Basiliximab application on liver recipients:a meta-analysis of randomized controlled trials
2017-04-17GuoQingZhangChengShuoZhangNingSunWuLvBaoMinChenandJiaLinZhang
Guo-Qing Zhang, Cheng-Shuo Zhang, Ning Sun, Wu Lv, Bao-Min Chen and Jia-Lin Zhang
Shenyang, China
Basiliximab application on liver recipients:
a meta-analysis of randomized controlled trials
Guo-Qing Zhang, Cheng-Shuo Zhang, Ning Sun, Wu Lv, Bao-Min Chen and Jia-Lin Zhang
Shenyang, China
BACKGROUND: The benefits of the application of basiliximab induction therapy in liver transplantation are not clear. The present meta-analysis was to evaluate the pros and cons of basiliximab use in liver transplantation.
DATA SOURCES: We searched the associated publications in English from July 1998 to December 2015 in the following databases: MEDLINE, PubMed, Ovid, EMBASE, Web of Science and Cochrane Library.
RESULTS: Basiliximab significantly decreased the incidence of de novo diabetes mellitus after liver transplantation (RR=0.56; 95% CI: 0.34-0.91; P=0.02). Subgroup analysis showed that basiliximab in combination with steroids-free immunosuppressant significantly decreased the incidence of biopsy-proven acute rejection (RR=0.62; 95% CI: 0.39-0.97; P=0.04) and new-onset hypertension (RR=0.62; 95% CI: 0.42-0.93; P=0.02).
CONCLUSIONS: Basiliximab may be effective in reducing de novo diabetes mellitus. What is more, basiliximab in combination with steroids-free immunosuppressant shows statistical benefit to reduce biopsy-proven acute rejection and de novo hypertension.
(Hepatobiliary Pancreat Dis Int 2017;16:139-146)
liver transplantation;
basiliximab;
induction;
immunosuppression;
meta-analysis
Introduction
Induction immunosuppression[1]is a short-term, intense therapy administered around the time of transplantation to prevent acute rejection (AR) in the first month post-transplant. Induction therapy is used widely in a variety of solid organ transplantations.[2-9]Since basiliximab, a strong immunosuppressant, was approved for clinical use,[10]researches have shown that basiliximab induction therapy reduces the incidence of acute cellular rejection without increasing the risk of infection or other side effects in renal transplantation.[11-15]However, the role of basiliximab induction therapy in liver transplantation remains contentious. The present meta-analysis was to evaluate the basiliximab induction therapy in liver transplantation and mainly on biopsy-proven AR (BPAR), graft survival, mortality and other adverse events.
Methods
Literature search
A systematic literature published in English from July 1998 (basiliximab was first approved for clinical use in 1998) to December 2015 was searched in the following databases: MEDLINE, PubMed, Ovid, EMBASE, Web of Science and Cochrane Library. The keywords used were“liver transplantation”, “basiliximab”, “simulect”, “interleukin-2 receptor antagonist”, “anti CD-25 monoclonal antibody”, and abbreviations thereof. The key words were combined with appropriate Boolean operators. And for further relevant articles, we also checked the reference lists in all identified trials.
Inclusion and exclusion criteria
All prospective, controlled, randomized studies published as full papers or abstracts in which basiliximab was used to treat liver transplant recipients were reviewed. The inclusion criteria were: 1) patients underwent primary liver transplantation from a living or cadaveric donor; 2) the experimental group received basiliximabin combination with steroids-based/free immunosuppressant; 3) the control group received standard steroids induction therapy; 4) the stated primary outcomes and secondary outcomes were reported and all studies had a minimum follow-up duration of 12 months.
To avoid intrinsic bias, we excluded nonrandomized controlled studies and pharmacological studies that did not provide data on clinical outcome due to their short follow-up duration (less than 12 months). We also excluded trials with patients who underwent multi-organ transplantation or re-transplantation.
Outcomes
The primary outcomes analyzed were BPAR rate, graft survival, and mortality. Secondary outcomes were new-onset of hypertension and diabetes mellitus, overall infection, cytomegalovirus (CMV) infection, recurrence of hepatocellular carcinoma (HCC) and hepatitis C virus (HCV).
Data extraction and study quality
Data extraction was performed independently by two authors using a standardized form; any disagreement was resolved by discussion among all of the authors. The methodology quality of each trial was assessed according to the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention, including blinding, randomization, allocation concealment, intention-totreat (ITT) analysis, completeness of follow-up, and the method of handling missing values.
Statistical analysis
All statistical analyses were performed using RevMan 5.3 provided by Cochrane Collaboration. Pooled relative risk (RR) with 95% confidence intervals (CI) were calculated for each principal dichotomous variable outcome using either the fixed effects model or random effects model, where values of <1 favored basiliximab regimens and values of >1 favored basiliximab-sparing regimens. We analyzed heterogeneity among studies using Cochrane’s Q test and calculating I2, with P<0.05 used to denote statistical significance, and with I2calculated to measure the proportion of total variation in the estimates of treatment effect that was due to heterogeneity beyond chance.
Results
Trial selection
Database searches yielded 192 entries (Fig. 1), of which 20 were excluded as duplicates. Of the 172 publications that qualified for abstract review, 158 were excluded because they were not controlled trials. The remaining 14 trials underwent full article review and a further 8 publications were excluded mainly because they were entirely retrospective, or because of the short follow-up duration.
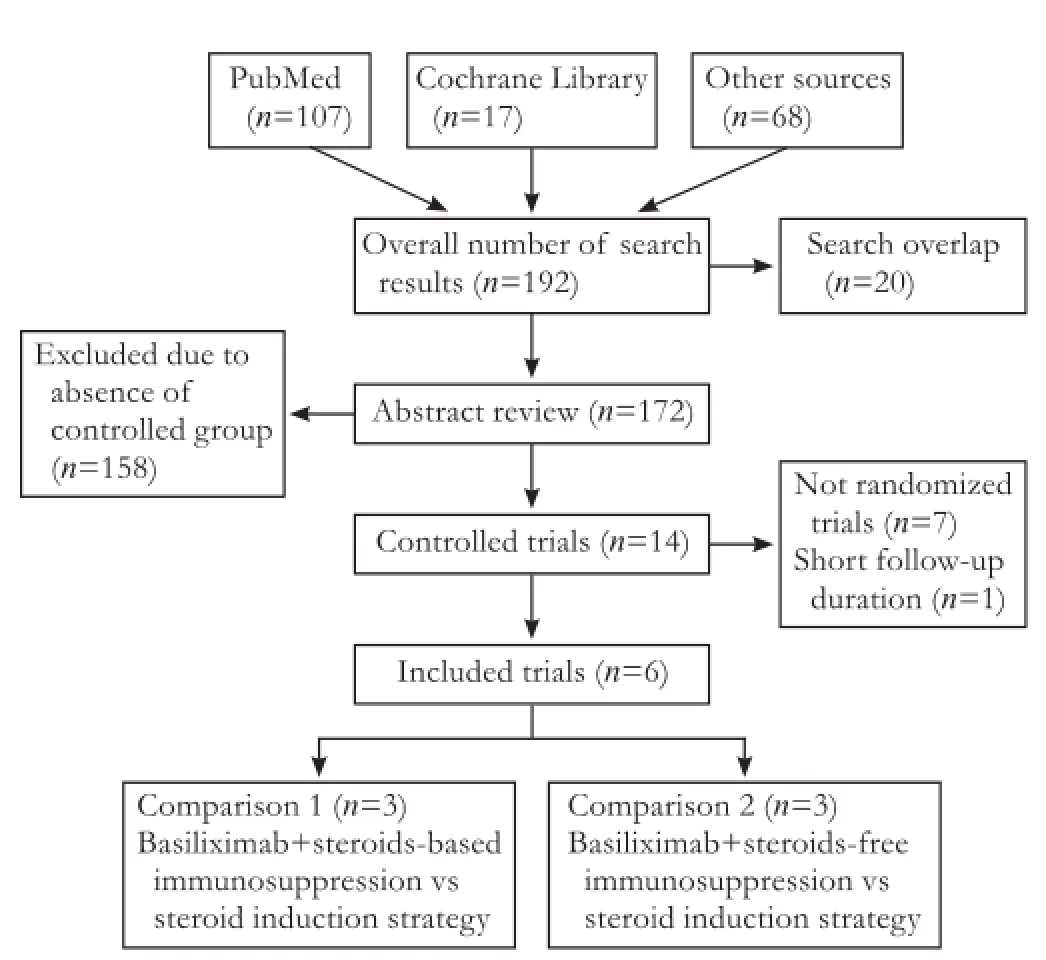
Fig. 1. Flow chart of publication search and selection.
A total of 6 randomized controlled trials (RCTs)[16-21]fulfilled the inclusion criteria, one[18]was in conference abstract. Among 887 patients in these trials, 446 treated with basiliximab regimens and 441 with basiliximabsparing regimens.
Included trials in the systematic review
Previous literatures[22-25]showed that in liver transplantation, basiliximab was used in addition to standard immunosuppressant to reduce other immunosuppressive drugs, such as calcineurin inhibitor (CNI) and corticosteroids. We have therefore classified the 6 included trials into 2 comparisons: comparison 1, basiliximab is added to the steroids-based immunosuppressant, which is compared to steroid; comparison 2, basiliximab is added to the steroids-free immunosuppressant, which is compared to steroid.
Table 1 shows the characteristics of the included RCTs. Three trials[16,20,21]compared basiliximab in combination with steroids-based immunosuppressant to standard steroids; the other three trials[17-19]compared basiliximab in combination with steroids-free immunosuppressant to standard steroids. Four studies[16,17,20,21]were restricted to adult patients and one[19]included only pediatric patients, whereas the study of De Simone et al[18]does not give any details on patients’ age.
The trials had greatly varied follow-up durations from 12 to 114 months: four trials[16,18,19,21]reported outcomes at 12 months, one[17]at 21.6 months and the remaming one[20]at 76-114 months.
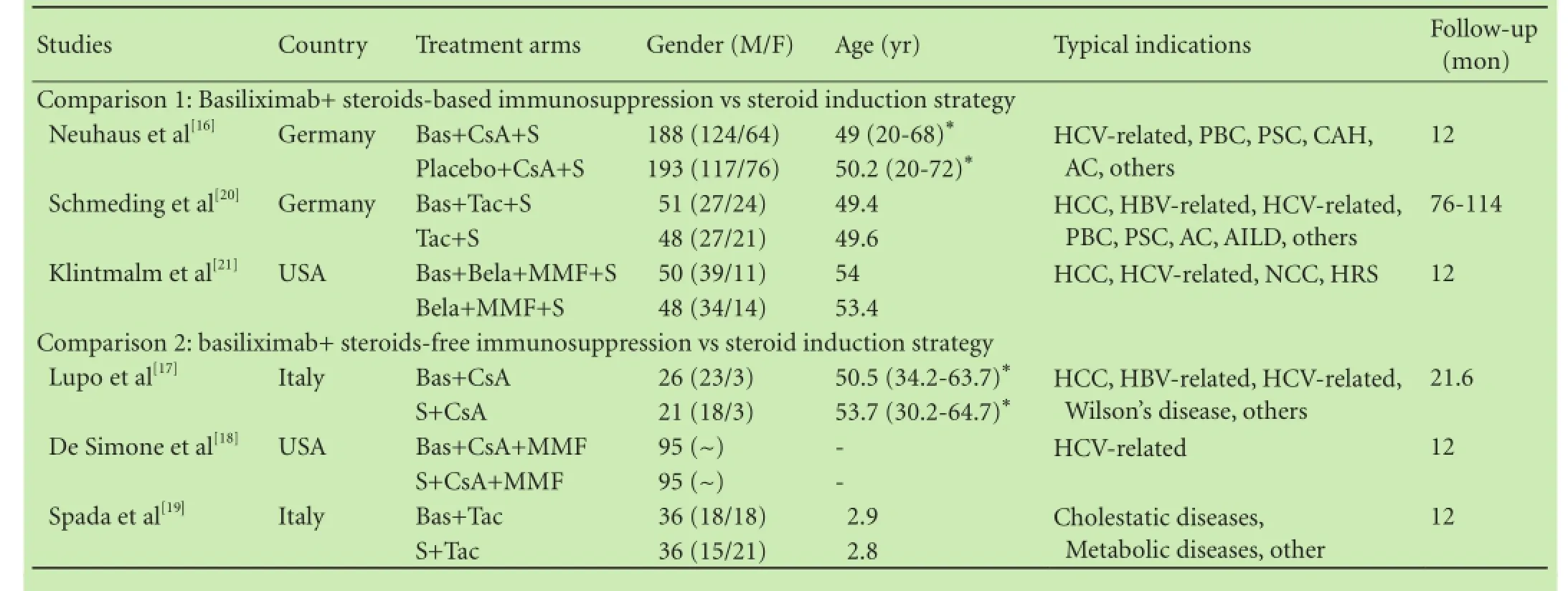
Table 1. The characteristics of the included RCTs stratified by the 2 pre-specified comparison groups

Table 1. The characteristics of the included RCTs stratified by the 2 pre-specified comparison groups (continued)
Quality of trials included in the systematic review
Table 2 shows the quality assessment of the included RCTs. Description of details of trial methodology was incomplete for the majority of the 6 published trials. All the 6 studies were randomized and one[16]was randomized, double blinded and placebo-controlled. Allocation concealment was found to be adequate in three trials.[16,17,21]ITT analysis was stated and performed in three studies.[16,17,19]Most authors[16,18-21]also did not state how missing values were handled. Agreement between authors for this qualitative assessment of the trials was 100%.

Table 2. The quality assessment of the included RCTs
Primary outcomes
BPAR
All of the 6 studies reported data on BPAR. Metaanalysis suggests that the therapeutic effect on BPAR has no significant difference between the basiliximab and basiliximab-sparing groups within 1 year (RR=0.89; 95% CI: 0.75-1.06; P=0.19; Fig. 2). Basiliximab in combination with steroids-free immunosuppressant significantly decreased BPAR (38%) within 1 year compared with steroids (RR=0.62; 95% CI: 0.39-0.97; P=0.04; Fig. 2).
Graft survival and mortality of patients
Basiliximab did not impact graft survival (RR=1.02; 95% CI: 0.97-1.08; P=0.45) or mortality (RR=0.83; 95% CI: 0.58-1.18; P=0.30) of patients. Subgroup analysis did not reveal significant difference (graft survival: comparison 1: RR=1.04; 95% CI: 0.98-1.12; P=0.22. comparison 2: RR=0.98; 95% CI: 0.89-1.08; P=0.69. Mortality of patients: comparison 1: RR=0.88; 95% CI: 0.57-1.36; P=0.55. comparison 2: RR=0.73; 95% CI: 0.39-1.37; P=0.33).
Secondary outcomes
De novo hypertension and diabetes mellitus
Two trials[18,19]reported data on new-onset hypertension and four trials[17,18,20,21]reported data on de novo diabetes mellitus. Our meta-analysis showed that de novo hypertension was significantly reduced using additional basiliximab in combination to steroids-free immunosuppressant in the follow-up period (RR=0.62; 95% CI: 0.42-0.93; P=0.02; Fig. 3). However, the RR of the four studies on de novo diabetes mellitus had a statistically significant heterogeneity (χ2=9.41, P=0.02, I2=68%) which is due to the study of Klintmalm et al.[21]If we only analyzed the other three studies, the risk reduction of de novo diabetes mellitus is greater (RR=0.56; 95% CI: 0.34-0.91; P=0.02; test of heterogeneity: χ2=0.45, P=0.80, I2=0%; Fig. 4). However, neither comparison 1 (RR=0.47; 95% CI: 0.17-1.28; P=0.14) nor comparison 2 (RR=0.59; 95% CI: 0.33-1.04; P=0.07) showed that basiliximab benefits de novo diabetes mellitus.
Overall infection and CMV infection
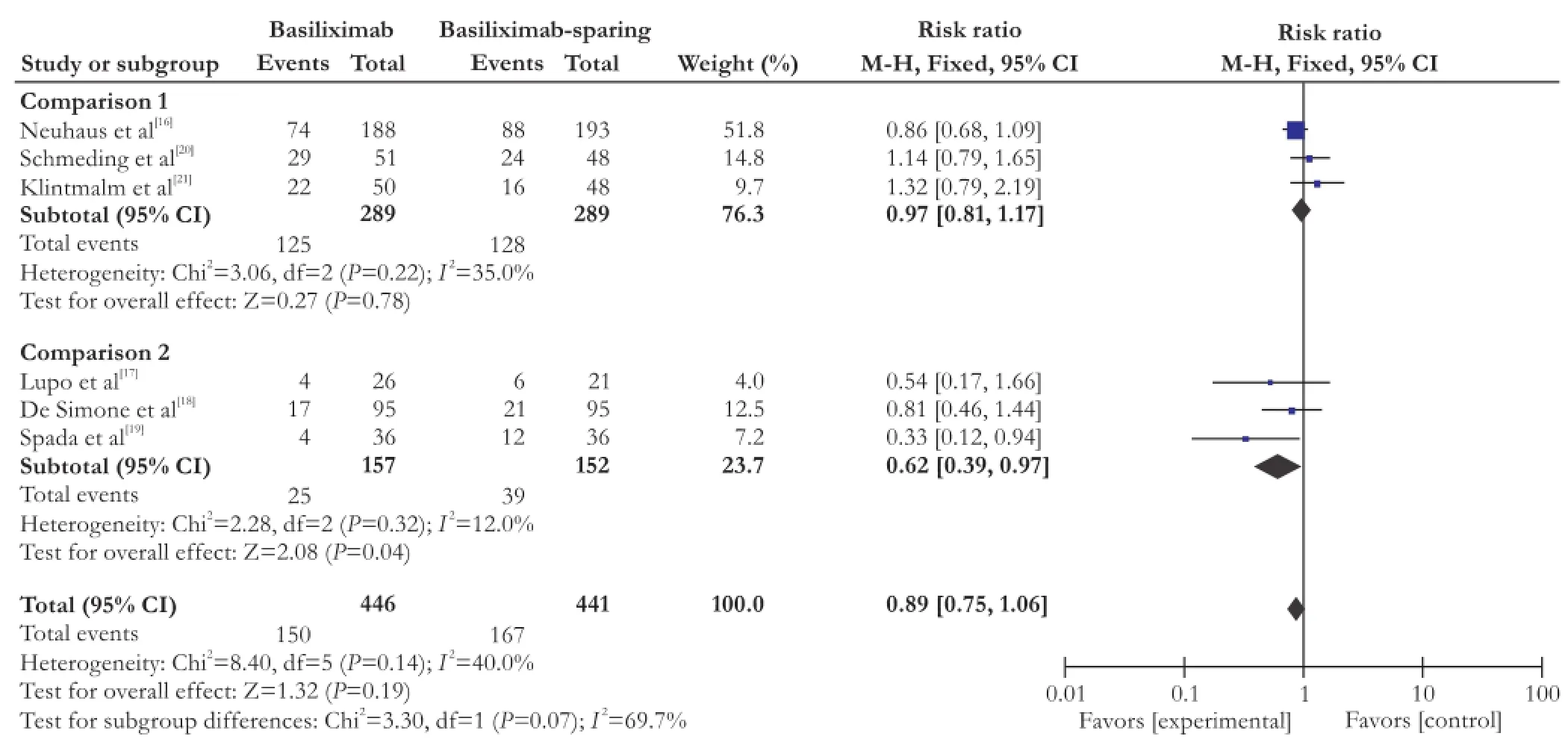
Fig. 2. Forest plot of BPAR within 12 months of all included studies.
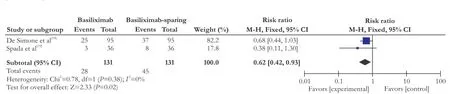
Fig. 3. The forest plot of de novo hypertension for patients who used basiliximab.
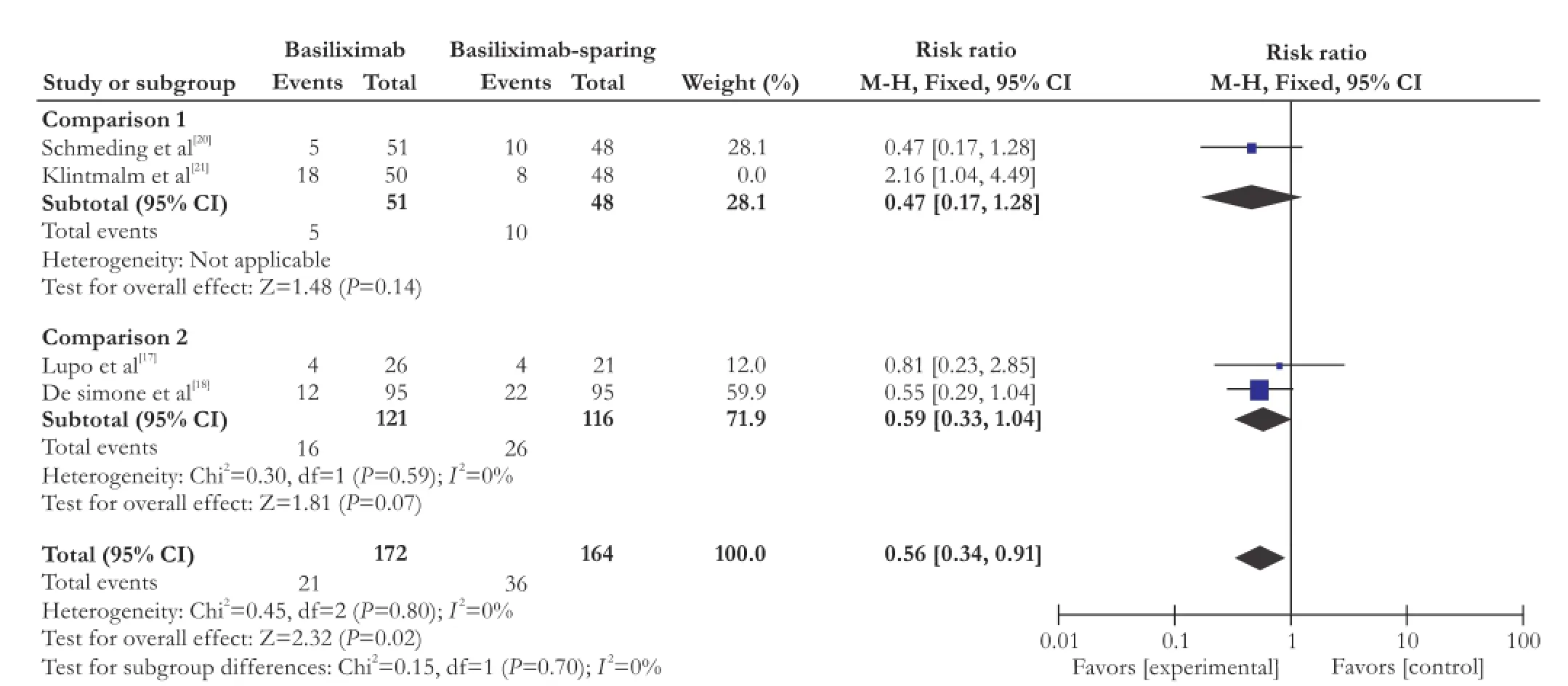
Fig. 4. The forest plot of de novo diabetes mellitus for patients who used basiliximab.
Data on overall infection was available in 4 trials[16,17,19,21]including 598 participants and on CMV infection was available in 3 trial[16,17,21]including 526 participants. Our meta-analysis showed that there was no significant difference between basiliximab treatment and basiliximabsparing groups on overall infection (RR=0.78; 95% CI: 0.56-1.07; P=0.12) and CMV infection (RR=1.12; 95% CI: 0.62-2.02; P=0.70). Subgroup analysis of overall infection showed similar outcomes (comparison 1: RR=0.90; 95% CI: 0.75-1.09; P=0.27. comparison 2: RR=0.65; 95% CI: 0.26-1.62; P=0.36).
Recurrence of HCV and HCC
Four trials[16,17,19,21]reported the recurrence of HCV and four[16,17,20,21]reported the recurrence of HCC. There were no statistically significant difference between the two treatment arms (HCV: RR=0.99; 95% CI: 0.70-1.41; P=0.98. HCC: RR=1.52; 95% CI: 0.53-4.35; P=0.44). Subgroup analysis of HCV recurrence obtained similar outcomes (comparison 1: RR=1.28; 95% CI: 0.62-2.67; P=0.50. comparison 2: RR=0.79; 95% CI: 0.56-1.11; P=0.17).
Discussion
Basiliximab,[27]a monoclonal anti-interleukin-2 receptor (anti-IL-2R) antibody and an antagonist of alpha-receptor of activated T-cells (CD-25), has been used in immunosuppression protocols based on cyclosporin A, tacrolimus, belatacept or azathioprine.[21,28-30]In the setting of renal transplantation, numerous studies have demonstrated a significant reduction in the incidence of acute and chronic cellular rejection without increasing the risk of infection or other side effects. However, the efficacy and safety in the liver transplantation were not conclusive. A systematic analysis by Wang et al[31]showed that anti-IL-2R reduced BPAR within 1 year which favored its application (RR=0.82; 95% CI: 0.68-0.99; P=0.04). However, concomitant immunosuppression in this metaanalysis is different among trial groups, which may be a main limitation of this review. A study by Penninga et al[32]showed no significant differences regarding acute rejection, mortality, graft loss, infection, HCV recurrence when IL-2R antagonist induction was compared with corticosteroid induction. However subgroup analysis for basiliximab was not described in detail.
According to our meta-analysis, basiliximab tends to reduce BPAR in liver transplantation. Subgroup analysis showed that only steroids-free immunosuppression strategy (comparison 2) had a significantly 38% reduction of BPAR within 1 year. Although the risk of BPAR is significantly reduced when basiliximab is applied (comparison 2), we did not observe a statistically significant difference in mortality or graft survival.
There is a major concern in organ transplantation that any addition of immunosuppressant will reduce immunity to infection and malignant disease. Lee et al[33]reported that induction therapy with basiliximab may have a negative impact with respect to early HCC recurrence within 1 year in high-risk patients. However, more studies had shown that basiliximab did not increase the incidence of adverse effects or infections. Ramirez et al[34]demonstrated that induction therapy with basiliximab improves graft survival after orthotopic liver transplantation without increasing the incidence of CMV, post-transplant lympho-proliferative disorders or HCV recurrence. A study by Humar et al[35]showed that basiliximab induction therapy might be associated with lower hepatitisC recurrence. This analysis did not show evidence that basiliximab increased risk of overall infection, CMV infection, HCV recurrence and HCC recurrence. Reasons below may account for these non-statistically differences: 1) there might be no significant intergroup differences in blood lymphocyte counts between the two groups; 2) basiliximab potentially reduced or even eliminated steroid application in liver transplantation. However, it is important to emphasize that the tendency toward more CMV infections, more hepatitis C (in adult patients) and more HCC recurrence observed in basiliximab induction therapy patients raises the possibility of over-immunosuppression in some patients.
Basiliximab is widely used in a variety of solid organ transplantations and has been promoted as a beneficial asset to immunosuppressive protocols in order to reduce acute rejection rate, potentially reducing or even eliminating steroid application and reducing CNI dosage and thereby minimizing steroids or CNI-related side effects such as infections, metabolic disorders, cardiovascular complications and nephrotoxicity after transplantation.[11,23,29,30,36-42]This analysis also looked at the possibility of reducing concomitant steroids medication when using basiliximab as some published studies reported and found that the steroid-free immunosuppression strategy shows a statistically significant 38% reduction of BPAR and de novo hypertension, favoring the use of basiliximab in combination with steroids-free immunosuppressant, which may indicate the possibility of reducing/avoiding concomitant steroid medication when using basiliximab in liver transplantation.
Although we did not list renal function as an outcome in our meta-analysis, several trials have reported the significant renal-sparing efficacy of basiliximab.[43,44]Lin et al[44]reported that basiliximab administration improved postoperative renal function by delaying the start and decreasing the dosage of tacrolimus. And it was particularly beneficial in pre-transplant recipients with renal dysfunction because the addition of basiliximab would provide a sufficient window for recovery of renal function. A RCT by Schmeding et al[20]reported that a slight improvement in renal function could be detected for basiliximab treated recipients even basiliximab was added to a CNI-based immunosuppression strategy.
The principal induction therapy agent used in pediatric liver transplantation is basiliximab. Gras and his colleagues[45]reported that basiliximab in combination with steroids-free immunosuppressant significantly decreased BPAR (P=0.007) and viral infection (P=0.045). Spada et al[19]also reached similar conclusions: 87.7% of patients are free from BPAR in the basiliximab group, which is significantly higher than that of 67.7% of patients in the steroid group. Overall infection was also significantly reduced in the basiliximab group. Thus, basiliximab is superior to steroid in pediatric liver recipients.
The main limitation of our research is the relatively small number of RCTs and the diversity of immunosuppression regimen. More RCTs are needed to draw a clear conclusion.
In conclusion, basiliximab does not increase the risk of infection and malignant disease; basiliximab may be effective in reducing de novo diabetes mellitus. What is more, basiliximab in combination with steroids-free immunosuppression strategy reduces acute rejection and de novo hypertension.
Contributors: ZGQ participated in design, data collection, studies selection, data analysis/interpretation, and statistics and wrote the first draft. ZCS and SN performed the research and wrote the first draft. LW and CBM collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. ZJL is the guarantor.
Funding: This study was supported by a grant from the Science and Technology project of Shenyang (F13-212-9-00).
Ethical approval: This study was approved by the Ethics Committee of the First Affiliated Hospital of China Medical University.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Turner AP, Knechtle SJ. Induction immunosuppression in liver transplantation: a review. Transpl Int 2013;26:673-683.
2 Sudan DL, Chinnakotla S, Horslen S, Iyer K, Fox I, Shaw B, et al. Basiliximab decreases the incidence of acute rejection after intestinal transplantation. Transplant Proc 2002;34:940-941.
3 Grego K, Arnol M, Bren AF, Kmetec A, Tomaziĉ J, Kandus A. Basiliximab versus daclizumab combined with triple immunosuppression in deceased donor renal graft recipients. Transplant Proc 2007;39:3093-3097.
4 Grundy N, Simmonds J, Dawkins H, Rees P, Aurora P, Burch M. Pre-implantation basiliximab reduces incidence of early acute rejection in pediatric heart transplantation. J Heart Lung Transplant 2009;28:1279-1284.
5 Kandus A, Arnol M, Omahen K, Oblak M, Vidan-Jeras B, Kmetec A, et al. Basiliximab versus daclizumab combined with triple immunosuppression in deceased donor renal transplantation: a prospective, randomized study. Transplantation 2010;89:1022-1027.
6 Swarup R, Allenspach LL, Nemeh HW, Stagner LD, Betensley AD. Timing of basiliximab induction and development of acute rejection in lung transplant patients. J Heart Lung Transplant 2011;30:1228-1235.
7 Niederhaus SV, Kaufman DB, Odorico JS. Induction therapy in pancreas transplantation. Transpl Int 2013;26:704-714.
8 Sweet SC. Induction therapy in lung transplantation. Transpl Int 2013;26:696-703.
9 Opelz G, Naujokat C, Daniel V, Terness P, Döhler B. Disas-sociation between risk of graft loss and risk of non-Hodgkin lymphoma with induction agents in renal transplant recipients. Transplantation 2006;81:1227-1233.
10 Basiliximab approved for use in renal transplant patients. Am J Health Syst Pharm 1998;55:1444-1445.
11 Thistlethwaite JR Jr, Nashan B, Hall M, Chodoff L, Lin TH. Reduced acute rejection and superior 1-year renal allograft survival with basiliximab in patients with diabetes mellitus. The Global Simulect Study Group. Transplantation 2000;70: 784-790.
12 Adu D, Cockwell P, Ives NJ, Shaw J, Wheatley K. Interleukin-2 receptor monoclonal antibodies in renal transplantation: meta-analysis of randomised trials. BMJ 2003;326:789.
13 Webster AC, Ruster LP, McGee R, Matheson SL, Higgins GY, Willis NS, et al. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev 2010: CD003897.
14 Keown P, Balshaw R, Khorasheh S, Chong M, Marra C, Kalo Z, et al. Meta-analysis of basiliximab for immunoprophylaxis in renal transplantation. BioDrugs 2003;17:271-279.
15 Webster AC, Playford EG, Higgins G, Chapman JR, Craig JC. Interleukin 2 receptor antagonists for renal transplant recipients: a meta-analysis of randomized trials. Transplantation 2004;77:166-176.
16 Neuhaus P, Clavien PA, Kittur D, Salizzoni M, Rimola A, Abeywickrama K, et al. Improved treatment response with basiliximab immunoprophylaxis after liver transplantation: results from a double-blind randomized placebo-controlled trial. Liver Transpl 2002;8:132-142.
17 Lupo L, Panzera P, Tandoi F, Carbotta G, Giannelli G, Santantonio T, et al. Basiliximab versus steroids in double therapy immunosuppression in liver transplantation: a prospective randomized clinical trial. Transplantation 2008;86:925-931.
18 De Simone P, De Carlis L, Grazi GL, Cuomo O, Calise F, Castagneto M, et al. Results of a multicenter, randomized, openlabel trial comparing basiliximab vs. steroids in HCV liver transplant patients. Am J Transplant 2007;7:312-312.
19 Spada M, Petz W, Bertani A, Riva S, Sonzogni A, Giovannelli M, et al. Randomized trial of basiliximab induction versus steroid therapy in pediatric liver allograft recipients under tacrolimus immunosuppression. Am J Transplant 2006;6:1913-1921.
20 Schmeding M, Sauer IM, Kiessling A, Pratschke J, Neuhaus R, Neuhaus P, et al. Influence of basiliximab induction therapy on long term outcome after liver transplantation, a prospectively randomised trial. Ann Transplant 2007;12:15-21.
21 Klintmalm GB, Feng S, Lake JR, Vargas HE, Wekerle T, Agnes S, et al. Belatacept-based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II randomized study. Am J Transplant 2014;14:1817-1827.
22 Liu CL, Fan ST, Lo CM, Chan SC, Ng IO, Lai CL, et al. Interleukin-2 receptor antibody (basiliximab) for immunosuppressive induction therapy after liver transplantation: a protocol with early elimination of steroids and reduction of tacrolimus dosage. Liver Transpl 2004;10:728-733.
23 Lladó L, Xiol X, Figueras J, Ramos E, Memba R, Serrano T, et al. Immunosuppression without steroids in liver transplantation is safe and reduces infection and metabolic complications: results from a prospective multicenter randomized study. J Hepatol 2006;44:710-716.
24 Verna EC, Farrand ED, Elnaggar AS, Pichardo EM, Balducci A, Emond JC, et al. Basiliximab induction and delayed calcineurin inhibitor initiation in liver transplant recipients with renal insufficiency. Transplantation 2011;91:1254-1260.
25 Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, Schemmer P, et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation--PROTECT. Am J Transplant 2012;12:1855-1865.
26 Hibi T, Shinoda M, Itano O, Obara H, Kitago M, Abe Y, et al. Steroid minimization immunosuppression protocol using basiliximab in adult living donor liver transplantation for hepatitis C virus-related cirrhosis. Hepatol Res 2015;45:1178-1184. 27 Nashan B. The IL2 pathway in clinical immunosuppression. Transplant Proc 2001;33:3072-3074.
28 Onrust SV, Wiseman LR. Basiliximab. Drugs 1999;57:207-214.
29 Calmus Y, Scheele JR, Gonzalez-Pinto I, Jaurrieta EJ, Klar E, Pageaux GP, et al. Immunoprophylaxis with basiliximab, a chimeric anti-interleukin-2 receptor monoclonal antibody, in combination with azathioprine-containing triple therapy in liver transplant recipients. Liver Transpl 2002;8:123-131.
30 Asensio M, Margarit C, Chavez R, Ortega J, Charco R, Iglesias J. Induction with basiliximab reduces acute rejection in pediatric liver transplant patients treated with tacrolimus and steroids. Transplant Proc 2002;34:1970-1971.
31 Wang XF, Li JD, Peng Y, Dai Y, Shi G, Xu W. Interleukin-2 receptor antagonists in liver transplantation: a meta-analysis of randomized trials. Transplant Proc 2010;42:4567-4572.
32 Penninga L, Wettergren A, Wilson CH, Chan AW, Steinbrüchel DA, Gluud C. Antibody induction versus corticosteroid induction for liver transplant recipients. Cochrane Database Syst Rev 2014:CD010252.
33 Lee JY, Kim YH, Yi NJ, Kim HS, Lee HS, Lee BK, et al. Impact of immunosuppressant therapy on early recurrence of hepatocellular carcinoma after liver transplantation. Clin Mol Hepatol 2014;20:192-203.
34 Ramirez CB, Doria C, di Francesco F, Iaria M, Kang Y, Marino IR. Basiliximab induction in adult liver transplant recipients with 93% rejection-free patient and graft survival at 24 months. Transplant Proc 2006;38:3633-3635.
35 Humar A, Crotteau S, Gruessner A, Kandaswamy R, Gruessner R, Payne W, et al. Steroid minimization in liver transplant recipients: impact on hepatitis C recurrence and post-transplant diabetes. Clin Transplant 2007;21:526-531.
36 Strassburg A, Pfister ED, Arning A, Nashan B, Ehrich JH, Melter M. Basiliximab reduces acute liver allograft rejection in pediatric patients. Transplant Proc 2002;34:2374-2375.
37 Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant 2008;22:1-15.
38 Varo E, Lopez A, Castroagudin JF, Delgado M, Conde R, Ferrer E, et al. Alternative immunosuppression for acute renal failure in liver transplantation: role of ultra-low dose tacrolimus and basiliximab. Transplant Proc 2002;34:1533-1534.
39 Neuberger JM, Mamelok RD, Neuhaus P, Pirenne J, Samuel D, Isoniemi H, et al. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: the‘ReSpECT’ study. Am J Transplant 2009;9:327-336.
40 A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. The U.S. Multicenter FK506 Liver Study Group. N Engl J Med 1994;331:1110-1115.
41 Morard I, Mentha G, Spahr L, Majno P, Hadengue A, Huber O, et al. Long-term renal function after liver transplantation is related to calcineurin inhibitors blood levels. Clin Transplant 2006;20:96-101.
42 Wilkinson A, Pham PT. Kidney dysfunction in the recipients of liver transplants. Liver Transpl 2005:S47-51.
43 Cantarovich M, Metrakos P, Giannetti N, Cecere R, Barkun J, Tchervenkov J. Anti-CD25 monoclonal antibody coverage allows for calcineurin inhibitor “holiday” in solid organ transplant patients with acute renal dysfunction. Transplantation 2002;73:1169-1172.
44 Lin CC, Chuang FR, Lee CH, Wang CC, Chen YS, Liu YW, et al. The renal-sparing efficacy of basiliximab in adult living donor liver transplantation. Liver Transpl 2005;11:1258-1264.
45 Gras JM, Gerkens S, Beguin C, Janssen M, Smets F, Otte JB, et al. Steroid-free, tacrolimus-basiliximab immunosuppression in pediatric liver transplantation: clinical and pharmacoeconomic study in 50 children. Liver Transpl 2008;14:469-477.
Received January 21, 2016
Accepted after revision October 20, 2016
My mission in life is not merely to survive, but to thrive; and to do so with some passion, some compassion, some humor, and some style.
—Maya Angelou
Author Affiliations: Department of Hepatobiliary and Transplantation Surgery, First Affiliated Hospital of China Medical University, Shenyang 110001, China (Zhang GQ, Zhang CS, Sun N, Lv W, Chen BM and Zhang JL); Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital of Zhengzhou University, Zhengzhou 450003, China (Zhang GQ)
Jia-Lin Zhang, MD, PhD, Department of Hepatobiliary and Transplantation Surgery, First Affiliated Hospital of China Medical University, Shenyang 110001, China (Tel: +86-24-83283308; Fax: +86-24-83282997; Email: jlz2000@yeah.net)
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(16)60183-2
Published online February 24, 2017.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- HIDA scan for functional gallbladder disorder: ensure that you know how the scan was done
- The association of non-alcoholic fatty liver disease and metabolic syndrome in a Chinese population
- Serum soluble ST2 is a promising prognostic biomarker in HBV-related acute-on-chronic liver failure
- Novel HBV mutations and their value in predicting efficacy of conventional interferon
- Long-term outcome of patients with chronic pancreatitis treated with micronutrient antioxidant therapy
- Pancreaticoduodenectomy for borderline resectable pancreatic head cancer with a modified artery-first approach technique
