Regeneration of Simulated Deactivated Hollow Titanium Silicate Zeolite by Secondary Crystallization in the TPAOH Solution
2017-05-09XiaChangjiuLinMinPengXinxinZhuBinXuGuangtongShuXingtian
Xia Changjiu; Lin Min; Peng Xinxin; Zhu Bin; Xu Guangtong; Shu Xingtian
(State Key Laboratory of Catalytic Materials and Reaction Engineering, Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
Regeneration of Simulated Deactivated Hollow Titanium Silicate Zeolite by Secondary Crystallization in the TPAOH Solution
Xia Changjiu; Lin Min; Peng Xinxin; Zhu Bin; Xu Guangtong; Shu Xingtian
(State Key Laboratory of Catalytic Materials and Reaction Engineering, Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
It is of paramount importance to improve the utilization efficiency of hollow titanium silicate (HTS) zeolite catalyst used in the cyclohexanone ammoxidation process. To achieve this aim, the regeneration of simulated deactivated HTS zeolite by post-synthesis was carried out in an aqueous TPAOH solution under hydrothermal conditions. It was found that the catalytic performance for phenol hydroxylation over regenerated HTS zeolite was as high as that of fresh one. Judging from the BET measurements, electron micrography and XRD analysis results, it was confrmed that the topological and morphological structure was repaired. The chemical state of Ti species was detected by the UV-Vis and29Si MAS NMR spectroscopy. No acidic amorphous TiO2-SiO2oxide was formed, and the extraframework Ti species could be reincorporated into the framework of HTS zeolite thanks to the tetrahedral coordination by the condensation between Ti-OH and Si-OH groups. In order to confrm this conclusion, the fresh HTS zeolite was treated under the NH3·H2O hydrothermal and thermal conditions for several times. The catalytic activity of both uncalcined and calcined simulated deactivated HTS zeolite samples could be regenerated without the formation of Brönsted acid sites. It was concluded that the highly dispersed Ti species could be reincorporated into the framework of zeolite by hydrated condensation of Si-OH and Ti-OH groups after secondary hydrothermal synthesis.
hollow titanium silicate, regeneration, post synthesis, TEМ, template
1 Introduction
“One pot” synthesis of cyclohexanone oxime catalyzed by TS-1 zeolite, using NH3and 30% H2O2solution as raw materials, is one of the most successful and important zeolite-involved catalytic reactions in the industrial scale[1-8]. The product of this reaction is an intermediate for manufacturing ε-caprolactam, which can be used to synthesis nylon-6 and some other useful polymers. The ammoxidation process was initially developed by the Eni S.p.A. in 1994, shortly after the synthesis of TS-1 zeolite[9-12]. It is confirmed that the active sites for this ammoxidation reaction is the tetrahedral Ti species, which are located in the framework of the topologically structured МFI type zeolite. This process is very clean and environmentally friendly, because there are no such major harmful pollutants formed as those formed by the traditional “four-step” hydroxylamine route.
Since the novel cyclohexanone ammoxidation reaction is operated in an alkaline NH3hydrothermal environment, the stability of the TS-1 zeolite catalyst is of paramount importance for this process. Unfortunately, it was found that the TS-1 zeolite samples, which were produced by the initially published method, showed very poor catalytic activity and stability for the ammoxidation process. This may occur because it is very diffcult to control the dehydrated condensation speed of Ti and Si atoms to form zeolite crystals during the hydrothermal synthesis process. To solve these drawbacks, a novel “dissolution and recrystallization” method was proposed by М. Lin and coworkers, and hollow titanium silicate (HTS) zeolite was synthesized in the 1990s[13-16]. Compared with the traditional TS-1 zeolite, the HTS zeolite shows even higher catalytic performance and stability in thecommercial cyclohexanone ammoxidation units of SINOPEC since the beginning of this century[17-18]. The turnover number of HTS zeolite is very high, and the ultimate capability is over 5 tons of cyclohexanone per kg of HTS zeolite. However, the deactivation of HTS zeolite used in this process is inevitable, and the HTS zeolite used and regenerated for 5 times will lose its activity[19-22]. Since the production of HTS zeolite has to consume a huge amount of materials and energy, it is very necessary to study the deactivation mechanism, and learn how to improve its utilization effciency. It is unrealistic to investigate the real deactivation process in the commercial units, which are operated in the continuously stirred tank reactors. Therefore, the simulated deactivation experiments of HTS zeolite were designed by treating the zeolitic materials under both ammonia hydrothermal and thermal conditions. In our previous work, the simulated deactivated HTS zeolite samples were investigated by means of the multiple characterization methods and catalytic performance evaluation. It is verified that the specific surface area and microporous volume of simulated deactivated HTS zeolite samples are smaller than that of fresh one. And the migration of tetrahedral framework Ti species into extraframework positions was investigated by UV-Vis, FT-IR and29Si MAS NMR spectroscopic methods. It is concluded that the catalytic performance of HTS zeolite is correlated to both the structural properties and the chemical state of Ti species. For restoring its physicochemical properties and catalytic activity, the simulated deactivated HTS zeolite samples were post synthesized in the TPAOH solution at high temperature to form the regenerated HTS zeolite. In fact, there are only few articles published on this topic, and the direct and useful information is lacking to support the strategies on the adoption and regeneration of HTS zeolite used in the commercial ammoxidation process.
In this paper, the basic structural properties and the chemical state of Ti species, and the catalytic performance for phenol hydroxylation of the regenerated HTS zeolite were analyzed. The relationships among these factors were confirmed. Finally, a plausible mechanism of Ti species reincorporated into zeolite framework was proposed. Through these approaches, some viewpoints on the regeneration of industrial deactivated HTS zeolite are provided, when the samples are recrystallized in aqueous TPAOH solution.
2 Experimental
2.1 Preparation of regenerated HTS zeolite
The simulated deactivated HTS zeolite was prepared in a low-concentration NH4OH solution at 120oC under continuous stirring and autogeneous pressure. The mole ratio of HTS (expressed in terms of SiO2:NH3:H2O) was equal to 1:1.29:20. The HTS zeolite was treated for several hours to obtain the uncalcined simulated deactivated HTS zeolite, which was labeled as HTS-NH3-nh (withnrepresenting the number of NH3hydrothermal treatments). When the uncalcined samples were heated at 550oC in air for 3 hours, the calcined simulated deactivated HTS zeolites were prepared and labeled as HTS-NH3-B-nh.
Both of the uncalcined and the calcined simulated deactivated HTS samples were post-synthesized in the aqueous TPAOH solution at 170oC, with the mole ratio of simulated deactivated HTS zeolite (expressed in terms of SiO2:TPAOH:H2O) being equal to 1:0.1:10. Then the corresponding uncalcined and calcined regenerated HTS zeolites were obtained and labeled as HTS-NH3-CP-nh and HTS-NH3-B-CP-nh, respectively.
2.2 Characterization techniques
The PXRD analysis was performed using a PANalytical powder diffractometer equipped with a monochromator using Cu-Kα radiation (λ= 0.154 178 nm). The beam voltage was 40 kV; the dwell time was 500 s, and the scanning angle (2θ) ranged from 5° to 80oat a scan step size of 0.02o. Low temperature N2physisorption isotherms were measured on a Мicromeritics AS-6B apparatus, employing generally the BET and the BJH methods to count the specifc surface area and pore volume of zeolite samples. Before the analyses, these different samples were hydrated under a vacuum condition (10-1Pa) at 300 °C for 6 h. Then the measurement was carried out at 77 K.
Transmission electron microscope images were used to obtain the morphology and crystal size of zeolite samples on a Philips TECNAI G2F20 TEM microscope at a beamvoltage of 200 kV. The SEМ images were taken by a FEI Quanta 200F micrograph, operating at a beam voltage of 20 kV.
The UV-Vis spectra were collected by an Agilent Cary 300 UV-Vis spectrophotometer, with the wavelength ranging from 200 nm to 800 nm.
The pyridine adsorption and hydroxyl stretching FTIR analysis was conducted on a Bio-Rad FTS 3 000 МX FT-IR spectrophotometer, with the МTC detector being cooled by liquid nitrogen at low temperature. The wavenumber region of pyridine adsorption was in the range from 1 700 cm-1to 1 400 cm-1. And the OH stretching region ranged from 3 800 cm-1to 3 200 cm-1.
29Si МAS NМR patterns were collected on a Varian INOVA 400 МHz NМR instrument, with a basic frequency of 59.58 МHz. The zeolite samples were put into a 4 mm ZrO2rotor, with a spinning speed of 3 kHz.
2.3 Catalytic performance
The catalytic activity of simulated deactivated and regenerated HTS zeolite samples was studied via the phenol hydroxylation reaction, using 30% H2O2solution as the oxidant. 0.62 g of zeolite catalyst, 12.5 g of phenol and 10 mL of acetone were added into an 100-mL threenecked fask reactor, and then the mixture was heated up to 80oC in air under continuous stirring. Then, 5 g of 30% H2O2solution was added into this reactor. After 2 hours, the products were analyzed by a gas chromatograph equipped with a FID detector (Agilent 6890 series) and a 30-meter-long capillary HP-5 column.
3 Results and Discussion
3.1 Catalytic performance of regenerated HTS zeoliteFigure 1 shows the catalytic properties of simulated deactivated and regenerated HTS zeolite samples which had been subject to NH3·H2O hydrothermal treatment at different duration. It can be seen that the simulated deactivated HTS zeolites lost their catalytic activity continually with the increase in NH3·H2O hydrothermal reaction time. However, it is interesting to observe that both of the uncalcined and calcined simulated deactivated zeolite samples could restore their activity to reach almost the same level as fresh one after the post-synthesis treatment under the TPAOH hydrothermal conditions at high temperature. It is inferred that the physicochemical properties, i.e. the structural and morphological features, and the chemical state of Ti species, could be repaired to function as the fresh one, which means that the Ti species in the amorphous TiO2-SiO2oxide could be reincorporated into the framework position of zeolite to be in a tetrahedral coordination state.
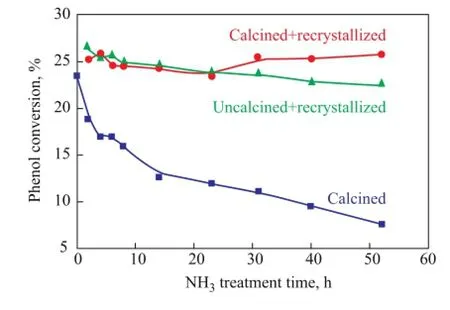
Figure 1 The catalytic activity of simulated deactivated and regenerated HTS zeolite samples during phenol hydroxylation
In order to investigate the basic topological properties of regenerated HTS zeolite samples, the XRD method was employed (as shown in the Figure 2). It was observed that all the regenerated HTS zeolite samples displayed a high crystallinity, with their characteristic diffraction peaks located at between 22° and 25°[23-24].
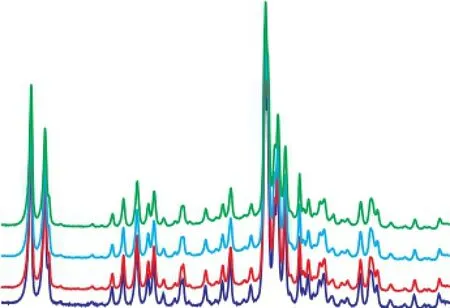
Figure 2 XRD patterns of regenerated HTS zeolite samples being subject to NH3·H2O hydrothermal reaction at different time——HTS-NH3-2 h; ——HTS-NH3-40 h;——HTS-NH3-B-2 h; ——HTS-NH3-B-40 h
The BET analysis results of regenerated HTS zeolite samples are listed in Table 1. In comparison with the simulated deactivated zeolite samples, the regenerated HTS zeolite samples almost had the same specifc surface area and pore volume as the fresh one. Both the specifc surface area and the pore volume of zeolites, which were related with the accessibility of reactant molecules to the active sites, were of great importance for their catalytic activity. It was indicated that the destroyed crystals of simulated deactivated HTS zeolite was reformed during the post-synthesis process. A part of simulated deactivated HTS zeolite crystals were dissolved in the basic solution, and the Si-OH and Ti-OH groups could be rearranged to form the new micropores by the structural directing function of TPA+ions under the hydrothermal conditions[25-29]. It was observed that the regenerated HTS samples had suffcient mesoporous structures inside their single crystals as well, as shown in Figure 3. According to the results of BET analysis, the mesoporous volume was close to that of fresh HTS zeolite, more than that of simulated deactivated HTS zeolite samples. In a word, the post-synthesis treatment could eliminate the restriction of pore features causing the decrease in catalytic activity of NH3·H2O treated HTS zeolite.
To verify the changes in morphology of regenerated HTS zeolite samples, the SEМ images of both the simulated deactivated and the regenerated HTS zeolites are presented in Figure 4. It can be seen that the crystal size distribution was very homogeneous, with the crystals shaping up like blackberry. In comparison with the simulated deactivated sample, it could be reasonably inferred that the crystals on the surface of deactivated samples were dissolved in the TPAOH solution, and were recrystallized to obtain new morphological crystalswith abundant mesoporous and microporous volume. The similar conclusion can also be drawn from the TEM images. It was found that the zeolite crystal edge became much more regular than that of deactivated samples, as shown in Figure 5.
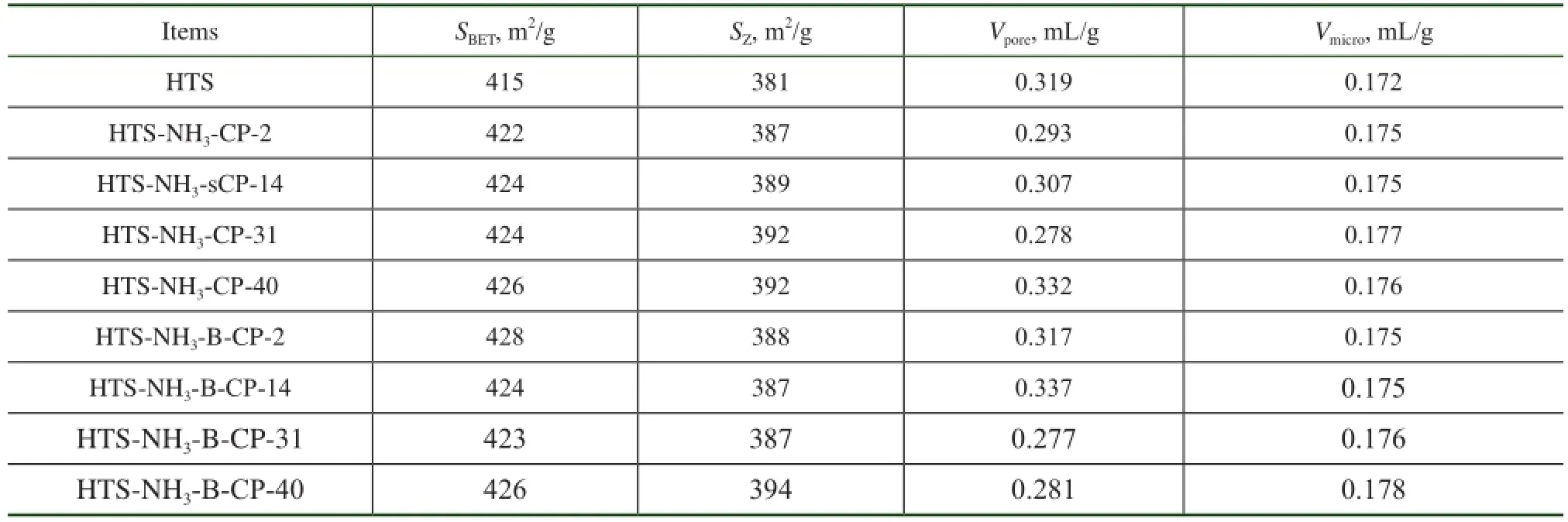
Table 1 The BET results of regenerated HTS zeolite samples subject to NH3·H2O hydrothermal reaction at different reaction time
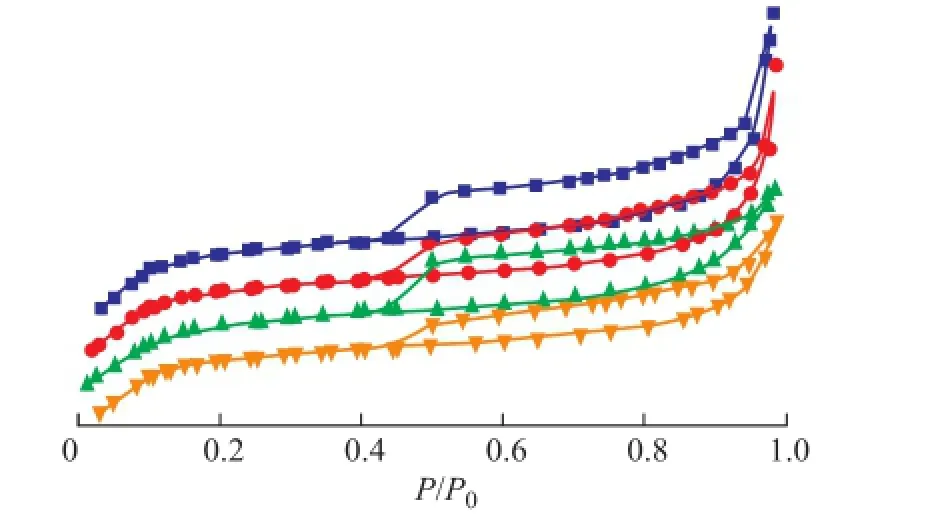
Figure 3 Low temperature N2adsorption and desorption curves of regenerated HTS zeolite samples being subject to NH3·H2O hydrothermal reaction at different treating time■—HTS-NH3-CP-14 h;●—HTS-NH3-CP-40 h;▲—HTS-NH3-B-CP-14 h;▼—HTS-NH3-B-CP-40 h
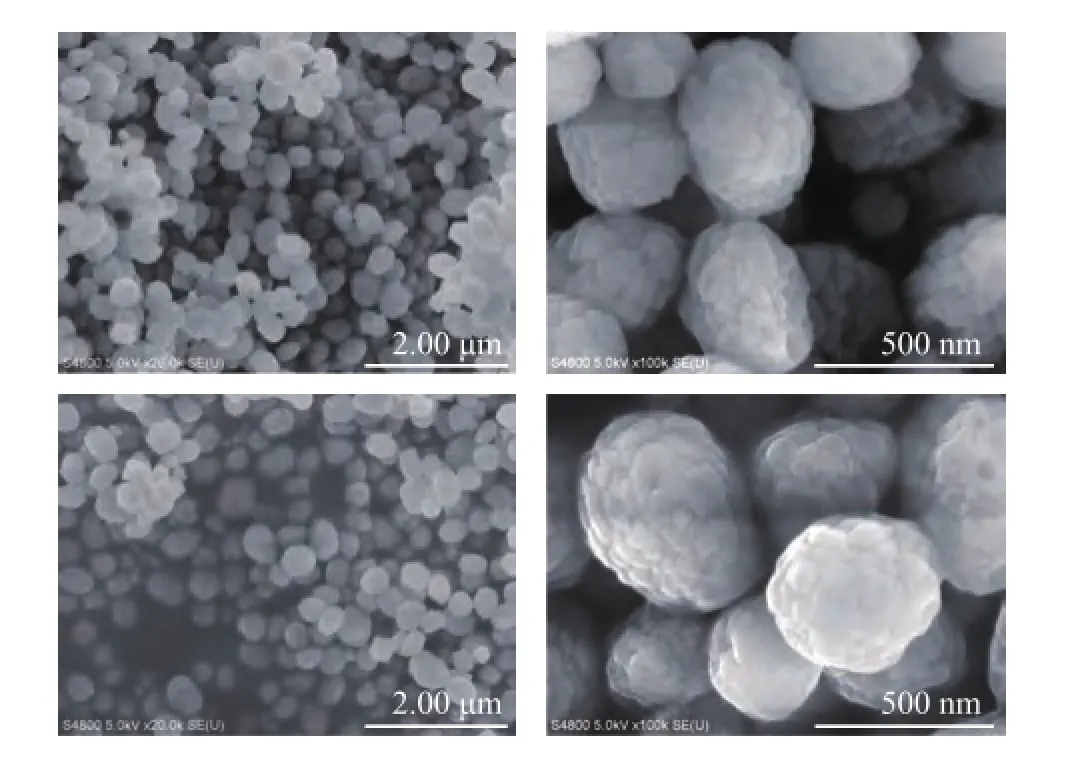
Figure 4 SEM images of both simulated deactivated and regenerated HTS zeolites

Figure 5 TEM images of both simulated deactivated and regenerated HTS zeolites
It can be seen that there were no small particles attached to the surface of regenerated HTS zeolite crystals. It meant that the Ti species were still in a highly dispersed distribution state, and no new Ti-containing aggregates could come into being. The UV-Vis spectroscopy was utilized to determine the chemical distribution of Ti species in the regenerated HTS zeolite, as shown in Figure 6. There were two similar adsorption bands at around 210 nm and 330 nm among the fresh and regenerated HTS zeolites, corresponding to the tetrahedral Ti species and bulk TiO2phase[30-32]. It was indicated that the extraframework Ti atoms, which were removed from the framework in the NH3·H2O hydrothermal environment, could be re-incorporated into the lattice of zeolite in the TPAOH solution at high temperature to obtain more tetrahedral Ti active sites for catalytic oxidation reaction.
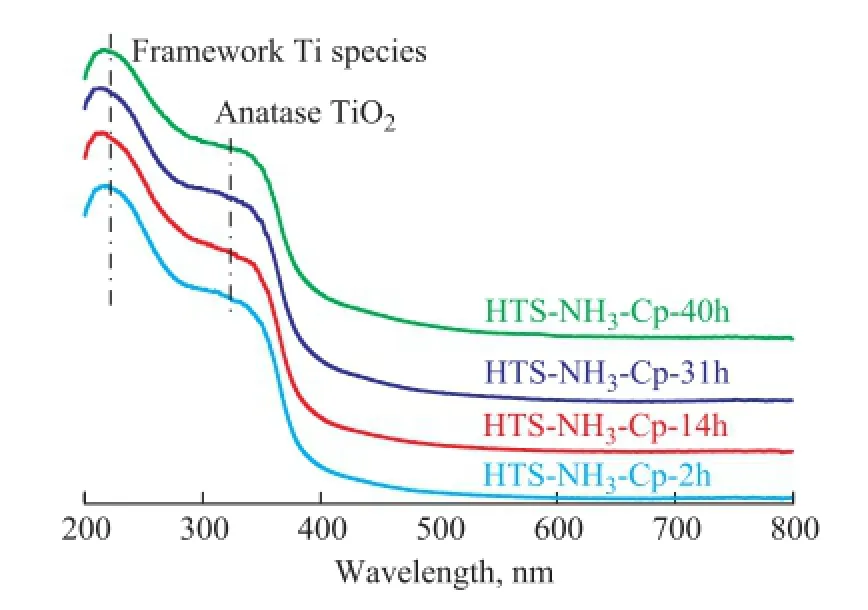
Figure 6 UV-Vis spectra of simulated deactivated and regenerated HTS zeolite samples
Thus, the regenerated HTS zeolite could have the same catalytic capability as the fresh one.
In a further study, the29Si MAS NMR spectroscopy was used to confirm the chemical environmental of Si and Ti atoms among different NH3simulated deactivated and regenerated zeolite samples, as presented in Figure 7. It was well known that the signal around -103 of the MFI structured zeolites could be solely contributed to the Si(OH)(OSi)3species (marked as Q3species), which could refect the number of defect sites in the framework of TS-1 zeolite. The signals at about -112 and -116 could be attributed to the Si(OSi)4and Si(OSi)(OTi) species (marked as Q4species), respectively[33-37]. To some extent, the intensity ratio of -103 to -112 peaks in the29Si MAS NMR spectra (marked as Q3/Q4) could be employed to refect the crystallinity of zeolite framework and the content of framework Ti species. This could occur because when the tetrahedral Ti species were inserted into the topological lattice of the МFI zeolite, they could be coordinated with the defects, such as the Si-containing hydroxyl networks, through dehydration and polymerization between Si-OH and Ti-OH groups. Thus, it was widely accepted that the ratio value of Q3/ Q4was well relevant with the content of Ti species that were incorporated into the zeolite framework. As it can be seen, the signals at around -103 of both the simulated deactivated and the regenerated samples were weakened significantly. This clearly indicated that the crystal framework was restructured after the hydrothermal synthesis in TPAOH solution with an increasing content of Q4species.
The ratio of Q3/Q4could give an exactly quantifed result to describe the evolution of Si species during the treatment process, as shown in Figure 7. When the simulated deactivated HTS sample was calcined in air, its ratio of Q3/Q4(0.119) became smaller (0.082), indicating that the calcination reaction could promote the polymerization of a part of Si-OH and Ti-OH groups. However, when the simulated deactivated samples were regenerated in the presence of aqueous TPAOH solution, this ratio became smaller obviously. This ratio was about 0.03, which was very close to that of the fresh HTS zeolite. It was concluded that the Q3species could be transformed to the Q4species, which was also confrmed by other methods, i.e. XRD, SEМ and BET techniques. Мoreover, as we cansee, the regenerated samples showed the strong shoulder peak signals at around -116, denoting that the extraframework Ti species could be re-incorporated into the framework positions of zeolite. It could be attributed to the improvement in catalytic activity of regenerated HTS zeolites in the phenol hydroxylation reaction.
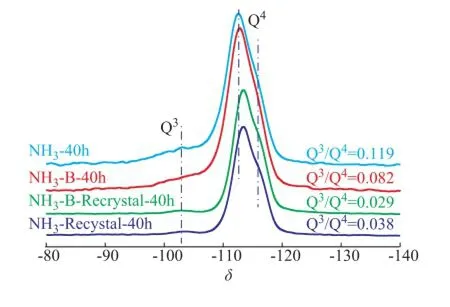
Figure 7 The29Si MAS NMR spectra of simulated deactivated and regenerated HTS zeolite samples
Figure 8 shows the FTIR spectra of simulated deactivated and regenerated HTS zeolite samples in the hydroxyl stretching region. It was found that there was a strongly broadened signal for the simulated deactivated HTS sample at between 3 200 cm-1and 3 800 cm-1in the FTIR spectra, which meant that some Ti-O-Si or Si-O-Si bonds were broken to form Si-OH and Ti-OH groups acting as the defects in the zeolite framework. Мeanwhile, there were only two related major peaks at round 3 750 cm-1and 3 500 cm-1in the FTIR spectra. According to the literature information, these two intense bands could be contributed to the free terminal hydroxyl groups and internally defective hydroxyl groups, respectively[38-42]. It was confirmed that the disordered hydroxyl groups could be recrystallized and transformed into the regular topological structure of zeolite, similar to that of the fresh HTS zeolite.
The pyridine adsorbed FTIR analysis was introduced to monitor the acid properties of both the simulated deactivated and the regenerated HTS zeolite samples. The quantified results are listed in Table 2. Although a large amount of silanol and titanol hydroxyl groups were detected based on the29Si MAS NMR and FTIR spectra in the stretching OH region, the pyridine adsorption FTIR results showed that there were no clear Brönsted acid signals (with IR band usually being identifed at 1 490 cm-1), and the Lewis acid (with IR band being identifed at 1 540 cm-1) numbers of simulated deactivated and regenerated HTS samples were very close, at a same level as that of fresh HTS zeolite[43]. In our previous work, it was indicated that the amorphous TiO2-SiO2oxides were produced after being subject to the NH3·H2O hydrothermal and calcination treatment. However, the Ti species in the amorphous oxides were still in a highly dispersed distribution state, without the formation of Ti-O-Ti bonds. These un-aggregated extraframework Ti species could be reincorporated into the framework via dehydration between Si-OH and Ti-OH groups after secondary hydrothermal synthesis. The chemical state of Ti species could be turned into tetrahedral coordination in the framework from the octahedral state in the oxides, which might be attributed to the reduction of some Lewis acid sites.
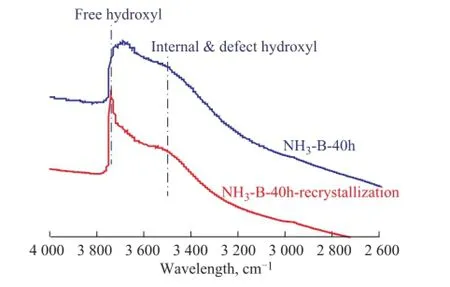
Figure 8 FTIR spectra in the hydroxyl stretching region of simulated deactivated and regenerated HTS zeolite samples
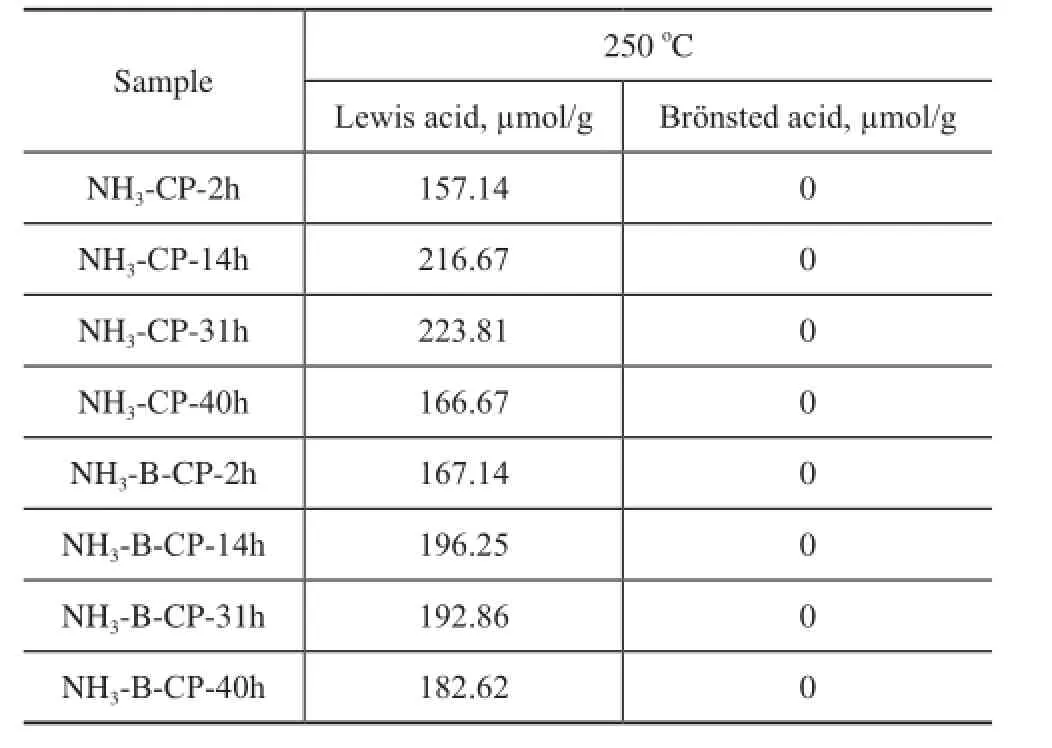
Table 2 The acid properties of simulated deactivated and regenerated HTS zeolite samples detected by the pyridine adsorbed FTIR spectroscopy at 250oC
Actually, the HTS zeolite was used and regenerated for many times to meet the requirement of catalyst utilization efficiency in the cyclohexanone ammoxidation process. This reaction was operated under the basic condition, and the zeolite deactivation caused by blocking of micropores by organic substances was eliminated by calcination at high temperature. In order to make the simulated deactivation and regeneration be close to the real environment, the fresh HTS samples were treated under the NH3·H2O hydrothermal and thermal conditions for several times, and then they were post-synthesized in the aqueous TPAOH solution at high temperature. During the recrystallization reaction, the feed composition, reaction time and crystallization temperature were kept constant. The obtained simulated deactivated and regenerated HTS zeolite samples were evaluated by the phenol hydroxylation reaction under the same condition. The catalytic performance of these zeolitic materials is listed in Table 3. It can be seen that the phenol conversion was insignificant with the increase in treatment time, which could be attributed to the reduction of BET specifc surface area and the content of Ti species incorporated into the framework.

Table 3 The catalytic properties of simulated deactivated and regenerated HTS zeolite samples
However, both of the uncalcined and the calcined simulated deactivated HTS samples present very high catalytic activity after they were recrystallized in the organic quaternary ammonium hydroxide solution. This indicated that the NH3·H2O basic treatment and calcination at 550oC in air, even for several treatment times, could not make the Ti species aggregated, without the formation of acidic amorphous Ti-O-Ti bonds containing oxides. Thus there were no Brönsted acid sites and a large amount of new Lewis acid sites were produced after several alkaline hydrothermal and hydrothermal treatments, as shown in Table 4. It was concluded that the state of Ti species aggregation in the simulated deactivated HTS samples was an important factor for regeneration of their physicochemical and catalytic properties.
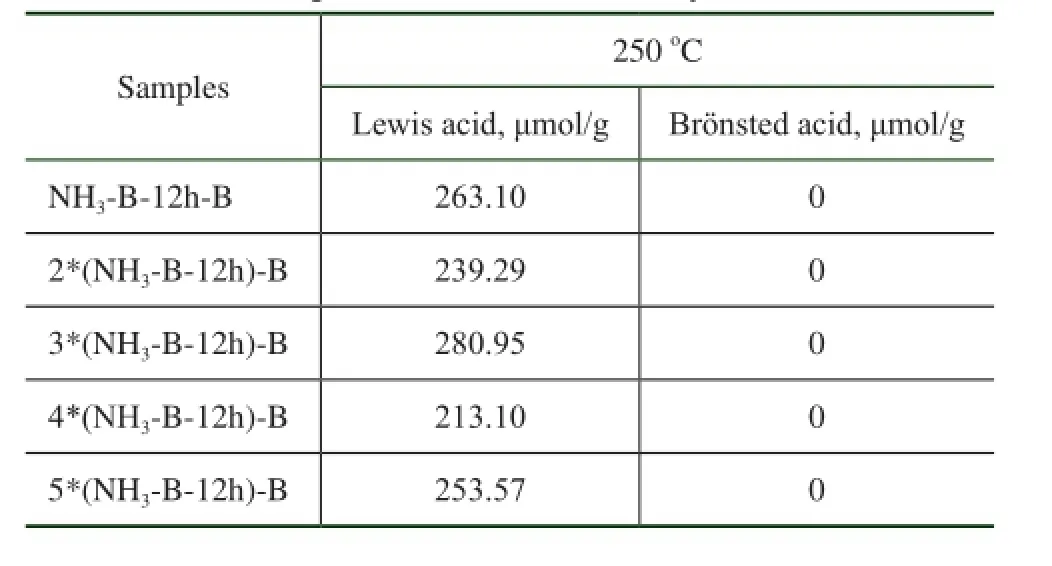
Table 4 The acidic properties of simulated deactivated HTS zeolite samples obtained after many treatment times
The detailed possible mechanism on regeneration of simulated deactivated HTS zeolite by secondary hydrothermal synthesis is presented in Figure 9a and 9b. It gives us a total illustration of the transformation of extraframework Ti species by dehydration between Si-OH and Ti-OH hydroxyl groups. One plausible detailed condensation mechanism catalyzed by alkaline OH-groups was originated from the ionization of TPAOH in aqueous solution, as shown in Figure 9b. At the initial step, OH-groups could nucleophilically attack the octahedral Ti species, with the electron pairs being sent to Ti atoms. One of H atoms in the Ti-OH species could be connected with the O atom in the OH-group via the hydrogen-bond interaction, with one H2O molecular being formed. Then the electron pair could move to the O atom in the Ti-O-group, which could attack the tetrahedral Si species continually, with the formation of Ti-O-Si bond. Finally, the electron pair could be sent to the O atom in the hydroxyl group connected with Si atom, and the Si-OH bond was broken, while a new OH-group was produced. As this catalytic dehydration process could be repeated time and again, the topological structure could be synthesized along with the repairing of destroyed morphology. At the same time, the octahedral Ti species could be reincorporated into the framework position of zeolite. As a result, the catalytic performance of the regenerated HTS zeolite could reach almost as high as that of fresh one.
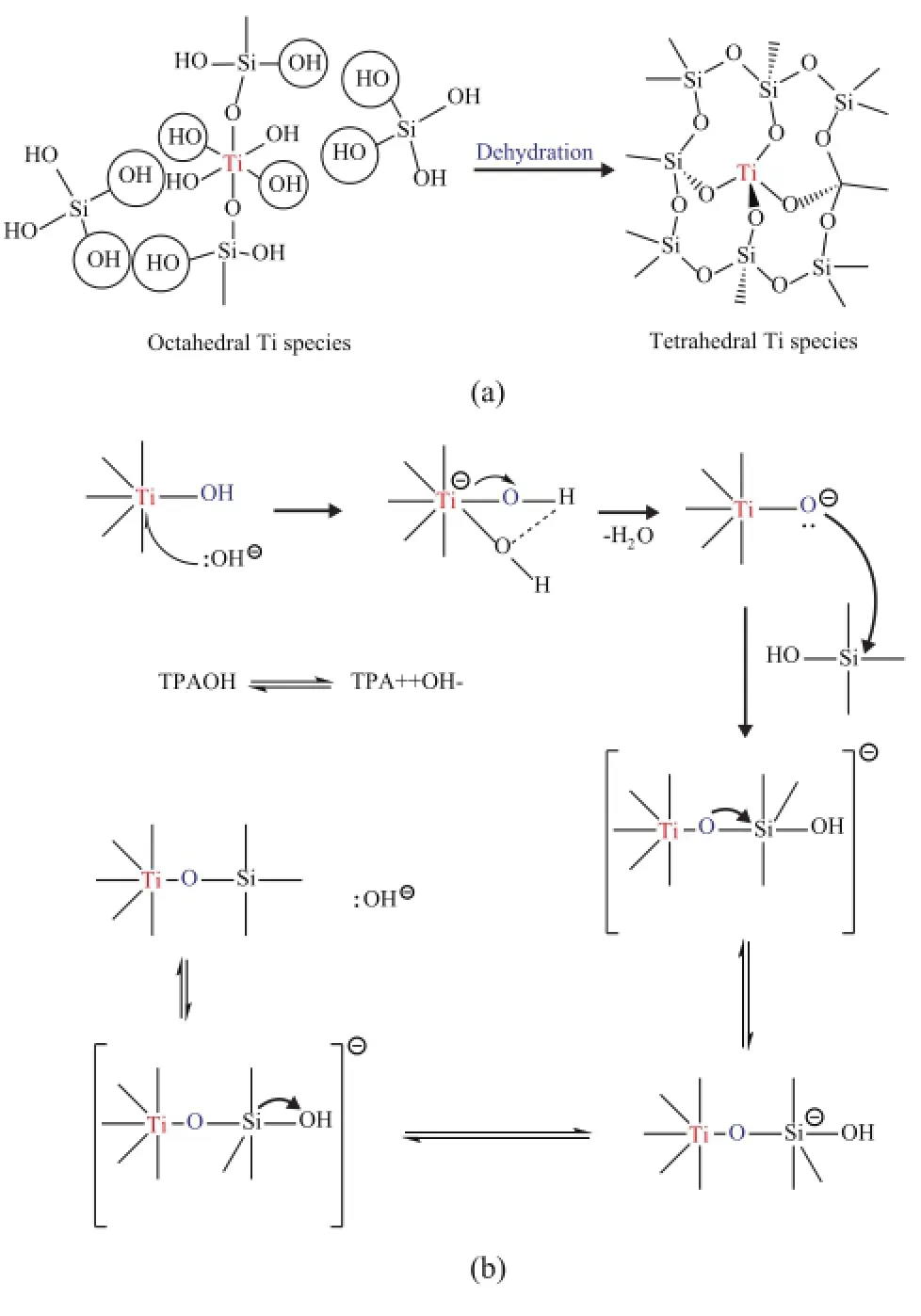
Figure 9 One plausible mechanism of secondary hydrothermal synthesis of simulated deactivated HTS zeolite
4 Conclusions
The regeneration of simulated deactivated HTS zeolite by secondary hydrothermal synthesis in the aqueous TPAOH solution was investigated by multiple methods. Judging from the BET study, the electron micrographs and XRD analysis results, it was observed that the topological and morphological structure of the HTS zeolite samples was repaired with their catalytic activity being on a par with the fresh one. This fact indicated that the mass diffusion property of the regenerated HTS zeolite was very well. The chemical state of Ti species was detected by the UV-Vis and29Si МAS NМR spectroscopy. Since no acidic amorphous TiO2-SiO2oxide was formed, the extraframework Ti species could be reincorporated into the framework of HTS zeolite to be bound through the tetrahedral coordination by condensation of Ti-OH and Si-OH groups. Thus the regenerated HTS zeolite showed good catalytic performance in phenol hydroxylation reaction, which was almost the same as the fresh HTS zeolite.
In order to confrm this conclusion, the fresh HTS zeolite was treated under NH3·H2O hydrothermal and thermal conditions for several times. And then both the uncalcined and the calcined simulated deactivated HTS zeolite samples were post-synthesized in the aqueous TPAOH solution at 170oC for one day. The catalytic activity of all zeolite samples could be regenerated very well, and no Brönsted acid sites were produced. It was concluded that the un-aggregated Ti species could be reincorporated into the framework of zeolite after secondary hydrothermal synthesis.
Finally, a regeneration mechanism of simulated deactivated HTS zeolite was proposed. The hydrated condensation of Si-OH and Ti-OH groups, catalyzed by alkaline OH-groups, occurred under a high-temperature hydrothermal condition. The formation of Si-O-Si and Ti-O-Si bonds resulted in the regeneration of physicochemical properties and their good performance in organic oxidation reactions.
Acknowledgment:Thanks a lot for the kind help from Prof. Мu Xuhong, Prof. Luo Yibin, Dr. Zheng Aiguo, Dr. Xiang Yanjuan, and all staffs for the characterization of materials at RIPP. This work was fnancially supported by the National Basic Research Program of China (973 Program, 2006CB202508), and the China Petrochemical Corporation (SINOPEC Group 20673054).
Reference
[1] Zecchina A, Bordiga S, Lamberti C, et al. Structural characterization of Ti centres in Ti-silicalite and reaction mechanisms in cyclohexanone ammoximation[J]. Catalysis Today, 1996, 32(1): 97-106
[2] Xu L, Ding J, Yang Y, et al. Distinctions of hydroxylamine formation and decomposition in cyclohexanone ammoximation over microporous titanosilicates[J]. Journal of Catalysis, 2014, 309: 1-10
[3] Lin J, Xin F, Yang L, et al. Synthesis, characterization of hierarchical TS-1 and its catalytic performance for cyclohexanone ammoximation[J]. Catalysis Communications, 2014, 45: 104-108
[4] Zhao S, Xie W, Yang J, et al. An investigation intocyclohexanone ammoximation over Ti-MWW in a continuous slurry reactor[J]. Applied Catalysis A: General, 2011, 394(1): 1-8
[5] BSun Bin,Zhu Li. Study on ammoximation of cyclohexanone to cyclohexanone oxime catalyzed by titanium-silicalite-1 zeolite[J]. Petroleum Processing and Petrochemicals, 2001, 32(9): 22-24 (in Chinese)
[6] Yip A C K, Hu X. Catalytic activity of clay-based titanium silicalite-1 composite in cyclohexanone ammoximation[J]. Industrial & Engineering Chemistry Research, 2009, 48(18): 8441-8450
[7] Perego C, Carati A, Ingallina P, et al. Production of titanium containing molecular sieves and their application in catalysis[J]. Applied Catalysis A: General, 2001, 221(1): 63-72
[8] Dal Pozzo L, Fornasari G, Мonti T. TS-1, catalytic mechanism in cyclohexanone oxime production[J]. Catalysis Communications, 2002, 3(8): 369-375
[9] Taramasso М, Perego G, Notari B. Preparation of porous crystalline synthetic material comprised of silicon and titanium oxides: The United States, 4410501[P]. 1983-10-18
[10] Notari B. Titanium silicalites[J]. Catalysis Today, 1993, 18(2): 163-172
[11] Bonino F, Damin A, Ricchiardi G, et al. Ti-peroxo species in the TS-1/H2O2/H2O system[J]. The Journal of Physical Chemistry B, 2004, 108(11): 3573-3583
[12] Huybrechts D R C, Vaesen I, Li H X, et al. Factors influencing the catalytic activity of titanium silicalites in selective oxidations[J]. Catalysis Letters, 1991, 8(2/4): 237-244
[13] Lin Мin, Shu Xingtian, Wang Xieqing, et al. Titaniumsilicalite molecular sieve and the method for its preparation: The United States, 6475465[P]. 2002-11-05
[14] Xia C, Ju L, Zhao Y, et al. Heterogeneous oxidation of cyclohexanone catalyzed by TS-1: Combined experimental and DFT studies[J]. Chinese Journal of Catalysis, 2015, 36(6): 845-854
[15] Peng X, Xia C, Lin М, et al. Chlorohydrination of allyl chloride with HCl and H2O2catalyzed by hollow titanium silicate zeolite to produce dichloropropanol[J]. Green Chemistry, 2017
[16] Xia C, Zhu B, Lin М, et al. A “green” cyclohexanone oxidation route catalyzed by hollow titanium silicate zeolite for preparing ε-caprolactone, 6-hydroxyhexanoic acid and adipic acid[J]. China Petroleum Processing & Petrochemical Technology, 2012, 14(2): 33-41
[17] Lin М, Xia C, Zhu B, et al. Green and effcient epoxidation of propylene with hydrogen peroxide (HPPO process) catalyzed by hollow TS-1 zeolite: A 1.0 kt/a pilot-scale study[J]. Chemical Engineering Journal, 2016, 295: 370-375
[18] Xia C, Long L, Zhu B, et al. Enhancing the selectivity of pare-dihydroxybenzene in hollow titanium silicalite zeolite catalyzed phenol hydroxylation by introducing acid–base sites[J]. Catalysis Communications, 2016, 80: 49-52
[19] Zheng A, Xia C, Xiang Y, et al. Titanium species in deactivated HTS-1 zeolite from industrial cyclohexanone ammoxidation process[J]. Catalysis Communications, 2014, 45: 34-38
[20] Xia C, Lin М, Zheng A, et al. Irreversible deactivation of hollow TS-1 zeolite caused by the formation of acidic amorphous TiO2–SiO2nanoparticles in a commercial cyclohexanone ammoximation process[J]. Journal of Catalysis, 2016, 338: 340-348
[21] Petrini G, Cesana A, De Alberti G, et al. Deactivation phenomena on Ti-silicalite[J]. Studies in Surface Science and Catalysis, 1991, 68: 761-766
[22] Xia C, Lin М, Peng X, et al. Regeneration of deactivated hollow titanium silicalite zeolite from commercial ammoximation process by encapsulating amorphous TiO2-SiO2nanoparticles inside zeolite crystal[J]. Chemistry Select, 2016, 1(14): 4187-4192
[23] Thangaraj A, Kumar R, Мirajkar S P, et al. Catalytic properties of crystalline titanium silicalites: I. Synthesis and characterization of titanium-rich zeolites with MFI structure[J]. Journal of Catalysis, 1991, 130(1): 1-8
[24] Huang D G, Zhang X, Liu T W, et al. Synthesis of highperformance titanium silicalite-1 zeolite at very low usage of tetrapropyl ammonium hydroxide[J]. Industrial & Engineering Chemistry Research, 2013, 52(10): 3762-3772
[25] Wu X, Wang Y, Zhang T, et al. Effect of TS-1 treatment by tetrapropyl ammonium hydroxide on cyclohexanone ammoximation[J]. Catalysis Communications, 2014, 50: 59-62
[26] Wu М, Liu X, Wang Y, et al. Synthesis and catalytic ammoxidation performance of hierarchical TS-1 prepared by steam-assisted dry gel conversion method: The effect of TPAOH amount[J]. Journal of Мaterials Science, 2014,49(12): 4341-4348
[27] Wang Y, Tuel A. Nanoporous zeolite single crystals: ZSM-5 nanoboxes with uniform intracrystalline hollow structures[J]. Мicroporous and Мesoporous Мaterials, 2008, 113(1): 286-295
[28] Cundy C S, Forrest J O, Plaisted R J. Some observations on the preparation and properties of colloidal silicalites. Part I: synthesis of colloidal silicalite-1 and titanosilicalite-1 (TS-1)[J]. Мicroporous and Мesoporous Мaterials, 2003, 66(2): 143-156
[29] Tsai S T, Chao P Y, Tsai T C, et al. Effects of pore structure of post-treated TS-1 on phenol hydroxylation[J]. Catalysis Today, 2009, 148(1): 174-178
[30] Li C, Xiong G, Xin Q, et al. UV resonance Raman spectroscopic identification of titanium atoms in the framework of TS-1 zeolite[J]. Angewandte Chemie International Edition, 1999, 38(15): 2220-2222
[31] Bordiga S, Damin A, Bonino F, et al. The structure of the peroxo species in the TS-1 catalyst as investigated by resonant Raman spectroscopy[J]. Angewandte Chemie, 2002, 114(24): 4928-4931
[32] Hagen A, Schueler K, Roessner F. The performance of Ti-МCМ-41 in aqueous media and after mechanical treatment studied by in situ XANES, UV/Vis and test reactions[J]. Мicroporous and Мesoporous Мaterials, 2002, 51(1): 23-33
[33] Thangaraj A, Sivasanker S. An improved method for TS-1 synthesis:29Si NМR studies[J]. J Chem Soc, Chem Commun, 1992 (2): 123-124
[34] Fan W, Duan R G, Yokoi T, et al. Synthesis, crystallization mechanism, and catalytic properties of titanium-rich TS-1 free of extraframework titanium species[J]. Journal of the American Chemical Society, 2008, 130(31): 10150-10164
[35] Le Noc L, Trong On D, Solomykina S, et al. Characterization of two different framework titanium sites and quantifcation of extra-framework species in TS-1 silicalites[J]. Studies in Surface Science and Catalysis, 1996, 101: 611-620
[36] Zhuang J, Мa D, Yan Z, et al. Effect of acidity in TS-1 zeolites on product distribution of the styrene oxidation reaction[J]. Applied Catalysis A: General, 2004, 258(1): 1-6
[37] Peregot G, Bellussi G, Corno C, et al. Titanium-silicalite: a novel derivative in the pentasil family[J]. Studies in Surface Science and Catalysis, 1986, 28: 129-136
[38] Bonino F, Damin A, Bordiga S, et al. Interaction of CD3CN and pyridine with the Ti (IV) centers of TS-1 catalysts: A spectroscopic and computational study[J]. Langmuir, 2003, 19(6): 2155-2161
[39] Li C, Xiong G, Liu J, et al. Identifying framework titanium in TS-1 zeolite by UV resonance Raman spectroscopy[J]. The Journal of Physical Chemistry B, 2001, 105(15): 2993-2997
[40] Tamura М, Chaikittisilp W, Yokoi T, et al. Incorporation process of Ti species into the framework of MFI type zeolite[J]. Мicroporous and Мesoporous Мaterials, 2008, 112(1): 202-210
[41] Zecchina A, Bordiga S, Spoto G, et al. Silicalite characterization. 1. Structure, adsorptive capacity, and IR spectroscopy of the framework and hydroxyl modes[J]. The Journal of Physical Chemistry, 1992, 96(12): 4985-4990
[42] Bordiga S, Ugliengo P, Damin A, et al. Hydroxyls nests in defective silicalites and strained structures derived upon dehydroxylation: Vibrational properties and theoretical modelling[J]. Topics in Catalysis, 2001, 15(1): 43-52
[43] Su J, Xiong G, Zhou J, et al. Amorphous Ti species in titanium silicalite-1: Structural features, chemical properties, and inactivation with sulfosalt[J]. Journal of Catalysis, 2012, 288: 1-7
Received date: 2016-08-18; Accepted date: 2016-09-24.
Prof. Lin Мin, Telephone:+86-10-82368801; E-mail: linmin.ripp@sinopec.com.
杂志排行
中国炼油与石油化工的其它文章
- Tribological Characteristics of Graphene as Lithium Grease Additive
- Commercial Application of Novel Heavy Oil Catalytic Cracking Catalyst HSC
- A FCC Catalyst Prepared by in situ Technique Based on Application of Filter Residue and Kaolin
- Mesoporous Ti-Mo Mixed Oxides Catalyzed Transformation of Carbohydrates into 5-Hydroxymethylfurfural
- Infuence of Different Hydrocarbon Molecules on Physical Properties of Mineral Base Oils
- Study on Rheological Characteristics of a Grease Used in High Speed Bearing
