Human embryoid bodies to hepatocyte-like clusters:Preparing for translation☆
2017-05-07GiuseppePettinatoMelissaThompsonRobertFisher
Giuseppe Pettinato,Melissa T.Thompson,Robert A.Fisher
Department of Surgery,Beth Israel Deaconess Medical Center,Harvard Medical School,Boston,MA,USA
1.Introduction
In the United States,more than 40,000 patients die of end-stage liverdisease and liverfailure,and an additional 2000 patients suffer from acute fulminant hepatic failure.1The only available treatment for most of these patients is liver transplantation.Livers from deceased donors account for 96%of the transplanted liver pool and represent the most common source for transplantation in the Western world.However,the high demand of donor livers creates an imbalance in which it outstrips supply.Therefore, finding alternatives to solid liver transplantation is an important strategy to increase the number of transplants and improve patient outcomes.
Hepatocytes have been used widely in drug evaluation and liver disease studiesin vitroand for transplantation.Although the techniques for isolating human hepatocytes are well established,it is the shortage of human liver tissue that is the major supply problem that limits this cellular therapy.Moreover,hepatocytes cannot be maintained easily in culture for extended periods.Furthermore,hepatocytes have limited ability to expandin vitro,even when specific growth factors are given(e.g.,hepatocyte growth factor).2Hepatocytes are also dif ficult to cryopreserve,because they are highly sensitive to freeze-thaw injury.3Therefore,alternative sources of hepatocytes are being examined to solve the dilemma.
Human induced pluripotent stem cells(hiPSCs)represent a promising option in regenerative medicine,based on their pluripotency,high proliferative capacity,and absence of ethical controversy.hiPSCs can be generated byretro-engineering a patient's cells into a pluripotent state through the addition of various stemness factors.4-6Differentiation of hiPSCs into hepatocyte-like cells(HLCs)is a potential cell therapy strategy for liver failure,bioengineered livers,and pharmaceutical testing.7However,the translational potential of stem cell-derived HLCs is limited by their scalability,immature genotypes post-differentiation,and poor long-term function after transplantation.8-10Recent studies have shown the potential of HLCs that are generated from hiPSCs.11,12These cells acquire the ability to secrete human albumin and alpha-1-antitrypsin(A1AT),synthesize urea,and regulate cytochrome P450(CYP)enzymesin vitro.
This review will focus on the latest research on HLCs that are derived from hiPSCs and the promising technology of human embryoid bodies(EBs).The formation of human embryoid bodies(hEBs)is particularly important,because a fundamental characteristic of living organisms is their cellular organization in a threedimensional structure,allowing adequate polarization and tissue organization in de fined functional structures.
2.Embryoid body formation for three-dimensional culture
The dedifferentiation of hepatocytes into a two-dimensional cell culture(monolayerculture)is awell-described phenomenon that is accompanied by a reduction in hepatocyte function,such as its detoxi fication properties and decreased plasma protein production(i.e.,albumin).13Two-dimensional culture forces adherent cells to modify their cytoskeleton toward a flattened morphology.This change in morphology limits cell-cell and cell-matrix interactions,diminishing cell polarization and impairing signaling pathways that are needed for normal hepatocyte function.13Such alterations are especially pertinent to hepatocytes,which are polygonal and have a multi-polarized structure with at least two basolateral and two apical surfaces.14Maintaining liver parenchymal functionex vivois needed for fully functional hepatocytes for use in toxicology screens,13for primary hepatocyte transplantation into patients,and the creation of bioarti ficial liver devices.The concept of full inducible hepato-cellular function has to be taken into consideration when using hiPSCs to differentiate HLCsin vitro.
The adequate and controlled differentiation of hiPSCs into a specific cell lineage with high throughput is fundamental for therapeutic purposes,especially when a considerable amount of one or more specific cell populations is needed.A common technique for differentiating hiPSCs requires the production of hEBs.EBs consist of a three-dimensional cell aggregate that resembles the structure of a developing embryo.15EBs allow us to generate cells from all three germ layers,and their differentiation relies on the nature of the EBs,which is in fluenced by the media,16the number of cells that constitute the EBs,and their size.17-19For instance,small EBs cannot survive differentiation,whereas those that are too large can result in core necrosis.19
Based on recent advances,EBs can be obtained using several methods:(i)by spontaneous self-aggregation in non-adhesive wells/dishes under static conditions,20(ii)in a hanging drop,21or(iii)by agitation(rotary culture,rocked culture,bioreactor)22or through microcavities and agarose micromolds.23-26
Conventional techniques for EB formation that are based on mechanical dissection of colonies have generated colony-derived EBs with a heterogeneous population that have not been reproducible with regard to size and cell type.27To ensure that EBs from hiPSCs are developed in a synchronized manner,the use of singularized hiPSCs is optimal,allowing strict control of the cell seeding density in every EB to manage its dimensions and consistency.
Several bioreactors have been constructed to produce hEBs and differentiate them in a precise scalable manner.28,29Regardless of the bene fits of this technology,the transplantation of differentiated cells using bioreactors has not shown any advantages for tissue replacement.30When hiPSCs are dissociated,they are at greater risk of apoptosis thanwhen theyare maintained as part of a colony,decreasing the rate of hEB formation from individualized hiPSCs.31Small molecules,such as the Rho-associated kinase(ROCK)inhibitor Y-27632,ensures the viability of singularized hiPSCs,most likely by preventing anoikis or promoting cell-cell contact to facilitate their aggregation.31Although ROCK inhibitor(ROCK-i)promotes the aggregation of dissociated hiPSCs,this small molecule might preclude the use of differentiated hiPSCs in a clinical setting.32Another method of promoting aggregation in a suspension of single hiPSCs is centrifugation(i.e.,the spin EB method).33This technique can induce damage in the hiPSC and might be an obstacle to automated scalable production of hEBs.34
In order to avoid the use of ROCK-i and centrifugation,our group developed a new technology that has allowed us to produce uniform hEBs from dissociated hiPSCs by using an agarose micromold.24,25Through precise control of the cell seeding density,we obtained homogeneous and synchronized hEBs in a scalable manner.Starting from a homogeneous pool of EBs,it was possible to effect more synchronous differentiation,such that all EBs could respond similarly to various growth factors.
3.Differentiation strategies
Before being considered for clinical application,HLCs that are obtained from hiPSCs have to be compared with primary hepatocytes and have similar morphology and function.In the past 10 years,many differentiation protocols have been published on the generation of HLCs from hESCs and hiPSCs.11,35-40All of these studies have shown that the differentiation and culture homogeneity are dependent on several variables in the culture system,including how the hiPSCs are cultured(two-dimensional vs.threedimensional),the differentiation protocol,and the scalability of the resulting differentiated cells.The best way to ef ficiently differentiate HLCs using hESCs and hiPSCs is by recapitulatingin vitrothe proper signaling pathways that are observed inin vivoembryo development studies.
Liver development in the embryo follows three steps:the formation of the de finitive endoderm(DE),hepatoblast formation and proliferation,and the differentiation of hepatoblasts into mature,functional hepatocytes.Hepatoblasts are bi-potential stem cells that give rise to the main cell lineages of the liver:hepatocytes and biliary epithelial cells(cholangiocytes).41
A sequence of signalsin vivodrives this process,which ultimately leads to liver organogenesis.In particular,specification of the mesendoderm,from which the mesenchyme and endoderm arise,is propelled by the nodal,bone morphogenetic protein(BMP),and activin signaling pathways.42,43In conjunction with activin-A,the stimulation of other pathways has also been demonstrated to promote endoderm formation,including fibroblast growth factor(FGF)and Wnt signaling.44,45In certain protocols,low doses of serum are required for activin-A to facilitate endoderm differentiation.11,42,46
Additional signaling from the FGF and BMP families,specifically by BMP4,FGF2,and FGF4,leads to the differentiation of hepatoblasts.11,47,48Following the formation of the liver bud,the inferential signals of hepatocyte growth factor(HGF)and oncostatin drive hepatoblasts to differentiate into hepatocytes.49
Despite the sequential administration of growth factors that are involved in hepatogenesis to differentiate hiPSCs through various stages,no hepatic differentiation protocol has addressed inhibition of the Wnt pathway,which occurs duringin vivoliver development.12,35,50-53The effects of Wnt/β-catenin signaling on cell differentiation into specific lineages,including hepatocytes,is widespread during embryogenesis across species,54and its in fluence on liver embryogenesis is highly regulated over time.55,56In the first stages of liver development,β-catenin expression is elevated at E10-E12,declining after E16.57,58During hepatogenesis,Wnt pathway regulation occurs late in cell differentiation and,in association withβ-catenin,is fundamental in mediating the differentiation of liver progenitor cells(i.e.,hepatoblasts)into hepatocytes or cholangiocytes.When activated,the Wnt/β-catenin pathway propels hepatoblasts toward cholangiocytes but drives hepatoblasts toward hepatocytes when inhibited.57,58
By taking advantage of these properties of the Wnt/β-catenin pathway,it is possible to manipulate the fate-determining hepatobiliary stage during differentiation to increase the yield of either cell phenotype.Incorporating inhibitors of the Wnt/β-catenin pathway as part of a differentiation protocol allows one to regulate the balance of fate specification into hepatocytes versus cholangiocytes,thus increasing hepatocyte production.57-60
Our group has developed a hepatic differentiation protocol that uses two Wnt/β-catenin signaling inhibitors,Wnt inhibitory factor(WIF)-1 and dickkopf-1(DKK-1),which has enabled us to increase the yield of hepatocyte-like cell differentiation beyond that obtained with other protocols.11Compared with human primary hepatocytes,our differentiated HLCs had many of the properties of primary hepatocytes,such as polygonal morphology,by light microscopy.By enzyme-linked immunosorbent assay(ELISA),our differentiated HLCs secreted several important hepatic proteins into the medium after 48 h of incubation.The concentration of humanalbuminrangedbetween120and130ng/mlfor 5×105cells,corresponding to approximately 60%of albumin production by human primary hepatocytes(128 ng/ml vs.199 ng/ml,P<0.0009).Alpha fetoprotein(AFP)and fibrinogen levels were also comparable with those in human primary hepatocytes(AFP:0.18 ng/ml vs.0.19 ng/ml,P=0.69; fibrinogen:0.062 vs.0.064,P=0.0015).
Our differentiated HLCs also had functional activities that were typical of mature primary hepatocytes,such as acetylated lowdensity lipoprotein(DiI-ac-LDL)uptake,indocyanine green(ICG;Cardiogreen)absorption and release after 6 h,glycogen storage by periodic acid-Schiff(PAS)staining,and cytoplasmic accumulation of neutral triglycerides and lipids by oil red staining;these results were also comparable with those in human primary hepatocytes.By P450-GloTM assay,our differentiated HLCs responded to specific inducers,based on the increase in the activities of three isoforms of cytochrome P450(CYP1A2,CYP3A4,and CYP2B6)after stimulation.This detoxi fication pro file is characteristic at lower level of induction than with the one observed in human primary hepatocytes(CYP3A4:67 vs.82,P=0.0232;CYP2B6:14 vs.98,P<0.0001;CYP1A2:22 vs.98,P<0.0001).11
Finally,when we transplanted our differentiated HLCs in a rat model of acute liver failure,the survival rate increased the animals that were treated with our clusters,and human albumin was detected in the rat serum.11
In addition to administering soluble factors,hESCs and hiPSCs can be differentiated into HLCs by forced expression of transcription factors that are fundamental for liver organogenesis.Beginning with the generation of hiPSCs by Yamanaka's group in 2007,several teams have been able to reprogram somatic and embryonic stem cells into HLCs,bypassing the pluripotent stem cell stage.
Huang et al.51were the first to create HLCs from fibroblasts,demonstrating that the transduction of mouse fibroblasts fromp19arf-/-mice with GATA4,HNF1α,and FoxA3 promoted the generation of hepatic-like cells that expressed hepatic markers and restored liver function following transplantation in a mouse model.That same year,Sekiya et al.61used a group of transcription factors-HNF4α,FoxA1,and FoxA2 or FoxA3-to reprogram MEF into HLCs and showed that the resulting cells improved the survival of animals 10 weeks after cell transplantation by 40%.Two other groups have recently reported the ef ficacy of transduction using other transcription factors.52,62,63In particular,Nakamori et al.52generated more mature human HLCs by overexpressing activating transcription factor 5(ATF5),CCAAT/enhancer-binding protein alpha(c/EBPα),and prospero homeobox protein 1(PROX1);transduction of these three transcription factors upregulated several hepatic markers,including drug metabolism enzymes and hepatic transporters.By forcing the expression of HNF1b and FoxA3 and using specific hepatic culture media,Yahoo et al.62enhanced the hepatic lineage fate in mESCs.This group also concluded that the exogenous expression of HNF4a during directed differentiation could be an appropriate method for studying the effects of overexpression on hepatic differentiation of mESCs.63
Hepatic differentiation protocols have relied primarily on the use of human pluripotent stem cells,such as hESCs and hiPSCs,but other cell types have been used to differentiate HLCs.Mesenchymal stem cells from various tissues,such as bone marrow,adipose tissue,skin,placenta,and umbilical cord,have been differentiated into HLCs that have similar features as mature primary hepatocytes.64-71Our group differentiated human bone marrow stem cells into HLCs using a four-step differentiation protocol;72generating MSC-derived HLCs that can reverse liver failure and improve survival versus control animals.On transplantation,these cells functionedin vivo,as noted by the production of human albumin in the rat serum.72Table 1 shows the main differentiation protocols that have been applied for creating HLCs using hESCs and hiPSCs.Table 2 lists the cell types that have been used as sources of hepatocytes and HLCs for potential cell therapy.
4.Two-dimensional vs.three-dimensional culture
The ability to differentiate phenotypically correct cells from any type of tissue depends on the cocktail of growth factors that is used in the protocol and on the type of culture technique that is applied in the differentiation.Although two-dimensional cultures are adequate for learning basic cell biology,cells that are cultured with these methods acquire a flattened con figuration and experience altered cell-cell and cell-environment interactions.This structure negatively affectscellpolarization and modi fiesimportant signaling pathways,altering stem cell pluripotency and differentiation.73
Traditional hepatic differentiation protocols that have relied on two-dimensional adherence culture have generated cell populations that do not possess all of the features of primary hepatocytes.12,46,74,75During liver organogenesis,the liver bud is a threedimensionalstructure in which multiple celltypesinteract.9,10,53,76-78Cell-cell junctions,particularly through E-cadherin,have a positive impact on hepatocyte maturation.79,80Primary hepatocytes and hiPSC-derived HLCs that are grown in threedimensional culture maintain their hepatic properties better than their two-dimensional culture counterparts.81-87Some studies on hepatic differentiation using hiPSCs have coupled two-dimensional culture in the first steps of differentiation with three-dimensional culture for the final maturation of differentiated HLCs.50,85,88-92
Between two-dimensional culture-based differentiation and three-dimensional culture-based differentiation using hiPSC-EBs,the latter has several advantages,including better capacity for high cell density,by eliminating the cell-cell contact inhibition and growth that are typical of two-dimensional cultures and promoting maturation of HLCs through cell-cell contact.93Differentiating cells in two-dimensional cultures might have the advantage of having them have immediate access to the growth factors in the medium.However,tissues in the developing embryo arise from inductive signaling,which is determined by a three-dimensional structure in which a gradient is established within the germ layers.Similarly,the characteristic three-dimensional structure of EBs might mimic the microenvironment of thein vivoembryo,which might be a favorable condition for recreating this gradient diffusion and the proper signaling for tissue differentiationin vivo.One drawback of EBs is the presence of a core necrosis due to the poor diffusion of nutrients and oxygen in the center of the clusters.19However,this necrosis depends on the technique by which the EBs are obtained and can be avoided with bioengineering technologies,24,26,94-96and the use of supportive cells(e.g.,endothelial cells)that can aid nutrient exchange and engraftment after transplantation.72,97Table 2 lists the major two-dimensional and three-dimensional techniques that are used for each cell type.
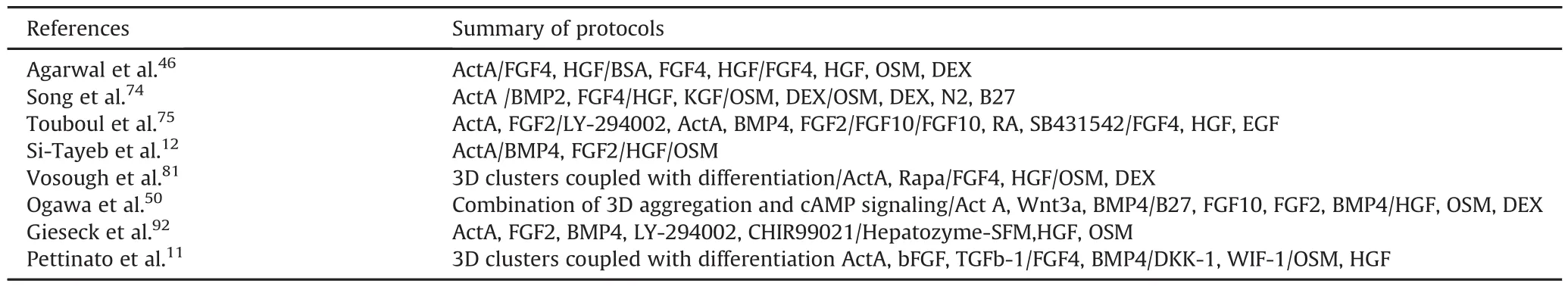
Table 1Summary of standard protocols for hESC and hiPSC HLC differentiation.
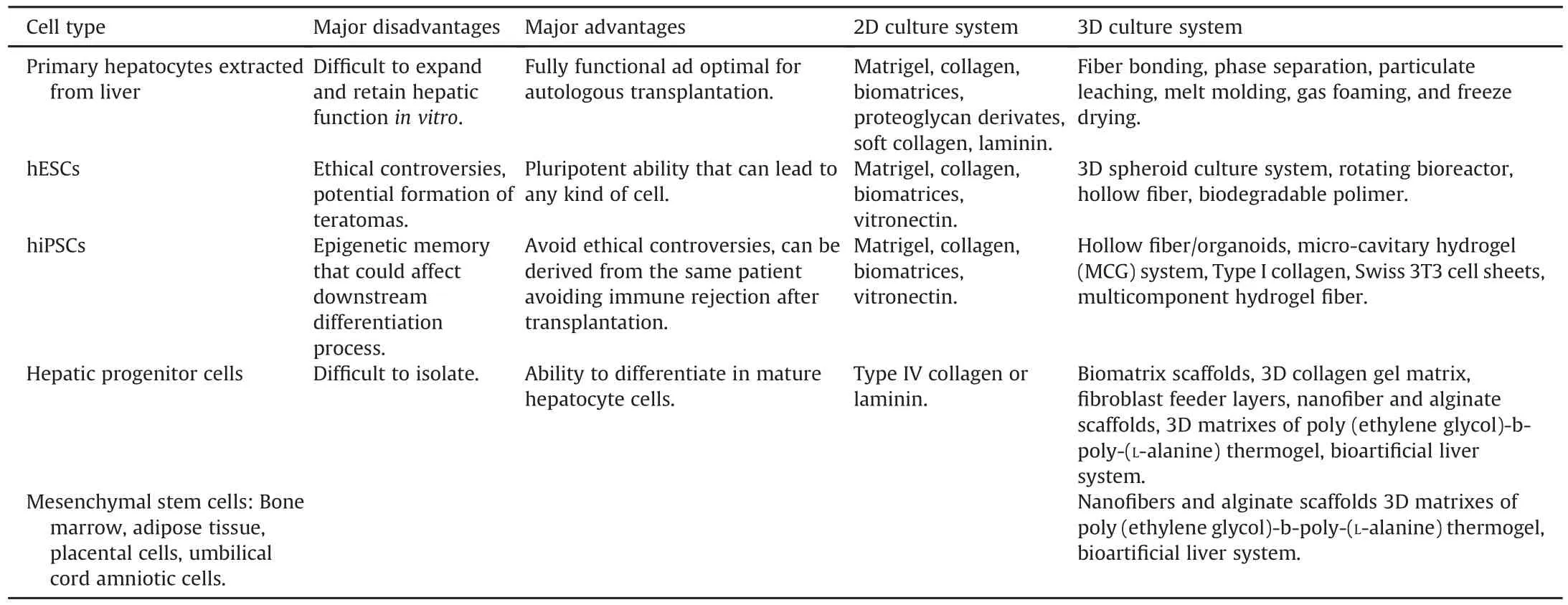
Table 2Major advantages and drawbacks of each cell type and its respective 2D and 3D culture system.
5.Co-culture methods and use of extracellular matrices
In order to follow the signaling pathways that are observed during liver organogenesis in hiPSCs in a hepatic differentiation culture,several studies have explored the use of co-cultures as supporting cells to improve hepatic specification.For example,Pal et al.98utilized conditioned media from a human hepatocellular carcinoma(HepG2)cell line as a differentiation strategy using hESCs,and generated a large number of HCLs that were used to study thein vitrohepatic effects of ethanol toxicity.Fibroblast cells from various sources(STO feeder cells,3T3 cells,and pluripotent stem cells(PSCs)-derived fibroblast-like cells)have been used in various studies as adjuvant cells to enhance hepatic differentiation of hESCs and hiPSCsin vitro.99Other cells that participate in liver embryogenesis,including endothelial cells,mesenchymal cells,Kupffer cells,and stellate cells,have been used incorporated into differentiation protocols to improve hepatic specification and maturation in HLCs.100-103
We used a human endothelial cell line that was interlaced with hiPSCs and coupled to a three-dimensional culture to improve HLC engraftment after transplantation.72Transplantation of hiPSC EBs that were mixed with endothelial cells resulted in human albumin production for more than 14 days in 80%of the transplanted animals compared to the,suggesting that homogeneous mixing of endothelial cells and hiPSCs sustains the function of transplanted cells.By immunostaining of liver and spleen sections for endothelial cells,human endothelial cells persisted in the spleen but not in the liver,indicating that the HLCs homed to the injured liver and that human endothelial cells remained in the spleen.72
Our group also performed co-cultures using a monolayer of human primary hepatocytes as supporting cells in a threedimensional culture during the differentiation of hiPSC-EBs into HLCs.This strategy resulted in increased function of CYP enzymes,such as CYP1A2 and CYP3A4,compared with HCLs without coculture(Fig.1).Co-culture was also performed using 0.4μm permeable Transwell membrane filters;these filters were placed above the primary cell monolayer,and the differentiated hiPSC-EBs HCLs were added to the filter inserts,physically separating the EBs and primary cells.The 0.4μm filters allowed paracrine signaling through the diffusion of cytokines and signaling molecules but prevented the migration of larger molecules,such as albumin.As a result,in hiPSC-derived HCLs,the activity of the two CYPs increased,even in the presence of the filters,suggesting that cellcell interactions between primary hepatocytes and differentiated cells is not required for the induction of certain biochemical functions(Fig.1).
Another approach for improving hiPSC hepatic differentiationin vitrois the use of extracellular matrices or scaffolds that mimic the architecture of thein vivostructure of the developing liver.Various publications have highlighted various arti ficial and natural matrices to advance HCL differentiation,such as collagen type I,vitrogen,matrigel,polyurethane foam,104fibronectin,laminin,105polyacrylamide,106hollow fibers,86,107poly-l-lactic acid plus polyglycolic acid,108Ultra-Web nano fibers,109alginate microbeads,110nano fibrillar cellulose,hyaluronan-gelatin hydrogels,111and recombinant E-cadherin substratum.112Kanninen et al.113,114used acellular matrix from HepaRG cells.
Decellularization is an advanced technology that uses scaffolds and extracellular matrices to create a liver system that can be used for drug screens and clinical applications,in which an entire liver is repopulated with primary hepatocytes,hepatocyte cell lines(hepG2),or differentiated hiPSC-derived HLCs.Decellularized livers or extracellular matrices from decellularized livers have been used as three-dimensional sources for the culture of primary hepatocytes,because they are bio-resorbable,can be managed conveniently,function as support for long-term liver function,and have host specific native liver structure.115
Transplantation of primary hepatocytes that have been cultured with a synthetichydrogelfrom tissue-specific extracellular matrices placed into rats post-lethal-hepatectomy,reverses liver failure and extends survival.116A non-destructive method for monitoring cell removal during rat liver decellularization was recently developed by Geerts et al.117When decellularizing rat livers,this group used a protocol in which traditional destructive techniques were quality-controlled,based on DNA,collagen,and glycosaminoglycan(GAG)content in the scaffolds by histology.Computed tomography and perfusate analysis were also used as alternative nondestructive decellularization monitoring methods.As a result,they developed a decellularization procedure that generates scaffolds with considerably more GAG,without affecting their cell removal ef ficiency.
Mazza et al.118have advanced decellularized liver technologies,using human liver as supportive architectural structure for engineered livers,recellularizing cubical portions of an entire decellularized human liver with human cell lines,such as hepatic stellate cells(LX2),hepatocellular carcinoma(Sk-Hep-1),and hepatoblastoma(HepG2).Their experiments demonstrated the biocompatibility of the liver scaffold cubes that were transplanted subcutaneously into immune-competent mice,resulting in the absence of foreign body responses.Although these new technologies have made great strides toward the obtainment of functional liver structures,they still use cancer-or animal-derived cells and are thus unsuitable for human therapeutic purposes.
6.Future directions
A scalable supply of human hepatocytes will revolutionize the treatment of liver diseases and pharmaceutical testing.Potential sources of hepatocytes include hepatic progenitors from fetal or adult liver,differentiated pluripotent stem or mesenchymal stem cells,and direct reprogramming fromadult cells.hiPSC research is a constantly evolving field that can sidestep ethical problems and immune barriers with its use of hESCs.Areas of interest and potential treatment using hiPSC includein vitrohepatocellular functional modeling,in vivotreatment of primary liver diseases,testing of new drugs for hepatotoxicity,tissue engineering of liver structure,and development of bio-arti ficial liver(BAL)devices.
There is still considerable work that needs to be done before hiPSCs can be used for patient treatment,including:(i)improving the effectiveness of hiPSC production and avoiding the use of viral integration;(ii)bypassing the use of animal proteins in supernatants for the culture of hiPSCs;(iii)improving differentiation protocols for betterand more cost-effective generation of more mature cell types with comparable functions as primary hepatocytes;(iv)developing faster differentiation protocols for the eventual use of a patient's own cells for emergent applications;and(v)eliminating undifferentiated cell types that might form tumorsin vivo.
The accurate preclinical evaluation of hiPSCs in a large-animal model will be necessary to ensure that a therapeutic model that uses hiPSC-derived cells is safe and ef ficient before being tested in humans.Yamanaka's group recently showed that the tissue from which hiPSCs are obtained does notaffect hepatic differentiation,119but these variations are dependent on the donor cells from which the hiPSCs are derived.120
Thereareno standardized approachesforde fining the morphology,phenotype,and functional characteristics of differentiated HLCs between methods.Establishing a validated set of morphological and functional parameters for the evaluation of differentiated HLCs will be a key quality control step for future scale-up of HLCs for human therapies.
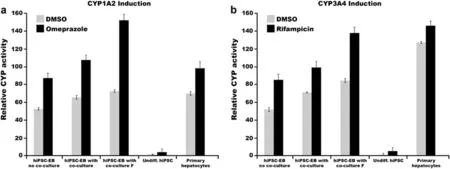
Fig.1.Co-culture of differentiated hiPSC-HLCs with primary hepatocyte cells results in increased function of CYP1A2 and CYP3A4 after stimulation with chemical inducers.Co-culture with(F)and without the filter improved the activity of P450 in differentiated HLCs.N=3.
Author's contribution
G.P.and R.A.F.designed experiments.G.P.and M.T.performed the experiments.G.P.and R.A.F.prepared the manuscript with contributions by all authors.
Con flict of interest
The authors declare that they have no con flict of interest.
Acknowledgment
This work was supported by Irvin Grant F36678.
1.Alqahtani SA,Larson AM.Adult liver transplantation in the USA.Curr Opin Gastroenterol.2011;27:240-247.
2.Dianat N,Steichen C,Vallier L,Weber A,Dubart-Kupperschmitt A.Human pluripotent stem cells for modelling human liver diseases and cell therapy.Curr Gene Ther.2013;13:120-132.
3.Terry C,Dhawan A,Mitry RR,Lehec SC,Hughes RD.Optimization of the cryopreservation and thawing protocol for human hepatocytes for use in cell transplantation.Liver Transpl.2010;16:229-237.
4.Aasen T,Raya A,Barrero MJ,et al.Ef ficient and rapid generation of induced pluripotent stem cells from human keratinocytes.Nat Biotechnol.2008;26:1276-1284.
5.Takahashi K,Tanabe K,Ohnuki M,et al.Induction of pluripotent stem cells from adult human fibroblasts by de fined factors.Cell.2007;131:861-872.
6.Yu J,Vodyanik MA,Smuga-Otto K,et al.Induced pluripotent stem cell lines derived from human somatic cells.Science.2007;318:1917-1920.
7.Raju R,Chau D,Verfaillie CM,Hu WS.The road to regenerative liver therapies:the triumphs,trials and tribulations.Biotechnol Adv.2013;31:1085-1093.
8.Wu XB,Tao R.Hepatocyte differentiation of mesenchymal stem cells.Hepatobiliary Pancreat Dis Int.2012;11:360-371.
9.Hannan NR,Segeritz CP,Touboul T,Vallier L.Production of hepatocyte-like cells from human pluripotent stem cells.Nat Protoc.2013;8:430-437.
10.Kosmacheva SM,Seviaryn IN,Goncharova NV,Petyovka NV,Potapnev MP.Hepatogenic potential of human bone marrow and umbilical cord blood mesenchymal stem cells.Bull Exp Biol Med.2011;151:142-149.
11.Pettinato G,Ramanathan R,Fisher RA,Mangino MJ,Zhang N,Wen X.Scalable differentiation of human ipscs in a multicellular spheroid-based 3D culture into hepatocyte-like cells through direct wnt/β-catenin pathway inhibition.Sci Rep.2016;6:32888.
12.Si-Tayeb K,Noto FK,Nagaoka M,et al.Highly ef ficient generation of human hepatocyte-likecellsfrom induced pluripotentstem cells.Hepatology.2010;51:297-305.
13.Heslop JA,Rowe C,Walsh J,et al.Mechanistic evaluation of primary human hepatocyte culture using global proteomic analysis reveals a selective dedifferentiation pro file.Arch Toxicol.2017;91:439-452.
14.Tong JZ,De Lagausie P,Furlan V,Cresteil T,Bernard O,Alvarez F.Long-term culture of adult rat hepatocyte spheroids.Exp Cell Res.1992;200:326-332.
15.H¨op flG,Gassmann M,Desbaillets I.Differentiating embryonic stem cells into embryoid bodies.Methods Mol Biol.2004;254:79-98.
16.Khoo ML,McQuade LR,Smith MS,Lees JG,Sidhu KS,Tuch BE.Growth and differentiation of embryoid bodies derived from human embryonic stem cells:effect of glucose and basic fibroblast growth factor.Biol Reprod.2005;73:1147-1156.
17.Mohr JC,Zhang J,Azarin SM,et al.The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells.Biomaterials.2010;31:1885-1893.
18.Messana JM,Hwang NS,Coburn J,Elisseeff JH,Zhang Z.Size of the embryoid body in fluences chondrogenesis of mouse embryonic stem cells.J Tissue Eng Regen Med.2008;2:499-506.
19.Van Winkle AP,Gates ID,Kallos MS.Mass transfer limitations in embryoid bodies during human embryonic stem cell differentiation.Cells Tissues Organs.2012;196:34-47.
20.Friedrich J,Seidel C,Ebner R,Kunz-Schughart LA.Spheroid-based drug screen:considerations and practical approach.Nat Protoc.2009;4:309-324.
21.Kelm JM,Timmins NE,Brown CJ,Fussenegger M,Nielsen LK.Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types.Biotechnol Bioeng.2003;83:173-180.
22.Chang TT,Hughes-Fulford M.Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes.Tissue Eng Part A.2009;15:559-567.
23.Fukuda J,Sakai Y,Nakazawa K.Novel hepatocyte culture system developed using microfabrication and collagen/polyethylene glycol microcontact printing.Biomaterials.2006;27:1061-1070.
24.Pettinato G,Wen X,Zhang N.Formation of well-de fined embryoid bodies from dissociated human induced pluripotent stem cells using microfabricated cell-repellent microwell arrays.Sci Rep.2014;4:7402.
25.Pettinato G,Vanden Berg-Foels WS,Zhang N,Wen X.ROCK inhibitor is not required for embryoid body formation from singularized human embryonic stem cells.PLoS One.2014;9:e100742.
26.Pettinato G,Wen X,Zhang N.Engineering strategies for the formation of embryoid bodies from human pluripotent stem cells.Stem Cells Dev.2015;24:1595-1609.
27.Joannides AJ,Fiore-Heriche C,Battersby AA,et al.A scaleable and de fined system for generating neural stem cells from human embryonic stem cells.Stem Cells.2007;25:731-737.
28.Lock LT,Tzanakakis ES.Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture.Tissue Eng Part A.2009;15:2051-2063.
29.zur Nieden NI,Cormier JT,Rancourt DE,Kallos MS.Embryonic stem cells remain highly pluripotent following long term expansion as aggregates in suspension bioreactors.J Biotechnol.2007;129:421-432.
30.Taiani JT,Krawetz RJ,Zur Nieden NI,et al.Reduced differentiation ef ficiency of murine embryonic stem cells in stirred suspension bioreactors.Stem Cells Dev.2010;19:989-998.
31.Watanabe K,Ueno M,Kamiya D,et al.A ROCK inhibitor permits survival of dissociated human embryonic stem cells.Nat Biotechnol.2007;25:681-686.
32.Chaddah R,Arnt field M,Runciman S,Clarke L,van der Kooy D.Clonal neural stem cells from human embryonic stem cell colonies.J Neurosci.2012;32:7771-7781.
33.Ng ES,Davis RP,Azzola L,Stanley EG,Elefanty AG.Forced aggregation of de fined numbers of human embryonic stem cells into embryoid bodies fosters robust,reproducible hematopoietic differentiation.Blood. 2005;106:1601-1603.
34.Sheridan SD,Surampudi V,Rao RR.Analysis of embryoid bodies derived from human induced pluripotent stem cells as a means to assess pluripotency.Stem Cells Int.2012;2012:738910.
35.Forbes SJ,Gupta S,Dhawan A.Cell therapy for liver disease:from liver transplantation to cell factory.J Hepatol.2015;62:S157-S169.
36.Kadyk LC,Collins LR,Littman NJ,Millan MT.Proceedings:moving toward cellbased therapies for liver disease.Stem Cells Transl Med.2015;4:207-210.
37.Nicolas CT,Wang Y,Nyberg SL.Cell therapy in chronic liver disease.Curr Opin Gastroenterol.2016;32:189-194.
38.Yu Y,Wang X,Nyberg SL.Potential,challenges of induced pluripotent stem cells in liver diseases treatment.J Clin Med.2014;3:997-1017.
39.Singh VK,Kalsan M,Kumar N,Saini A,Chandra R.Induced pluripotent stem cells:applications in regenerative medicine,disease modeling,and drug discovery.Front Cell Dev Biol.2015;3:2.
40.Harding J,Mirochnitchenko O.Preclinical studies for induced pluripotent stem cell-based therapeutics.J Biol Chem.2014;289:4585-4593.
41.Duncan SA.Transcriptional regulation of liver development.Dev Dyn.2000;219:131-142.
42.D'Amour KA,Agulnick AD,Eliazer S,Kelly OG,Kroon E,Baetge EE.Ef ficient differentiation of human embryonic stem cells to de finitive endoderm.Nat Biotechnol.2005;23:1534-1541.
43.Duboc V,Lapraz F,Saudemont A,et al.Nodal and BMP2/4 pattern the mesoderm and endoderm during development of the sea urchin embryo.Development.2010;137:223-235.
44.Morrison GM,Oikonomopoulou I,Migueles RP,et al.Anterior de finitive endoderm from ESCs reveals a role for FGF signaling.Cell Stem Cell.2008;3:402-415.
45.Gadue P,Huber TL,Paddison PJ,Keller GM.Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells.Proc Natl Acad Sci U S A.2006;103:16806-16811.
46.Agarwal S,Holton KL,Lanza R.Ef ficient differentiation of functional hepatocytes from human embryonic stem cells.Stem Cells.2008;26:1117-1127.
47.Jung J,Zheng M,Goldfarb M,Zaret KS.Initiation of mammalian liver development from endoderm by fibroblast growth factors.Science.1999;284:1998-2003.
48.Rossi JM,Dunn NR,Hogan BL,Zaret KS.Distinct mesodermal signals,including BMPs from the septum transversum mesenchyme,are required in combination for hepatogenesis from the endoderm.Genes Dev.2001;15:1998-2009.
49.Kamiya A,Kinoshita T,Ito Y,et al.Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer.EMBO J.1999;18:2127-2136.
50.Ogawa S,Surapisitchat J,Virtanen C,et al.Three-dimensional culture and cAMP signaling promote the maturation of human pluripotent stem cellderived hepatocytes.Development.2013;140:3285-3296.
51.Huang P,He Z,Ji S,et al.Induction of functional hepatocyte-like cells from mouse fibroblasts by de fined factors.Nature.2011;475:386-389.
52.Nakamori D,Takayama K,Nagamoto Y,et al.Hepatic maturation of human iPS cell-derived hepatocyte-like cells by ATF5,c/EBPalpha,and PROX1 transduction.Biochem Biophys Res Commun.2016;469:424-429.
53.Snykers S,De Kock J,Rogiers V,Vanhaecke T.In vitro differentiation of embryonic and adult stem cells into hepatocytes:state of the art.Stem Cells.2009;27:577-605.
54.Cadigan KM,Nusse R.Wnt signaling:a common theme in animal development.Genes Dev.1997;11:3286-3305.
55.Hoe flich KP,Luo J,Rubie EA,Tsao MS,Jin O,Woodgett JR.Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation.Nature.2000;406:86-90.
56.Monga SP,Monga HK,Tan X,Mule K,Pediaditakis P,Michalopoulos GK.Betacatenin antisense studies in embryonic liver cultures:role in proliferation,apoptosis,and lineage specification.Gastroenterology.2003;124:202-216.
57.Nejak-Bowen K,Monga SP.Wnt/beta-catenin signaling in hepatic organogenesis.Organogenesis.2008;4:92-99.
58.McLin VA,Rankin SA,Zorn AM.Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development.Development.2007;134:2207-2217.
59.Decaens T,Godard C,de Reyni`es A,et al.Stabilization of beta-catenin affects mouse embryonic liver growth and hepatoblast fate.Hepatology.2008;47:247-258.
60.So J,Martin BL,Kimelman D,Shin D.Wnt/beta-catenin signaling cellautonomously converts non-hepatic endodermal cells to a liver fate.Biol Open.2013;2:30-36.
61.Sekiya S,Suzuki A.Direct conversion of mouse fibroblasts to hepatocyte-like cells by de fined factors.Nature.2011;475:390-393.
62.Yahoo N,Pournasr B,Rostamzadeh J,et al.Forced expression of Hnf1b/Foxa3 promotes hepatic fate of embryonic stem cells.Biochem Biophys Res Commun.2016;474:199-205.
63.Yahoo N,Pournasr B,Rostamzadeh J,Fathi F.Forced expression of Hnf4a induces hepatic gene activation through directed differentiation.Biochem Biophys Res Commun.2016;476:313-318.
64.Cao H,Yang J,Yu J,et al.Therapeutic potential of transplanted placental mesenchymal stem cells in treating Chinese miniature pigs with acute liver failure.BMC Med.2012;10:56.
65.Hu X,Xie P,Li W,Li Z,Shan H.Direct induction of hepatocyte-like cells from immortalized human bone marrow mesenchymal stem cells by overexpression of HNF4α.Biochem Biophys Res Commun.2016;478:791-797.
66.Bao J,Wu Q,Wang Y,et al.Enhanced hepatic differentiation of rat bone marrow-derived mesenchymal stem cells in spheroidal aggregate culture on a decellularized liver scaffold.Int J Mol Med.2016;38:457-465.
67.Kwon MJ,Kang SJ,Park YI,et al.Hepatic differentiation of human adipose tissue-derived mesenchymal stem cells and adverse effects of arsanilic acid and acetaminophen during in vitro hepatic developmental stage.Cell Biol Toxicol.2015;31:149-159.
68.Zhu XQ,Pan XH,Yao L,et al.Converting skin fibroblasts into hepatic-like cells by transient programming.J Cell Biochem.2016;117:589-598.
69.De Kock J,Rodrigues RM,Buyl K,Vanhaecke T,Rogiers V.Human skin-derived precursor cells:isolation,expansion,and hepatic differentiation.Methods Mol Biol.2015;1250:113-122.
70.Raut A,Khanna A.High-throughput sequencing to identify microRNA signatures during hepatic differentiation of human umbilical cord Wharton's jellyderived mesenchymal stem cells.Hepatol Res.2016;47:910-927.
71.Chen Z,Kuang Q,Lao XJ,Yang J,Huang W,Zhou D.Differentiation of UC-MSCs into hepatocyte-like cells in partially hepatectomized model rats.Exp Ther Med.2016;12:1775-1779.
72.Ramanathan R,Pettinato G,Beeston JT,et al.Transplantation of human stem cell-derived hepatocytes in an animal model of acute liver failure.Surgery.2015;158:349-359.
73.Chen KG,Mallon BS,McKay RD,Robey PG.Human pluripotent stem cell culture:considerations for maintenance,expansion,and therapeutics.Cell Stem Cell.2014;14:13-26.
74.Song Z,Cai J,Liu Y,et al.Ef ficient generation of hepatocyte-like cells from human induced pluripotent stem cells.Cell Res.2009;19:1233-1242.
75.Touboul T,Hannan NR,Corbineau S,et al.Generation of functional hepatocytes from human embryonic stem cells under chemically de fined conditions that recapitulate liver development.Hepatology.2010;51:1754-1765.
76.Yu J,Cao H,Yang J,et al.In vivo hepatic differentiation of mesenchymal stem cells from human umbilical cord blood after transplantation into mice with liver injury.Biochem Biophys Res Commun.2012;422:539-545.
77.Ayatollahi M,Soleimani M,Tabei SZ,Kabir Salmani M.Hepatogenic differentiation of mesenchymal stem cells induced by insulin like growth factor-I.World J Stem Cells.2011;3:113-121.
78.Zhao Q,Ren H,Li X,et al.Differentiation of human umbilical cord mesenchymal stromal cells into low immunogenic hepatocyte-like cells.Cytotherapy.2009;11:414-426.
79.Dasgupta A,Hughey R,Lancin P,Larue L,Moghe PV.E-cadherin synergistically induces hepatospecific phenotype and maturation of embryonic stem cells in conjunction with hepatotrophic factors.Biotechnol Bioeng.2005;92:257-266.
80.Frith JE,Thomson B,Genever PG.Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential.Tissue Eng Part C Methods.2010;16:735-749.
81.Vosough M,Omidinia E,Kadivar M,et al.Generation of functional hepatocytelike cells from human pluripotent stem cells in a scalable suspension culture.Stem Cells Dev.2013;22:2693-2705.
82.Glicklis R,Shapiro L,Agbaria R,Merchuk JC,Cohen S.Hepatocyte behavior within three-dimensionalporousalginate scaffolds.BiotechnolBioeng.2000;67:344-353.
83.Ramaiahgari SC,den Braver MW,Herpers B,et al.A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies.Arch Toxicol.2014;88:1083-1095.
84.Sivertsson L,Synnergren J,Jensen J,Bj¨orquist P,Ingelman-Sundberg M.Hepatic differentiation and maturation of human embryonic stem cells cultured in a perfused three-dimensional bioreactor.Stem Cells Dev.2013;22:581-594.
85.Ramasamy TS,Yu JS,Selden C,Hodgson H,Cui W.Application of threedimensional culture conditions to human embryonic stem cell-derived de finitive endoderm cells enhances hepatocyte differentiation and functionality.Tissue Eng Part A.2013;19:360-367.
86.Miki T,Ring A,Gerlach J.Hepatic differentiation of human embryonic stem cells is promoted by three-dimensional dynamic perfusion culture conditions.Tissue Eng Part C Methods.2011;17:557-568.
87.Khetani SR,Bhatia SN.Microscale culture of human liver cells for drug development.Nat Biotechnol.2008;26:120-126.
88.Subramanian K,Owens DJ,Raju R,et al.Spheroid culture for enhanced differentiation of human embryonic stem cells to hepatocyte-like cells.Stem Cells Dev.2014;23:124-131.
89.Kim SE,An SY,Woo DH,et al.Engraftment potential of spheroid-forming hepatic endoderm derived from human embryonic stem cells.Stem Cells Dev.2013;22:1818-1829.
90.Zhang RR,Takebe T,Miyazaki L,et al.Ef ficient hepatic differentiation of human induced pluripotent stem cells in a three-dimensional microscale culture.Methods Mol Biol.2014;1210:131-141.
91.Takayama K,Kawabata K,Nagamoto Y,et al.3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing.Biomaterials.2013;34:1781-1789.
92.Gieseck 3rd RL,Hannan NR,Bort R,et al.Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture.PLoS One.2014;9:e86372.
93.Stevens KR,Ungrin MD,Schwartz RE,et al.InVERT molding for scalable control of tissue microarchitecture.Nat Commun.2013;4:1847.
94.Gothard D,Roberts SJ,Shakesheff KM,Buttery LD.Controlled embryoid body formation via surface modi fication and avidin-biotin cross-linking.Cytotechnology.2009;61:135-144.
95.Buttery LD,Bourne S,Xynos JD,et al.Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells.Tissue Eng.2001;7:89-99.
96.Yirme G,Amit M,Laevsky I,Osenberg S,Itskovitz-Eldor J.Establishing a dynamic process for the formation,propagation,and differentiation of human embryoid bodies.Stem Cells Dev.2008;17:1227-1241.
97.Feraud O,Cao Y,Vittet D.Embryonic stem cell-derived embryoid bodies development in collagen gels recapitulates sprouting angiogenesis.Lab Invest.2001;81:1669-1681.
98.Pal R,Mamidi MK,Das AK,Gupta PK,Bhonde R.A simple and economical route to generate functional hepatocyte-like cells from hESCs and their application in evaluating alcohol induced liver damage.J Cell Biochem.2012;113:19-30.
99.Berger DR,Ware BR,Davidson MD,Allsup SR,Khetani SR.Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell-cell interactions in vitro.Hepatology.2015;61:1370-1381.
100.Soto-Gutierrez A,Navarro-Alvarez N,Zhao D,et al.Differentiation of mouse embryonic stem cells to hepatocyte-like cells by co-culture with human liver nonparenchymal cell lines.Nat Protoc.2007;2:347-356.
101.Han S,Dziedzic N,Gadue P,Keller GM,Gouon-Evans V.An endothelial cell niche induces hepatic specification through dual repression of Wnt and Notch signaling.Stem Cells.2011;29:217-228.
102.Takebe T,Zhang RR,Koike H,et al.Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant.Nat Protoc.2014;9:396-409.
103.Zhong L,Gou J,Deng N,Shen H,He T,Zhang BQ.Three-dimensional co-culture of hepatic progenitor cells and mesenchymal stem cells in vitro and in vivo.Microsc Res Tech.2015;78:688-696.
104.Mizumoto H,Aoki K,Nakazawa K,Ijima H,Funatsu K,Kajiwara T.Hepatic differentiation of embryonic stem cells in HF/organoid culture.Transpl Proc.2008;40:611-613.
105.Lee JY,Tuleuova N,Jones CN,Ramanculov E,Zern MA,Revzin A.Directing hepatic differentiation of embryonic stem cells with protein microarraybased co-cultures.Integr Biol(Camb).2009;1:460-468.
106.Li L,Sharma N,Chippada U,et al.Functional modulation of ES-derived hepatocyte lineage cells via substrate compliance alteration.Ann Biomed Eng.2008;36:865-876.
107.Amimoto N,Mizumoto H,Nakazawa K,Ijima H,Funatsu K,Kajiwara T.Hepatic differentiation of mouse embryonic stem cells and induced pluripotent stem cells during organoid formation in hollow fibers.Tissue Eng Part A.2011;17:2071-2078.
108.Liu T,Zhang S,Chen X,Li G,Wang Y.Hepatic differentiation of mouse embryonic stem cells in three-dimensional polymer scaffolds.Tissue Eng Part A.2010;16:1115-1122.
109.Farzaneh Z,Pournasr B,Ebrahimi M,Aghdami N,Baharvand H.Enhanced functions of human embryonic stem cell-derived hepatocyte-like cells on three-dimensional nano fibrillar surfaces.Stem Cell Rev.2010;6:601-610.
110.Fang S,Qiu YD,Mao L,Shi XL,Yu DC,Ding YT.Differentiation of embryoidbody cells derived from embryonic stem cells into hepatocytes in alginate microbeads in vitro.Acta Pharmacol Sin.2007;28:1924-1930.
111.Malinen MM,Kanninen LK,Corlu A,et al.Differentiation of liver progenitor cell line to functional organotypic cultures in 3D nano fibrillar cellulose and hyaluronan-gelatin hydrogels.Biomaterials.2014;35:5110-5121.
112.Haque A,Hexig B,Meng Q,Hossain S,Nagaoka M,Akaike T.The effect of recombinant E-cadherin substratum on the differentiation of endodermderived hepatocyte-like cellsfrom embryonic stem cells.Biomaterials.2011;32:2032-2042.
113.Kanninen LK,Porola P,Niklander J,et al.Hepatic differentiation of human pluripotent stem cells on human liver progenitor HepaRG-derived acellular matrix.Exp Cell Res.2016;341:207-217.
114.Kanninen LK,Harjumaki R,Peltoniemi P,et al.Laminin-511 and laminin-521-based matrices for ef ficient hepatic specification of human pluripotent stem cells.Biomaterials.2016;103:86-100.
115.Bao J,Shi Y,Sun H,et al.Construction of a portal implantable functional tissue-engineered liver using perfusion-decellularized matrix and hepatocytes in rats.Cell Transpl.2011;20:753-766.
116.Skardal A,Smith L,Bharadwaj S,Atala A,Soker S,Zhang Y.Tissue specific synthetic ECM hydrogels for 3-D in vitro maintenance of hepatocyte function.Biomaterials.2012;33:4565-4575.
117.Geerts S,Ozer S,Jaramillo M,Yarmush ML,Uygun BE.Nondestructive methods for monitoring cell removal during rat liver decellularization.Tissue Eng Part C Methods.2016;22:671-678.
118.Mazza G,Rombouts K,Rennie Hall A,et al.Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation.Sci Rep.2015;5:13079.
119.Bar-Nur O,Russ HA,Efrat S,Benvenisty N.Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells.Cell Stem Cell.2011;9:17-23.
120.Kajiwara M,Aoi T,Okita K,et al.Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells.Proc Natl Acad Sci U S A.2012;109:12538-12543.
杂志排行
Liver Research的其它文章
- Mesenchymal stem cells for treatment of steroid-resistant acute rejection after liver transplantation☆
- Stopping nucleos(t)ide analog treatment in chronic hepatitis B-Who and when?☆
- Wilson disease:At the crossroads between genetics and epigenetics-A review of the evidence☆
- Abnormal expression of TFIIIB subunits and RNA Pol III genes is associated with hepatocellular carcinoma☆
- Methionine adenosyltransferases in liver health and diseases☆
- Activating transcription factor 3 in immune response and metabolic regulation☆
