied, the effects of ultrasonic radiation on structural parameters of oil still remain unclear. This work aims to investigate the effect of ultrasonic radiation on the physical properties, the structural changes and the FCC performance of vacuum residue.
2 Experimental
2.1 Materials
Experiments have been carried out using vacuum residue delivered from the PetroChina Urumqi Petrochemical Company. Aluminum oxide neutral (FCP) was purchased from the Sinopharm Chemical Reagent Co., Ltd. Petroleum ether (AR), xylene (AR), toluene (AR), normal heptane (AR) and ethanol (AR) were purchased from the Shanghai Lingfeng Reagent Co., Ltd. All these reagents were of analytical reagent grade.
2.2 Ultrasonic experimental setup and procedure
Schematic representation of the ultrasonic experimental setup used in the study is given in Figure 1. In this set of experiments, the upgrading of vacuum residue was done by an ultrasonic horn reactor. The technical parameters of the reactor are as follows: frequency: 28±2 and 40±3 kHz; power range 200—1 200 W; temperature range: 30~90oC and system voltage range: 220 V±10%.
A definite amount of vacuum residue (150 mL) was placed in a 250 mL beaker and exposed directly to ultrasonic radiation for 7 min. After ultrasonic radiation, the oil samples were cooled down to an ambient temperature of 25 ºC. After all programs were completed, the properties of vacuum residue were measured.
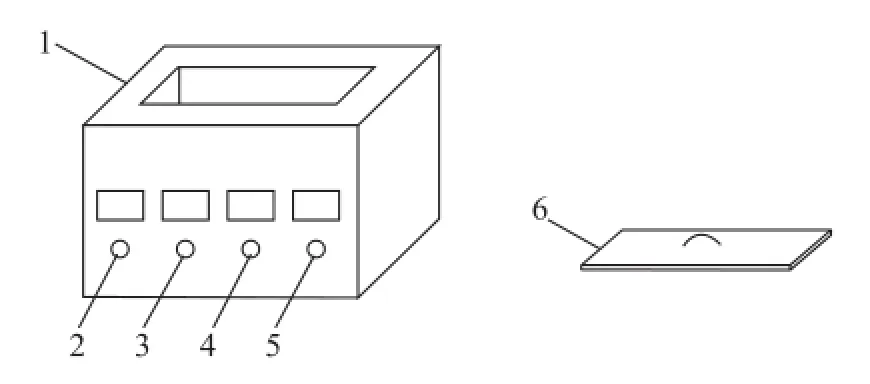
Figure 1 Experimental setup of the ultrasound reactor system1— Ultrasonic in strument; 2—Time; 3—Temperature; 4—Power; 5—Frequency; 6—Insulated cover
2.3 Characterization of the vacuum residue sample
The experiment was carried at 40 kHz and 800 W at 70oC for 7 min with the purpose of investigating the effects of ultrasonic radiation on the properties of oil residues. The vacuum residue was characterized according to the standards of the American Society for Testing and Мaterials (ASTМ). A primary characterization was conducted to determine the density (ASTM D5002-99), kinematic viscosity (ASTМ D-445), and carbon residue (ASTМ D189-06e1), while the contents of SARA fractions in vacuum residue were determined using column chromatographic separation technology according to ASTМ D4124-01 method.
2.4 Analysis of the structure of vacuum residue
The vacuum residue was analyzed by a Nicolet Magna 550 FT-IR instrument according to the manufacturer instructions. The detection wave number ranged between 500 cm-1and 4 000 cm-1with an accuracy of 2 cm-1.
1H-NMR analysis of vacuum residue was conducted with an Advanced 500 superconducting Fourier transform NМR spectrometer (Bruker, Switzerland) to determine the ratio of various H in the component.
A BCH-1 semi-automatic hydrocarbon meter (Jiangsu Jiangfen Electro Analytical Instrument Co., Ltd), KDN-C azotometer (Shanghai Qianjian Instrument Co., Ltd) and CLS-2 coulomb sulfurmeter (Jiangsu Jiangfen Electro Analytical Instrument Co., Ltd) were used to determine the content of C, H, N, and S of the samples according to the ASTМ D6784 method.
The relative molecular mass of the samples was determined at 60oC according to the VPO method in toluene using a Knauer-7000 vapor pressure osmometer. The improved Brown-Ladner method (B-L method) was used to calculate the average molecular structural parameters[22].
2.5 FCC Evaluation
The FCC reaction evaluation was performed using a fxedfluidized bed. This experimental apparatus is described in Figure 2 with a weight hourly space velocity of 10 h-1, when the raw material was mixed with the atomized water vapor. After preheating, the mixture was placed into the reactor where it reacted over the FCC catalyst (MLC-500-13) provided by the Sinopec Research Institute of Petroleum Processing.
After reaction, both products and stripped products were subject to condensation and were cooled to collect FCC off-gas and condensed fuid, respectively, followed by further oil-water separation. We used a simulated distillation to get the mass fraction of gasoline, diesel, and heavy oil fraction from liquid products. Next, we determined the yields of gasoline, diesel, and heavy oil according to the quality of the liquid product. Following the regeneration of the catalyst covered with carbon by O2, we measured the amount of smoke and the yield of coke was obtained by analyzing CO and CO2via chromatography.
The fractions of the FCC off-gas were analyzed by a HP-6890 refinery gas analyzer using a multi-dimensional gas chromatography technique. The analysis of fractions of liquid products was performed on a HP5880A gas chromatograph. The chromatographic column (3.17 mm ×508mm) was provided with a stainless steel packed column, the fxed phase of which was composed of 10% of UCW-982, and the supporter was made of (0.25—0.32) mm Chromosorb P-AW, coupled with a FID detector, while the temperature programmed parameters covered an initial temperature of 35oC and a fnal temperature of 360oC, with a heating rate of 10oC/min.
The balance computation of the content of the desired boiling point products was conducted to calculate the yield of every product obtained during VR conversion:
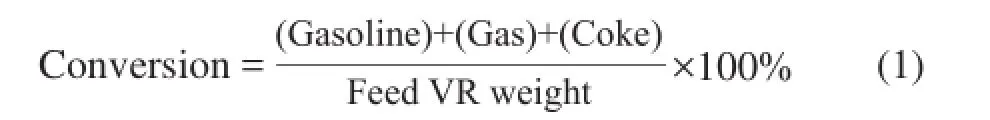
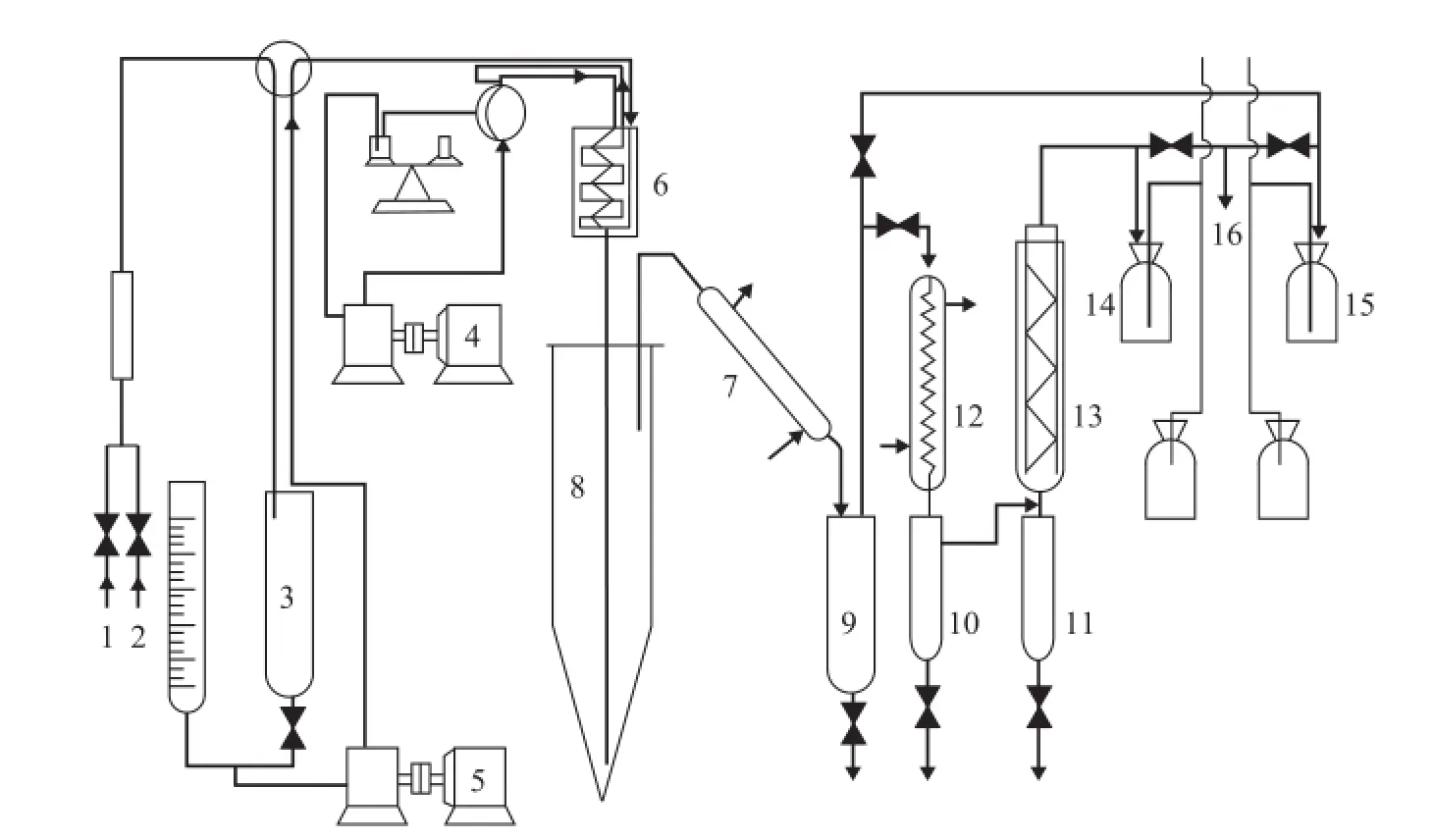
Figure 2 Schematic diagram of the technical pilot scale riser FCC apparatus1—O2; 2— Compressed air; 3—Distilled water; 4—Feedstock pump; 5—Water pump; 6—Heater; 7—Water cooler;8—Reactor; 9,10,11— Oil collector; 12—Water cooler; 13—Air cooler; 14—FCC off-gas; 15—Smoke; 16—Sampling site
3 Results and Discussion

Figure 3 Effect of ultrasonic radiation temperature on carbon residue content
3.1 Determination of ultrasonic radiation conditionsCarbon residue is a major indicator of carbon deposits formed in oil processing which are mainly derived from compounds with condensed ring and heterocyclicstructure in resins and asphaltenes. Therefore, the change in the resin and asphaltene content and the structure of oil after ultrasonic treatment will affect the content of carbon residue and processing performance of oil.
The effects of the ultrasonic radiation temperature, time and the ultrasonic power on the content of carbon residue were studied. The effect of ultrasonic radiation temperature on the content of carbon residue in vacuum residue sample was investigated under the conditions covering an ultrasonic power of 800 W and a treating time of 7 min.
With the radiation temperature increasing from 30oC to 70oC, the content of carbon residue in vacuum residue decreased. When the temperature stepped up to 70oC the carbon residue tended to level off. The results displayed that the radiation temperature of 70oC was a suitable temperature for ultrasonic radiation of vacuum residue oil (Figure 3). The percentage variation of carbon residue content in vacuum residue with an increasing ultrasonic radiation temperature at a constant power input (800 W) is shown in Figure 3.
It can be seen from Figure 4 that the carbon residue content decreased with an increasing radiation time. Carbon residue reduction has occurred very remarkably at the beginning of the ultrasound radiation and became constant after 7 min of radiation time. The optimized time for reduction of carbon residue has been specified at 7 min under the experimental conditions.
Figure 5 shows the change of carbon residue content with the increase in ultrasonic radiation power at 70oC for 7 min. The results showed that the content of carbon residue decreased significantly with an increasing ultrasonic radiation power. The optimized power for maximum reduction of carbon residue was observed at 800 W under the experimental conditions. Ultimately, the optimal processing conditions for ultrasonic radiation covered:a temperature of 70oC, a radiation time of 7 min, and a power of 800 W.
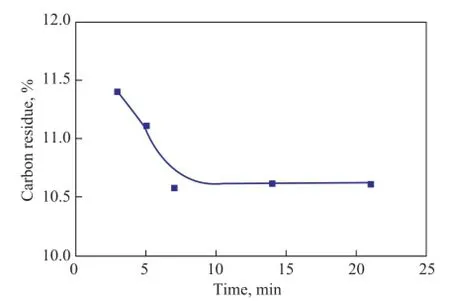
Figure 4 Effect of ultrasonic radiation time on carbon residue content
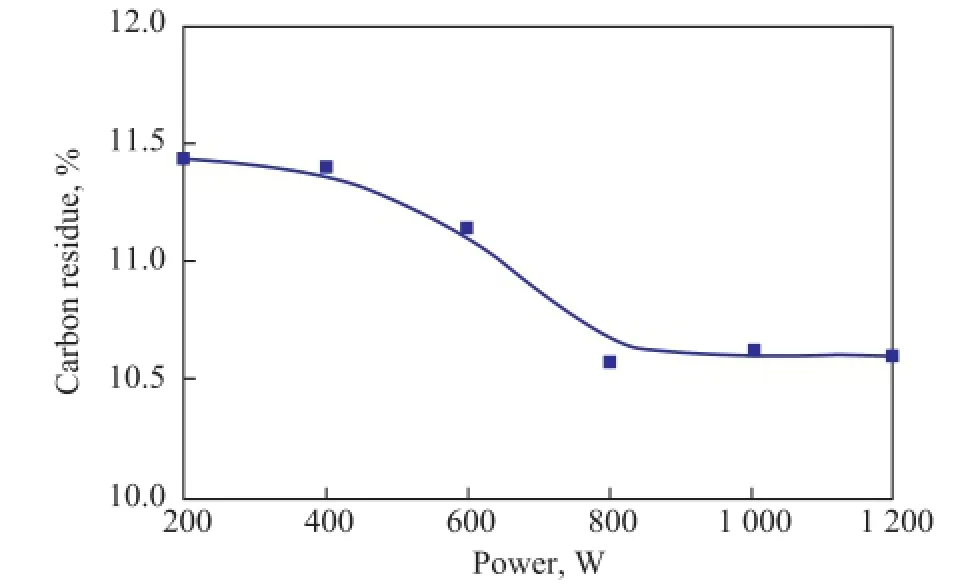
Figure 5 Effect of ultrasonic radiation power on carbon residue content
3.2 Influence of ultrasonic on the Properties of vacuum residue
The experiment was carried out at 40 kHz and 800 W at 70oC for 7 min with the purpose of investigating the effects of ultrasonic radiation on the properties of oil residue. As shown in Table 1, the viscosity of vacuum residue from 199 mPa.s decreased to 152 mPa.s, which was a reduction of 23.6%. The carbon residue value of vacuum residue decreased from 11.44% to 10.58%, which was a decrease of 7.75%. This result confirmed that ultrasound radiation can effectively reduce the viscosity and carbon residue content.
The viscosity, a measure of oil’s internal resistance to fow and shear, is the sum of the contribution of dispersion medium and dispersion phase. It is related to the particle shape, size and interaction of dispersion phase, and the interaction between dispersion medium and dispersion phase[17,23].
The viscosity of vacuum residue is usually affected by several factors, such as the number and size of branched chains, the number of rings , the content and structure of asphaltenes[25]. The latter two factors might matter most. For example, a decreasing total number of rings and the degree of association between asphaltenes and resins did reduce the complexity of asphaltene, which furthercontributed to the change of viscosity.
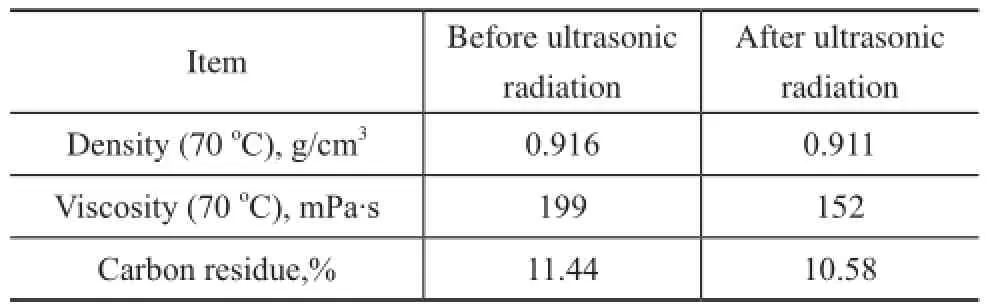
Table 1 Properties of vacuum residue before and after ultrasound radiation
3.3 Effect of ultrasonic radiation on the SARA fraction of vacuum residue
In order to confrm that ultrasonic radiation could change the content of carbon residue in vacuum residue, the contents of SARA fractions in the vacuum residue sample before and after ultrasonic radiation were measured for 7 min under the circumstances covering a temperature of 70oC and a power of 800 W.
It is shown in Table 2 that the contents of asphaltene, resin, and aromatics in the vacuum residue sample decreased by 16.4%, 12.4% and 4.8%, respectively, after ultrasonic radiation, while the content of saturates increased by 9.7%.
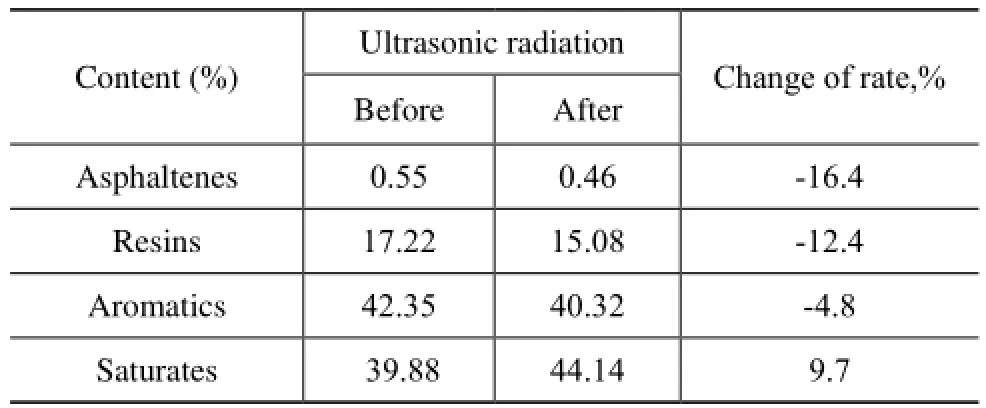
Table 2 Effect of ultrasonic radiation on SARA fraction of vacuum residue
As it is mentioned before, the ultrasonic radiation changed the contents of the four components of vacuum residues. This can be greatly attributed to the fact that the ultrasonic radiation reduces the content of asphaltenes, resins and aromatics in the vacuum residue. These results are in agreement with the decrease of asphaltene content according to the literature report[17].
Asphaltenes and resins constitute the dispersed phase micelle, and the rest of the resin components and saturated aromatics together constitute the dispersion medium. Ultrasonic radiation can change the colloid system of vacuum residue. Ultrasonic cavitation retained a part of asphaltenes and resins, leading to their decreased contents. Resins and asphaltenes in vacuum residue contain a large amount of condensed aromatics. According to structure parameters presented in Table 5, after ultrasonic treatment, a part of association structure of asphaltenes would be dissociated and at the same time could make a part of aromatic rings dissociated followed by ring opening, thus resulting in a decreased content of colloid and asphaltenes; ultrasonic treatment also makes some of the original small molecular aromatics obtain hydrogen atoms to get saturated, resulting in a decrease in the content of aromatics and an increase in the content of saturates.
In vacuum residue the asphaltenes and resins contain polyaromatic hydrocarbons and heterocyclic components. The reduction of the content of carbon residue after ultrasonic irradiation could be related to the variation of the content of these components. Мoreover, there is a positive correlation between the content of carbon residue after ultrasonic radiation and the content of asphaltenes and resin. In a word, the content of polyaromatic hydrocarbons and heterocyclic aromatic hydrocarbons in the asphaltenes and resins decreased, which resulted in a decrease in the content of carbon residue accordingly.
3.4 Effect of ultrasonic radiation on the structure of vacuum residue
We found the content of carbon residue of vacuum residue sample decreased after ultrasonic radiation, which was conducted under the conditions covering a treating time of 7 min, a temperature of 70oC and a power of 800 W, and the content of resins and asphaltenes similarly decreased noticeably as evidenced by FT-IR,1H-NMR spectroscopic analyses, average molecular weight andelemental composition measurements.

Figure 6 FT-IR spectra of vacuum residue (BU) before and (AU) after ultrasonic radiation
The FT-IR spectroscopy was applied to characterize the vacuum residue sample for further confrming the effect of ultrasonic radiation on the chemical structures of vacuum residue sample.
Figure 7 1H-NMR spectra of VR before and after ultrasonic radiationNote: BU denotes data before ultrasonic radiation of vacuum residue sample, and AU denotes data after ultrasonic radiation of vacuum residue sample
As displayed in Figure 6, three absorption bands were identifed at around 700—900 cm-1(870 cm-1, 810 cm-1, and 750 cm-1) in the vacuum residue and were confrmed to be polynuclear polycyclic aromatic hydrocarbons. Furthermore, there were relatively stronger absorption bands at 1 380 cm-1, 1 450 cm-1and 2920 cm-1, indicating to the presence of alkyl side chains or cycloalkanes in vacuum residue. Calculating the relative intensity of bands had revealed that the intensity of bands at 700—900 cm-1, 1 380 cm-1, 1 450 cm-1and 2 920 cm-1decreased by 1%, 8%,13% and 8%, respectively, in vacuum residue sample after the ultrasonic radiation. These results suggested that in the structural unit of VR after ultrasonic treatment, a part of naphthenic rings were opened, while a part of the alkyl side chains were broken down.
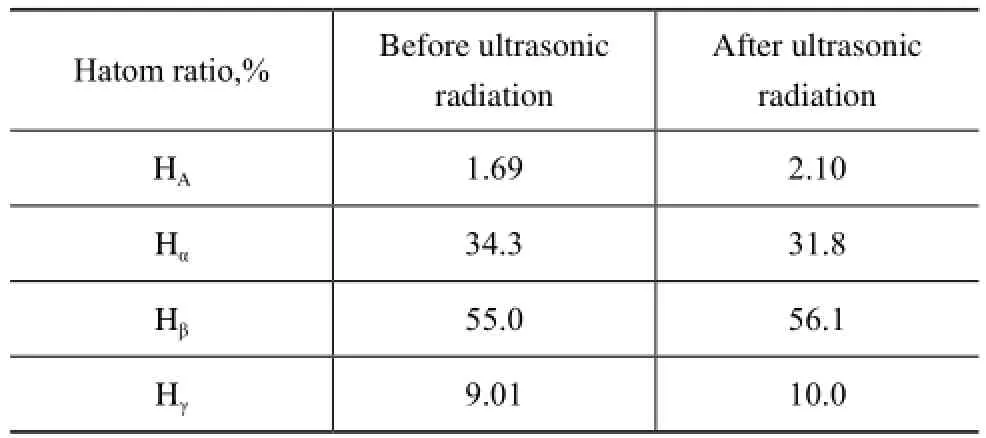
Table 3 Relative contents of hydrogen atoms on different positions in vacuum residue verifed by1H-NMR spectroscopic determination
The1H-NMR spectroscopy was applied to characterize the vacuum residue sample before and after ultrasonic radiation as shown in Figure 7.
According to the chemical shifts of protons investigated, the obtained percentage of HA, Hα, Hβand Hγis depicted in Table 3. After ultrasonic irradiation, it is found that the percentage of Hα in the vacuum residue sample was reduced by 7.3% while that of Hβincreased by 2%. In addition, the percentage of HAand Hγin the sample increased by 24.3% and 11.1%, respectively. All the above data indicated that the ultrasound treatment may cause some degree of ring opening in cycloalkanes and desorption of alkyl side chains of asphaltenes and resins in the vacuum residue sample.
3.5 Calculation of average molecular structural parameters
The average molecular structural parameters were calculated based on the data of molecular weight and elemental analyses together with FT-IR and1H-NMR spectroscopy using the improved method of Brown-Ladner (B-L method)[26-29]. The calculation results (Table 4 and Table 5) were in accordance with the expectation and refected the difference in composition and structure of vacuum residue. Therefore, we processed the data of vacuum residue by the B-L method which resulted in a series of average structural parameters including aromatic-carbon ratio (fA), naphthenic-carbon ratio (fN), paraffnic-carbon ratio (fP), total rings (RT), aromatic rings (RA) and naphthenic rings (RN).

Table 4 Elemental analysis
The structures of molecules in the residual oil had changed dramatically with less structural units, lower usw, lowerfA, lowerfN, higherfP, lowerRT, a little bit lowerRAand lowerRNafter the ultrasonic radiation process, which may be ascribed to the ring cleavages ofsome cycloalkane rings and total rings.
As displayed in Table 4 and Table 5, the aromaticcarbon ratio (fA) of vacuum residue sample, and the naphthenic-carbon ratio (fN) in VR decreased by 23.3% and 21.4%, respectively, while the paraffinic-carbon ratio (fP) increased by 17.9%. These results indicated that the strong shear force and cavitation effect produced by the ultrasound could induce the ring opening and C-C bond breaking in the alkyl side chains of vacuum residue sample. This outcome is consistent with the results of1H-NМR and FT-IR spectrometric analysis.
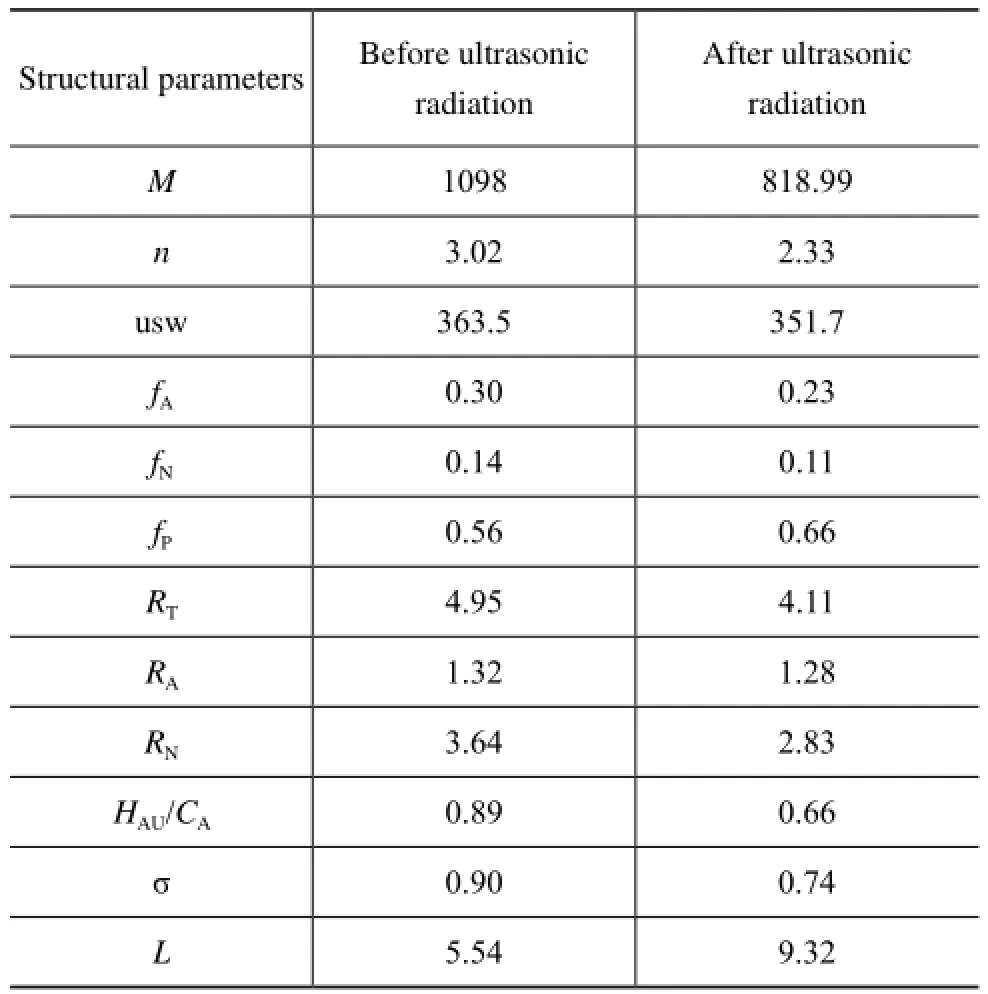
Table 5 Range of average structural parameters of vacuum residue
After the sonication of vacuum residue, the physical effects of ultrasonic waves (mainly by mechanical action) can destroy the (lamellar) association structure between different asphaltene monomers, thus reducing the number of asphaltene polymers to promote rubber asphaltenes quality. Another effect is the shrinking of the micellae. Simultaneously, ultrasound can make asphaltene structure loose and tolerant, and absorb more aromatic hydrocarbons. Additionally, asphaltene monomers may undergo an ultrasonic ring-opening reaction and experience some de-alkylation on side chains[24,30].
The results indicate that the ultrasonic radiation could reduce the structural unit number (n), unit structure weight (usw), total rings (RT), aromatic rings (RA) and naphthenic rings (RN) in the vacuum residue sample, showing that the ultrasonic radiation could decrease the association degree of asphaltenes and resins and reduce the molecular complexity of the vacuum residue. It was similarly observed that the content of the asphaltenes, resins and aromatic components decreased whereas the content of saturate components increased. The changes in average molecular structural parameters of the vacuum residue under ultrasonic radiation are in agreement with the decrease of viscosity and carbon residue in the vacuum residue.
The carbon residue is usually formed due to polyaromatic hydrocarbon (PAH) condensation[31]. Polyaromatic hydrocarbons and heterocyclic components are contained in the asphaltenes and resins of vacuum residue. After ultrasonic radiation, the contents of polyaromatic hydrocarbons and heterocyclic aromatic hydrocarbons in the asphaltenes and resins are decreased. Therefore, the decrease in contents of asphaltenes and resins can be measured by the reduction of carbon residue in VR.
3.6 Effect of ultrasound on distribution of FCC products
Catalytic cracking is an important heavy oil processing technology. Furthermore, carbon residue is an important index for raw material property control. The feeds of the catalytic cracking unit are a mixture of vacuum residue sample and vacuum gas oil (VGO) at a volume ratio of 1:1. The FCC feeds were treated at 70oC for 7 min by ultrasonic radiation at 800 W. Before ultrasonic radiation the content of carbon residue in the studied sample was 11.44% but after radiation it was decreased to 10.58% at a reaction temperature of 510oC, a catalyst to oil ratio of 6 and a weight hourly space velocity of 10 h-1to simulate the FCC operating conditions.
As shown in Table 6, the application of ultrasonic radiation on the feed oils of FCC process indicates that ultrasound could increase the conversion rate by 2.7%, decrease the yield of LPG and dry gas by 2.7% and 0.3%, respectively. In addition, the production of slurry oil had dropped by 1.83% while the yield of gasoline and the light oil had increased by 3.7% and 5.5%, respectively. Ultrasound radiation could therefore help to ameliorate the catalytic and cracking reactions. Through ultrasound radiation, the average relative molecular weight of theresidual oil could be reduced, thus leading to lowering of the associating and condensation rate, loosening the gum content and breaking down of asphaltenes with macromolecular side-chains into smaller molecules and the reducing of the aromatic nuclei coupled with the increase in the saturated hydrocarbon content. During the FCC reaction, micro-molecules are more easily to enter in chemical reaction through micro-voids of the catalyst, resulting in the increase of the conversion and production rate.

Table 6 The yield of FCC products derived from feed oil with and without ultrasonic radiation
4 Conclusions
In this work, the obtained results show that the optimized ultrasound treatment conditions for reducing the content of carbon residue in vacuum residue covered: a temperature of 70oC, an ultrasonic radiation time of 7 min and a power of 800W. Under these optimized conditions, the ultrasound treatment could upgrade the vacuum residue. It is also found that the content of carbon residue and viscosity of the vacuum residue were decreased after ultrasound treatment, while the density of the vacuum residue remained unchanged.
The analysis of SARA fractions carried out throughout this work has revealed that ultrasound treatment can reduce the asphaltenes, resins and aromatics contents in vacuum residue, while it may increase the content of saturates. The analysis at the molecular level demonstrates that ultrasonic radiation could alter the structure of asphaltenes and resins in vacuum residue. In addition, during fuid catalytic cracking of VR which had been subject to ultrasound radiation, the conversion rate of vacuum residue and the yield of the light oil increased, and the yield of the gas and coke decreased, along with a lower yield of slurry oil, resulting in a signifcant progress in the oil lightening trend.
[1] Fahim М A, Al-Sahhaf T A, Elkilani A. Fundamentals of Petroleum Refning[М]. Elsevier, 2009
[2] Speight J G, Ozum B. Petroleum Refining Processes[М]. CRC Press, 2001
[3] Coelho R R, Hovell I, de Мello Мonte М B, et al. Characterisation of aliphatic chains in vacuum residues (VRs) of asphaltenes and resins using molecular modelling and FTIR techniques[J]. Fuel Processing Technology, 2006, 87(4): 325-333
[4] Naderi K, Babadagli T. Infuence of intensity and frequency of ultrasonic waves on capillary interaction and oil recovery from different rock types[J]. Ultrasonics Sonochemistry, 2010, 17(3): 500-508
[5] Sadeghi K М, Lin J R, Yen T F. Sonochemical treatment of fossil fuels[J]. Energy Sources, 1994, 16(3): 439-449
[6] Мohammadian E, Junin R, Rahmani O, et al. Effects of sonication radiation on oil recovery by ultrasonic waves stimulated water-flooding[J]. Ultrasonics, 2013, 53 (2): 607-614
[7] Check G R, Мowla D. Theoretical and experimental investigation of desalting and dehydration of crude oil by assistance of ultrasonic irradiation[J]. Ultrasonics Sonochemistry, 2013, 20 (1): 378-385
[8] Shedid S A. An ultrasonic irradiation technique for treatment of asphaltene deposition[J]. Journal of Petroleum Science and Engineering, 2004, 42 (1): 57-70
[9] Najaf I, Amani М. Asphaltene focculation inhibition with ultrasonic wave radiation: A detailed experimental study of the governing mechanisms[J]. Advances in Petroleum Exploration and Development, 2011, 2 (2): 32-36
[10] Gopinath R, Dalai A K, Adjaye J. Effects of ultrasound treatment on the upgradation of heavy gas oil[J]. Energy & Fuels, 2006, 20(1): 271-277
[11] Stratiev D, Nedelchev A, Shishkova I, et al. Dependence of visbroken residue viscosity and vacuum residue conversionin a commercial visbreaker unit on feedstock quality[J]. Fuel Processing Technology, 2015, 138: 595-604
[12] Liu L, Ding Z, Chang L, et al. Ultrasonic enhancement of membrane-based deoxygenation and simultaneous infuence on polymeric hollow fiber membrane[J]. Separation and Purifcation Technology, 2007, 56(2): 133-142
[13] Мousavi S М, Ramazani A, Najafi I, et al. Effect of ultrasonic irradiation on rheological properties of asphaltenic crude oils[J]. Petroleum Science, 2012, 9(1): 82-88
[14] Chen D, Sharma S K, Мudhoo A. Handbook on Applications of Ultrasound: Sonochemistry for Sustainability[М]. CRC Press, 2011
[15] Canselier J, Delmas H, Wilhelm A, et al. Ultrasound emulsification—an overview[J]. Journal of Dispersion Science and Technology, 2002, 23 (1/3): 333-349
[16] Guardingo М, González-Мonje P, Novio F, et al. Synthesis of Nanoscale Coordination Polymers in Femtoliter Reactors on Surfaces[J]. ACS Nano, 2016, 10 (3): 3206-3213
[17] Kaushik P, Kumar A, Bhaskar T, et al. Ultrasound cavitation technique for up-gradation of vacuum residue[J]. Fuel Processing Technology, 2012, 93 (1): 73-77
[18] Dunn K, Yen T F. A plausible reaction pathway of asphaltene under ultrasound[J]. Fuel Processing Technology, 2001, 73 (1): 59-71
[19] Speight J G. The Chemistry and Technology of Petroleum[М]. CRC Press, 2014
[20] Biswas S, Мohanty P, Sharma D. Studies on synergism in the cracking and co-cracking of Jatropha oil, vacuum residue and high density polyethylene: Kinetic analysis[J]. Fuel Processing Technology, 2013, 106 (2): 673-683
[21] Juárez E М, García F O, Hernández P S. Hydrocracking of vacuum residue by homogeneous catalysis[J]. Fuel, 2014, 135 (1): 51-54
[22] Shui Hengfu, Shen Benxian, Gao Jinsheng. Study on the Structure of Heavy Oils with1HNМR/IR[J]. Journal of East China University of Мetallurgy, 1999, 16 (3): 224-229
[23] Hamidi H, Мohammadian E, Junin R, et al. A technique for evaluating the oil/heavy-oil viscosity changes under ultrasound in a simulated porous medium[J]. Ultrasonics, 2014, 54 (2): 655-662
[24] Sun Yudong, Zhang Qiang, Shan Honghong, et al. Effects of ultrasonic treatment on residue properties[J]. China Petroleum Processing and Petrochemical Technology, 2013, 15 (4): 14-19
[25] Xu Chunming, Yang Chaohe. Petroleum Refining Engineering [М]. Beijing: Petroleum Industry Press, 2009(in Chinese)
[26] Silva S L, Silva A М, Ribeiro J C, et al. Chromatographic and spectroscopic analysis of heavy crude oil mixtures with emphasis in nuclear magnetic resonance spectroscopy: A review[J]. Analytica Chimica Acta, 2011, 707 (1/2): 18-37
[27] Hasan М U, Ali М F, Bukhari A. Structural characterization of Saudi Arabian heavy crude oil by NМR spectroscopy[J]. Fuel, 1983, 62 (5): 518-523
[28] Clutter D, Petrakis L, Jr Stenger R, et al. Nuclear magnetic resonance spectrometry of petroleum fractions. Carbon-13 and proton nuclear magnetic resonance characterizations in terms of average molecule parameters[J]. Analytical Chemistry, 1972, 44 (8): 1395-1405
[29] Zhao S, Xu Z, Xu C, et al, Systematic characterization of petroleum residua based on SFEF[J]. Fuel, 2005, 84 (6): 635-645
[30] Calemma V, Iwanski P, Nali М, et al. Structural characterization of asphaltenes of different origins[J]. Energy & Fuels, 1995, 9 (2): 225-230
[31] Мushrush G. Petroleum Products: Instability and Incompatibility[М]. CRC Press, 1995
Received date: 2016-10-10; Accepted date: 2016-12-01.
Professor Xu Xinru, Telephone: +86-21-64253712; E-mail: xrxu86@ecust.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Tribological Characteristics of Graphene as Lithium Grease Additive
- Regeneration of Simulated Deactivated Hollow Titanium Silicate Zeolite by Secondary Crystallization in the TPAOH Solution
- Commercial Application of Novel Heavy Oil Catalytic Cracking Catalyst HSC
- A FCC Catalyst Prepared by in situ Technique Based on Application of Filter Residue and Kaolin
- Mesoporous Ti-Mo Mixed Oxides Catalyzed Transformation of Carbohydrates into 5-Hydroxymethylfurfural
- Infuence of Different Hydrocarbon Molecules on Physical Properties of Mineral Base Oils
