Serum soluble ST2 is a promising prognostic biomarker in HBV-related acute-on-chronic liver failure
2017-04-17ShaoWenJiangPengWangXiaoGangXiangRuiDongMoLanYiLinShiSanBaoJieLuandQingXie
Shao-Wen Jiang, Peng Wang, Xiao-Gang Xiang, Rui-Dong Mo, Lan-Yi Lin, Shi-San Bao, Jie Lu and Qing Xie
Shanghai, China
Serum soluble ST2 is a promising prognostic biomarker in HBV-related acute-on-chronic liver failure
Shao-Wen Jiang, Peng Wang, Xiao-Gang Xiang, Rui-Dong Mo, Lan-Yi Lin, Shi-San Bao, Jie Lu and Qing Xie
Shanghai, China
BACKGROUND: The IL-33/ST2 axis is involved in the pathogenesis of many diseases such as autoimmune diseases, cancer, and heart failure. However, studies of the IL-33/ST2 pathway in HBV-related acute-on-chronic liver failure (HBV-ACLF) are lacking. The present study aimed to determine the prognostic role of serum IL-33/soluble ST2 (sST2) in HBV-ACLF.
METHODS: Serum levels of IL-33 and sST2 in healthy controls (HC, n=18), chronic hepatitis B (CHB, n=27) and HBV-ACLF (n=51) patients at the 1st and 4th week after enrollment were detected using ELISA, and clinical data were collected. The follow-up of HBV-ACLF patients lasted for 6 months at least.
RESULTS: There was no significant difference of serum IL-33 level among HC, CHB and HBV-ACLF patients at week 1. However, serum sST2 level differed significantly among the three groups: highest in the HBV-ACLF group, moderate in the CHB group and lowest in the HC group. There was a reverse correlation between serum sST2 level and the survival of HBV-ACLF patients. The level of serum sST2 in HBV-ACLF survivors was significantly declined from week 1 to week 4 following the treatment, whereas that in HBV-ACLF nonsurvivors remained at a high level during the same period. Furthermore, serum sST2 level was significantly correlated with laboratory parameters and the most updated prognostic scores (CLIF-C OF score, CLIF-C ACLF score and ACLF grades). The receiver operating characteristics curves demonstrated that serum sST2 level was a good diagnostic marker for predicting the 6-month mortality in HBV-ACLF patients, comparable to the most updated prognostic scores. Serum sST2 cut-off points for predicting prognosis in HBV-ACLF patients were 76 ng/mL at week 1 or 53 ng/mL at week 4, respectively. HBV-ACLF patients with serum sST2 level above the cut-off point often had a worse prognosis than those below the cut-off point.
CONCLUSION: Serum sST2 may act as a promising biomarker to assess severity and predict prognosis of patients with HBV-ACLF and help for the early identification and optimal treatment of HBV-ACLF patients at high risk of mortality.
(Hepatobiliary Pancreat Dis Int 2017;16:181-188)
biomarker;
HBV-related acute-on-chronic liver failure;
interleukin-33;
prognosis;
soluble ST2
Introduction
Acute-on-chronic liver failure (ACLF) is gradually recognized as a separate clinical entity distinct from acute liver failure and chronic decompensation of liver cirrhosis.[1,2]In addition to the non-uniformed definition, there are also significant differences in the underlying chronic liver diseases of ACLF between the Eastern and Western patients: the major cause of ACLF in the Western countries is alcoholic cirrhosis, while chronic HBV infection makes up about 70% of the cause of ACLF in Asia.[3,4]Despite the different causes of ACLF, the Eastern and Western patients share common clinical characteristics, e.g. developing rapidly, lacking effective measures of assessing progression and predicting prognosis, limited definitive treatment except for liver transplantation, and a high mortality of 50%-90%.[5]Itis fundamentally important to differentiate ACLF from chronic decompensation of cirrhotic patients.[1]Liver function of chronic decompensation of cirrhotic patients is progressively deteriorating and almost irreversible, eventually developing into organ failure at some point. By contrast, ACLF patients often have a potential for reversibility to the original liver functional state if they receive proper treatment at the relatively early stage. However, part of ACLF patients may still show a further deterioration and die of multi-system organ failure despite intervention. Hence, it is of great value to find some promising biomarkers for the early identification and optimal treatment of ACLF patients at high risk of mortality.
Interleukin-33 (IL-33), a new member of the IL-1 family with pleiotropic roles, exists in two distinct isoforms as full-length IL-33 (proIL-33) and mature IL-33 (mtrIL-33), located in nucleus and extracellular environment, respectively.[6,7]IL-33 receptor, ST2, also has two isoforms: transmembrane ST2L expressed on the surface of target cells and soluble ST2 (sST) in serum.[8,9]ProIL-33, constitutively expressed in the nucleus of epithelial and endothelial cells, is associated with chromatin compaction and transcriptional repression.[10,11]When cells injury and necrosis or necroptosis occurs, proIL-33 is released as an alarmin via unconventional pathways and cleaved by serine proteases into mtrIL-33 with higher bioactivity. Subsequently, IL-33 binds to ST2L to form the binary IL-33-ST2 complex, activating intracellular signaling pathways. In contrast, serum sST2 blocks IL-33 function to prevent systemic IL-33 effects.[12]
IL-33/ST2 pathway not only induces Th2 mediated immunity in allergic diseases, helminth expulsion and fibrosis diseases, but also exerts a protective influence in antiviral and antitumor immunity through promoting Th1 immunity and CD8+T cell responses.[12,13]Recently, more and more studies demonstrated that IL-33/ST2 pathway is involved in pathogenesis of liver diseases, including viral hepatitis, liver fibrosis, and hepatocellular carcinoma and ConA-induced acute liver injury.[14-18]However, the precise role of IL-33/ST2 pathway in the progression of HBV-ACLF remains unclear, and the results of relevant studies are rare and even controversial partially.[19,20]Here, we sought to provide more convincing evidence for the involvement of IL-33/ST2 pathway in the progression of HBV-ACLF, and determine the prognostic accuracy of serum IL-33 or sST2 as a biomarker, which may contribute to reaching an agreement on their roles in HBV-ACLF.
Methods
Patients and design
Healthy controls (HC, n=18), chronic hepatitis B (CHB, n=27) and HBV-ACLF (n=51) patients were recruited from the Department of Infectious Diseases, Ruijin Hospital from May 2012 to May 2015. CHB patients met the diagnostic criteria as HBsAg positive for at least 6 months ahead of this study and an increased level of alanine aminotransferase (ALT). All the HBV-ACLF patients met the following diagnostic criteria: (i) HBsAg positive history longer than 6 months; (ii) serum total bilirubin (TBil) ≥171 µmol/L or a daily elevation ≥17.1 µmol/L; (iii) international normalized ratio (INR) ≥1.5; (iv) ascites and/or encephalopathy as determined by physical examination.[21]Exclusion criteria are as follows: (i) pregnancy; (ii) co-infected with human immunodeficiency virus (HIV); (iii) hepatocellular carcinoma, concomitant chronic hepatitis C, or other known liver diseases, including autoimmune hepatitis, drug-induced liver injury, metabolic disorders; (iv) severe systematic diseases.
Both the HBV-ACLF survivors and non-survivors received the same and standard treatment regimens during hospitalization.[21]The follow-up of HBV-ACLF patients started from the enrollment and lasted for 6 months. The end points included death and liver transplantation. There were 25 non-survivors (including 22 deaths and 3 liver transplantations) and 26 survivors among all HBV-ACLF patients at the end of follow-up. The study was approved by Scientific and Ethics Committee of Ruijin Hospital, in compliance with the Declaration of Helsinki 1975. Oral informed consent was obtained from all participants.
Clinical data and prognostic scores
Clinical data including age, gender, TBil, prothrombin time, INR, albumin, creatinine, ascites, encephalopathy and other parameters were collected from the hospital information system and patient physical examination.
Chronic Liver Failure Consortium organ failure score (CLIF-C OFs) and ACLF grades were evaluated according to Jalan et al’s report.[22]The mathematical formulas of CLIF-C ACLF score (CLIF-C ACLFs) were listed as follows: CLIF-C ACLFs=10×[0.33×CLIF-C OFs+0.04× age+0.63×ln (WBC count)-2], WBC: white blood cell.
Cytokine detection by ELISA
Blood was collected in coagulation promoting tubes at the 1st week of admission and after 4 weeks treatment. It should be noted that “week 1/week 4” was defined as the time point post initiation of ACLF in principle. However, it is extremely difficult to ensure that the date of enrollment is always consistent with the onset of the disease for all enrolled patients. For the patients suffering from ACLF for a period of time, “week 1/week 4” refers to the date since their first admission in our hospital. Serum was stored at -80 ℃ after centrifugation until use. Serum levels of IL-33 and sST2 were determined with human IL-33and ST2 ELISA Kits (R&D, USA) according to the manufacturer’s instructions. The lower detection limits of IL-33 and sST2 were 0.519 pg/mL and 5.1 pg/mL, respectively. Statistical analysis
All statistics were carried out using SPSS 16.0 statistical software. Quantitative variables were expressed as mean±SE. Categorical variables were expressed as number (percentage). The differences between two groups were evaluated by Student’s t test, Mann-Whitney U test or Chi-square test, while the differences among three or more groups were compared by one-way ANOVA test, Kruskal-Wallis test or Chi-square test, where appropriate. The correlation between two variables was assessed using Spearman’s rank correlation test. Predictive accuracy of any parameter for prognosis of HBV-ACLF patients was determined by receiver operating characteristics (ROC) curves and Kaplan-Meier survival analysis. The statistical significance between the areas under the ROC curves was evaluated using the DeLong method.[23]A P ≤0.05 (twotailed) was considered statistically significant.
Results
HBV-ACLF patients have a highest level of serum sST2 among all participants
The baseline characteristics of all participants were listed in Table 1. Furthermore, precipitating events in all enrolled HBV-ACLF patients were analyzed as follows: HBV re-activation accounted for 37.3% (19/51), alcohol consumption 11.8% (6/51), bacterial infection 7.8% (4/51), hepatotoxic drugs 5.9% (3/51), variceal bleeding 2.0% (1/51) and undefined event 35.3% (18/51). The most frequent precipitating event in our study was HBV reactivation, which usually resulted from irregular usage or inappropriate cease of oral antivirals and occurrence of drug-resistance.
As illustrated in Fig. 1A, the serum IL-33 levels were low and statistically indiscriminate among the HC, CHB and HBV-ACLF groups at week 1 after enrollment. Interestingly, serum sST2 level differed significantly among the three groups at week 1. The serum sST2 level of the HBV-ACLF group at week 1 was 82.19±7.61 ng/mL, which was significantly higher than that of the HC (9.86 ± 1.10 ng/mL, P<0.0001) and CHB groups (34.00±4.55 ng/mL, P<0.0001). Meanwhile, the serum sST2 level of the CHB group was also significantly higher than that of the HC group (P=0.0001) (Fig. 1B).
Serum sST2 levels in HBV-ACLF patients with different disease outcomes
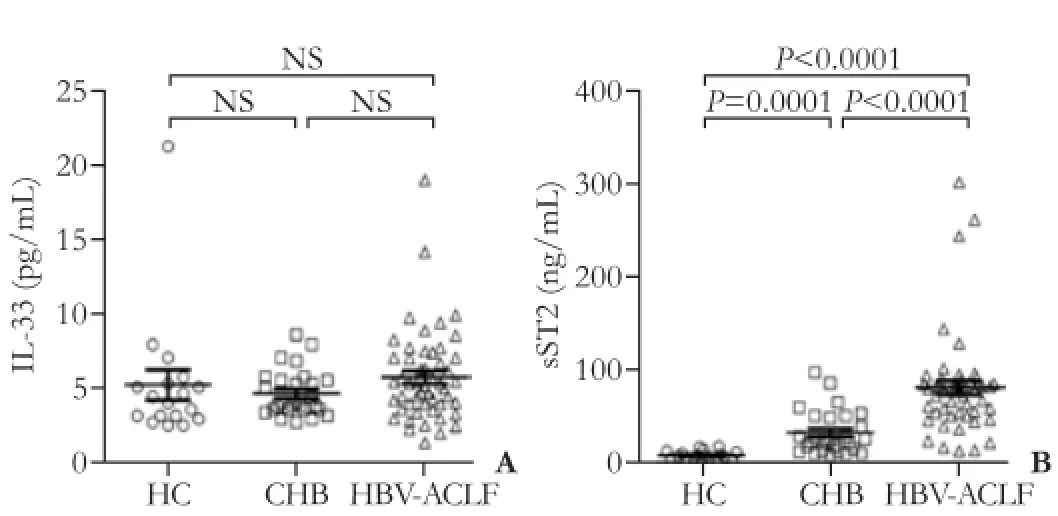
Fig. 1. The comparison of serum IL-33 (A) and sST2 (B) levels in healthy controls (HC), chronic hepatitis B (CHB), and HBV-related acute-on-chronic liver failure (HBV-ACLF) patients at week 1. Each point represents one participant. Values are expressed as mean± SE. The differences between two groups were calculated using the Student’s t test or Mann-Whitney U test, where appropriate.
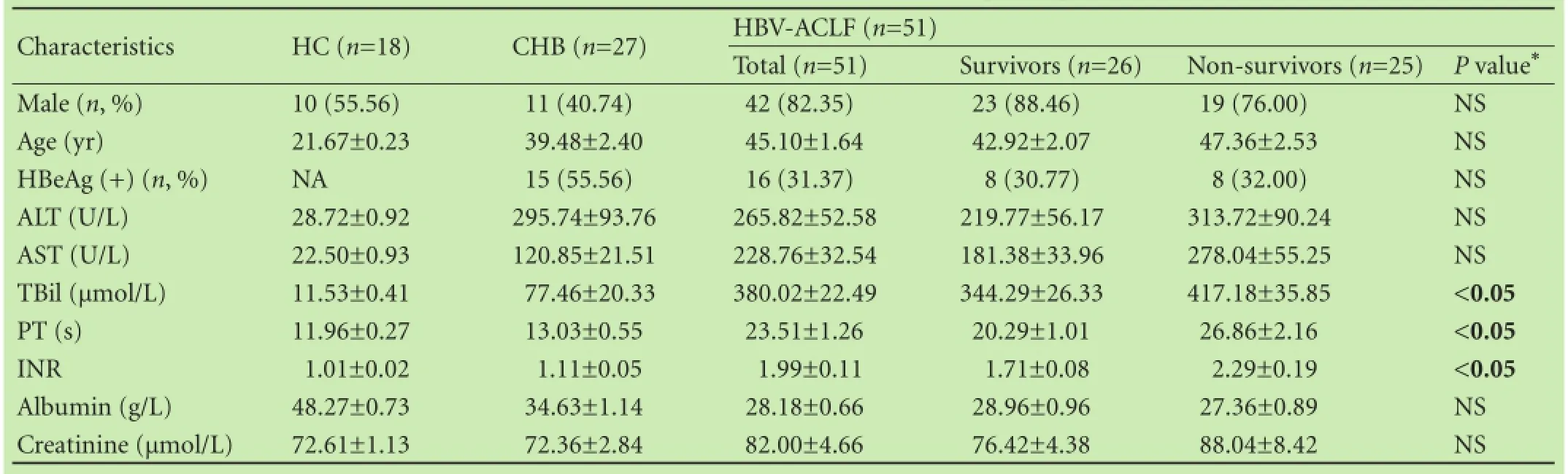
Table 1. Baseline characteristics of all enrolled participants

Fig. 2. The dynamic expression profiles of serum sST2 level in HBV-ACLF patients with different follow-up outcomes from week 1 to 4. A: The comparison of serum sST2 level between HBV-ACLF survivors (SR) and non-survivors (NSR) at week 1. B-D: The dynamic changes of serum sST2 level in HBV-ACLF survivors and nonsurvivors from week 1 to 4. P values were calculated using the Mann-Whitney U test or Wilcoxon matched pairs test, where appropriate.
The demographic and clinical features of survivors and non-survivors at week 1 were listed in Table 1. No significant difference was observed in gender, age and the levels of ALT, AST, albumin, and creatinine, while the levels of TBil, prothrombin time, and INR were obviously different between the two groups (Table 1). As shown in Fig. 2A, HBV-ACLF non-survivors had a higher level of serum sST2 than survivors at week 1 (101.07±13.30 vs 63.59±6.13 ng/mL, P<0.01). Furthermore, with the disease progression, serum sST2 level of HBV-ACLF survivors significantly declined from week 1 to week 4 (P<0.001) (Fig. 2B), whereas serum sST2 level of nonsurvivors remained almost unchanged and stayed at a high level (P>0.05) (Fig. 2C). Eventually, the gap of serum sST2 level between HBV-ACLF survivors and nonsurvivors widened further over time (P=0.0001) (Fig. 2D).
Serum sST2 level was significantly correlated with laboratory parameters
Serum sST2 level at week 1 was positively correlated with INR (P<0.0001) and WBC count (P=0.002), but not with TBil nor albumin (Fig. 3A-D). The correlations between serum sST2 level and INR or WBC count became more significant at week 4 (Fig. 3F and H). In addition, serum sST2 level was positively correlated with the level of TBil (P<0.001), but negatively correlated with the level of albumin (P<0.05) at week 4 (Fig. 3E and G).
Serum sST2 level was significantly correlated with the most updated prognostic scores
There were significant correlations between serum sST2 level versus CLIF-C OFs (P<0.001) and CLIF-C ACLFs (P<0.01) at week 1 (Fig. 4A and B); these correlations were stronger at week 4 (Fig. 4D and E). Furthermore, HBV-ACLF patients with Grade ≥2 had a signifi-cantly higher level of serum sST2 than those with Grade <2 both at week 1 and 4 (Fig. 4C and F).
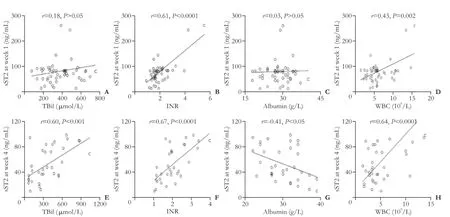
Fig. 3. The correlations between serum sST2 level and laboratory parameters. The correlations between serum sST2 at week 1 and levels of TBil (A), INR (B), albumin (C), and WBC count (D). The correlations between serum sST2 level at week 4 and levels of TBil (E), INR (F), albumin (G), and WBC count (H). P values were calculated using the Spearman’s rank correlation.

Fig. 4. The correlations between serum sST2 level and the most updated prognostic scores. The correlations between serum sST2 level at week 1 and CLIF-C OFs (A), CLIF-C ACLFs (B). C: The comparison of serum sST2 level at week 1 between patients with HBV-ACLF Grade <2 and those with Grade ≥2. The correlations between serum sST2 level at week 4 and CLIF-C OFs (D), CLIF-C ACLFs (E). F: The comparison of serum sST2 level at week 4 between patients with HBV-ACLF Grade <2 and those with Grade ≥2. P values were calculated using the Spearman’s rank correlation or the Mann-Whitney U test.
Serum sST2 had a comparable prognostic accuracy to the most updated prognostic scores for predicting 6-month mortality in HBV-ACLF patients
The ROC curves of sST2 and other two most updated prognostic scores were shown in Fig. 5. The corresponding area under ROC curve (AUC), 95% CI and P values were calculated and presented in Table 2. The AUC of serum sST2 was 0.721 at week 1 (95% CI: 0.576-0.866; P<0.01) and had no significant difference with that of the most updated prognostic scores (P>0.05). Similarly, serum sST2 had an AUC of 0.886 at week 4 (95% CI: 0.769-1.000; P<0.001), and its predictive ability was comparable to that of the most updated prognostic scores (P>0.05). Although the predictive ability of diverse prognostic scores at week 4 were superior to that at week 1 (P<0.05), there was no significant difference in predictive ability of serum sST2 between week 1 and week 4 (P>0.05).
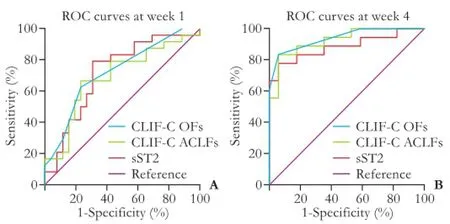
Fig. 5. Receiver operating characteristic (ROC) curves of serum sST2 and the most updated prognostic scores for predicting the 6-month mortality of patients with HBV-ACLF. ROC curves based on the data at week 1 (A) and week 4 (B).
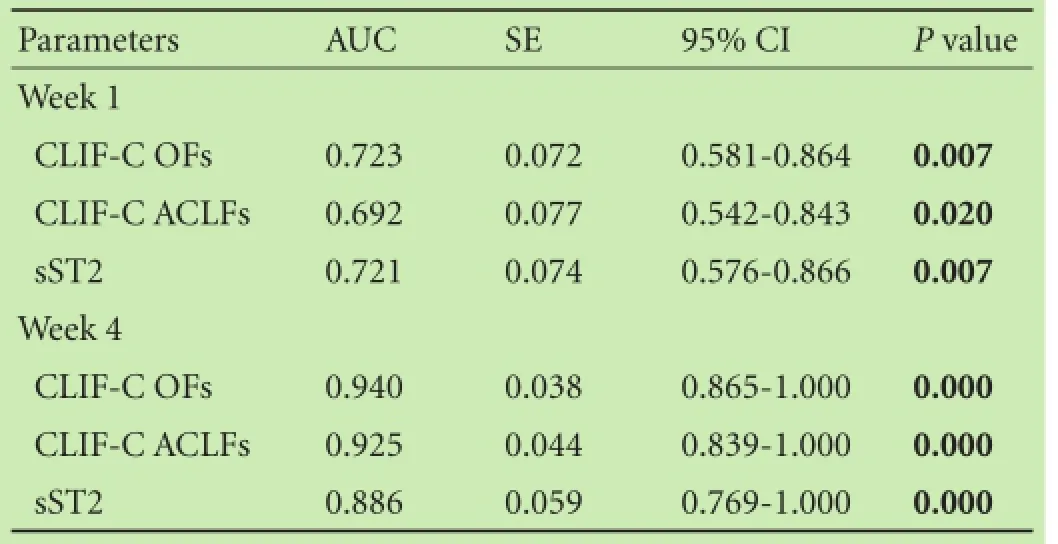
Table 2. Serum sST2 and the most updated prognostic scores for predicting the 6-month mortality of patients with HBV-ACLF
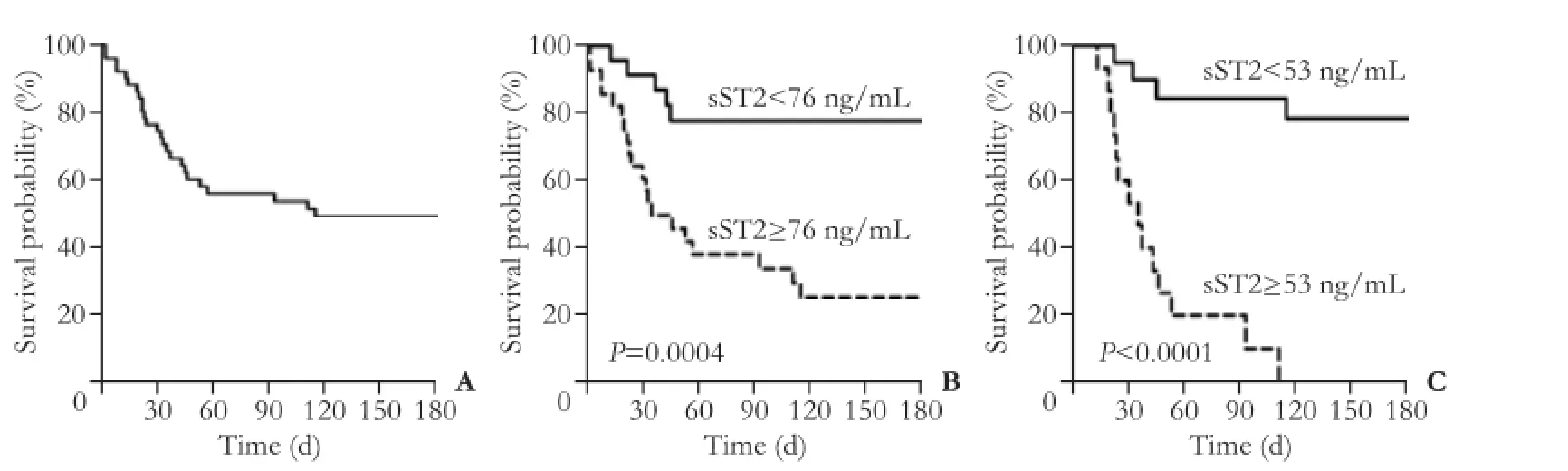
Fig. 6. Kaplan-Meier graphs of patients with HBV-ACLF according to different cut-off points of serum sST2 level. A: Kaplan-Meier graph showing the overall survival probability of the enrolled HBV-ACLF patients. Kaplan-Meier graphs showing the survival probability of two groups of HBV-ACLF patients stratified by serum sST2 level of 76 ng/mL at week 1 (B) and of 53 ng/mL at week 4 (C). P values were calculated using log-rank test.

Table 3. Diagnostic accuracy of different cut-off points of serum sST2 level at week 1 and 4 for predicting 6-month mortality in patients with HBV-ACLF
As shown in Fig. 6A, the 6-month survival rate of all enrolled HBV-ACLF patients was about 51%. At week 1, the cut-off point of serum sST2 level 76 ng/mL correctly identified 20 of 25 non-survivors in HBV-ACLF patients, with sensitivity of 80.0%, specificity of 69.2%, positive predictive value of 71.4% and negative predictive value of 78.3% (Table 3). Compared to the HBV-ACLF patients with serum sST2 <76 ng/mL at week 1, those with serum sST2 ≥ 76 ng/mL had a statistically poorer median survival time (>180 vs 35 days, P=0.0017, Fig. 6B); compared with the HBV-ACLF patients with serum sST2 <53 ng/mL at week 4, those with serum sST2 ≥53 ng/mL had a statistically poorer median survival time (>180 vs 35 days, P<0.0001, Fig. 6C).
Discussion
It has been demonstrated that IL-33/ST2 pathway is involved in various liver diseases,[14-18]and plays important and different roles in the specific diseases according to the type of target cells and the microenvironment.[13]For instance, IL-33 released from hepatocytes leads to the activation and expansion of IL-13-producing liverresident innate lymphoid cells (ILC2), which eventually promotes hepatic tissue fibrosis and remodeling.[16]Not strictly confined to the field of Th2 immunity, IL-33/ST2 pathway can also activate CD8+T cells and NK cells, and prolong the survival of patients with hepatocellular carcinoma.[17,24]Nevertheless, little is known about the role of IL-33/ST2 pathway in HBV-ACLF. The present study aimed to provide evidence for the involvement of IL-33/ ST2 pathway in the progression of HBV-ACLF, and investigate whether serum IL-33 or sST2 can be a prognostic biomarker of patients with HBV-ACLF.
Our data showed that serum IL-33 levels were not significantly different among HC, CHB and HBV-ACLF groups which were inconsistent with the other studies. Gao et al[19]found that serum IL-33 level in HBV-ACLF patients was higher than that in HC and CHB groups and was positively correlated with serum sST2. In comparison, Lei et al[20]reported that serum IL-33 level was more frequently detectable in patients with CHB than patients with HBV-ACLF which was similar to healthy controls. In addition, Lei et al did not find the correlation between serum IL-33 and sST2 levels, which was in accordance with our study.
Our data showed that HBV-ACLF patients had the highest baseline level of serum sST2 and the dynamic changes impacted their outcomes. Furthermore, it was noticed that serum sST2 level was significantly correlated with important laboratory parameters (TBil, prothrombin time, albumin and WBC count) and the most updated prognostic scores (CLIF-C OFs, CLIF-C ACLFs and ACLF grades), suggesting that serum sST2 level reflected the severity of HBV-ACLF. Finally, the ROC curves and Kaplan-Meier analysis proved that serum sST2 level had an equivalent accuracy for predicting the 6-month mortality in HBV-ACLF patients in comparison with the most updated prognostic scores. Considering the complicated calculation on the current existing prognostic scores, serum sST2, as a single laboratory parameter, is much simpler, more practical and suitable for easy clinic application. For those with serum sST2 level above the cut-off point, internal medicine usually turned out to be futile in saving lives, and liver transplantation is the only definitely effective treatment. The earlier liver transplantation performed, the better outcome the patient has. However, because of high cost and shortage of liver donors, liver transplantation could not be available forall ACLF patients. Hence, early prediction of a lethal outcome is essential for HBV-ACLF patients to choose optimal treatment strategy. The new parameter “sST2”helps doctors early recognize the ACLF patients at high risk in order to put liver transplantation on their agenda as soon as possible.
Although the exact underlying mechanisms of pathogenesis and progression of ACLF remain unclear, uncontrolled inflammatory response and immune dysfunction are considered as the predominant factor.[1]It is proposed that an inflammatory response might result in immune dysfunction, which makes patients susceptible to infection that further enhances a pro-inflammatory response in turn, eventually forming a vicious cycle. In the end, patients may shift from a pro-inflammatory to an anti-inflammatory condition and finally develop into immune paralysis, bringing about high rate of infection and mortality. The high level of serum sST2 observed in the present study is the very sign of immune paralysis in ACLF. On the one hand, serum sST2 serves as a decoy receptor restricting the bioactivity of extracellular IL-33 and inhibiting the activation of the target cells.[12]On the other hand, pro-inflammatory cytokines, like IL-6 and TNF-α abundant during liver failure, induce the production of serum sST2 in inflammatory condition.[25]In return, as a kind of powerful negative-feedback, serum sST2 can reduce the formation of pro-inflammatory cytokines and inhibit Th1-cell response, contributing to immune paralysis.[25,26]
Since CHB is the underlying etiology of HBV-ACLF, and reactivation of HBV infection is one of the major precipitating events in Asia, HBV is notably a fundamental factor in progression of the disease.[3]It has been reported that, in comparison to those with no therapy, ACLF patients with HBV reactivation had an improved survival if given antiviral treatment with tenofovir.[27]Wang et al[28]found that serum IL-33 level is significantly increased in CHB and that IL-33 could reduce the production of HBsAg, HBeAg and hepatitis B virions in vitro. Likewise, another study revealed that IL-33 drove protective antiviral CD8+T cell response in vivo.[29]However, as a decoy receptor, high level of serum sST2 in HBV-ACLF patients neutralizes the extracellular IL-33, leading to low level of IL-33 observed in HBV-ACLF patients. Perhaps low level of IL-33 is insufficient to induce antiviral immune response and reflects the state of impaired immunity, as can be seen in HIV infection,[30]prompting the occurrence and progression of HBV-ACLF.
Taken together, we had some unique points compared to the previous studies. Firstly, we included the most updated prognostic scores of CLIF-C OFs, CLIF-C ACLFs and ACLF grades in the current study, and proved that serum sST2 level had a comparable prognostic accuracy to the most updated prognostic scores for predicting 6-month mortality in HBV-ACLF patients. Since these new prognostic scores, specifically designed to stratify the risk of mortality in HBV-ACLF patients, are superior to nonspecific MELD, MELD-Na and Child-Pugh scores, our study provided more updated and convincing evidence for the predictive value of serum sST2 as a promising prognostic predictor in HBV-ACLF patients. Secondly, though we reached an agreement on the predictive value of serum sST2 in HBV-ACLF mortality with the previous study, we had different opinions on the optimal time points and cut-off value of serum sST2, both of which are so vital to a biomarker in clinical practice. Serum sST2 with the cut-off value of 76 ng/mL at week 1 is considered as the best option and most practical for predicting HBV-ACLF prognosis in our study in perspective of clinical application. However, we must admit that our study is a pilot one and the sample size of enrolled HBVACLF patients was relatively small.
In conclusion, the present study confirmed that IL-33/ST2 pathway is involved in the progression of HBV-ACLF. Serum sST2 level has a good prognostic accuracy for predicting the 6-month mortality in HBVACLF patients, comparable to the most updated prognostic scores. This data suggests that serum sST2 might be used as a promising biomarker to assess severity and predict prognosis of HBV-ACLF, helping for the early identification and optimal treatment of HBV-ACLF patients at high risk.
Contributors: JSW, LJ and XQ designed the study and drafted the manuscript. JSW, WP, MRD and LLY collected serum samples/ the clinical data and followed up the patients. JSW and WP performed ELISA assay and analyzed the data. XXG and BSS revised the manuscript. JSW and WP contributed equally to this article. XQ is the guarantor.
Funding: The study was supported by grants from the National Natural Science Foundation of China (81300316 and 81570535), the National Key Programs on Infectious Diseases of China (2012ZX10002004-003), the National Clinical Key Speciality Construction Project of China (Infectious Diseases), Shanghai Public Health Three-Year Action Project (15GWZK0102), and Project of Shanghai Municipal Health and Family Planning (20144329).
Ethical approval: The study was approved by Scientific and Ethics Committee of Ruijin Hospital (2012-87).
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, et al. Acute-on chronic liver failure. J Hepatol 2012;57:1336-1348.
2 Li H, Xia Q, Zeng B, Li ST, Liu H, Li Q, et al. Submassive hepatic necrosis distinguishes HBV-associated acute on chronic liver failure from cirrhotic patients with acute decompensation. J Hepatol 2015;63:50-59.
3 Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int 2009;3:269-282.
4 Olson JC, Wendon JA, Kramer DJ, Arroyo V, Jalan R, Garcia-Tsao G, et al. Intensive care of the patient with cirrhosis. Hepatology 2011;54:1864-1872.
5 Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif 2002;20:252-261.
6 Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A 2007;104:282-287.
7 Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem 2009;284:19420-19426.
8 Li H, Tago K, Io K, Kuroiwa K, Arai T, Iwahana H, et al. The cloning and nucleotide sequence of human ST2L cDNA. Genomics 2000;67:284-290.
9 Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem 2007;282:26369-26380.
10 Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One 2008;3: e3331.
11 Roussel L, Erard M, Cayrol C, Girard JP. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep 2008;9:1006-1012.
12 Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 2015;42: 1005-1019.
13 Villarreal DO, Weiner DB. Interleukin 33: a switch-hitting cytokine. Curr Opin Immunol 2014;28:102-106.
14 Liang Y, Jie Z, Hou L, Aguilar-Valenzuela R, Vu D, Soong L, et al. IL-33 induces nuocytes and modulates liver injury in viral hepatitis. J Immunol 2013;190:5666-5675.
15 Marvie P, Lisbonne M, L’helgoualc’h A, Rauch M, Turlin B, Preisser L, et al. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J Cell Mol Med 2010;14:1726-1739.
16 McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 2013;39:357-371.
17 Brunner SM, Rubner C, Kesselring R, Martin M, Griesshammer E, Ruemmele P, et al. Tumor-infiltrating, interleukin-33-producing effector-memory CD8(+) T cells in resected hepatocellular carcinoma prolong patient survival. Hepatology 2015;61:1957-1967.
18 Volarevic V, Mitrovic M, Milovanovic M, Zelen I, Nikolic I, Mitrovic S, et al. Protective role of IL-33/ST2 axis in Con A-induced hepatitis. J Hepatol 2012;56:26-33.
19 Gao S, Huan SL, Han LY, Li F, Ji XF, Li XY, et al. Overexpression of serum sST2 is associated with poor prognosis in acuteon-chronic hepatitis B liver failure. Clin Res Hepatol Gastroenterol 2015;39:315-323.
20 Lei Z, Mo Z, Zhu J, Pang X, Zheng X, Wu Z, et al. Soluble ST2 plasma concentrations predict mortality in HBV-related acuteon-chronic liver failure. Mediators Inflamm 2015;2015:535938.
21 Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Diagnostic and treatment guidelines for liver failure (2012 version). Zhonghua Gan Zang Bing Za Zhi 2013;21:177-183.
22 Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol 2014;61:1038-1047.
23 DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-845.
24 Gao K, Li X, Zhang L, Bai L, Dong W, Gao K, et al. Transgenic expression of IL-33 activates CD8(+) T cells and NK cells and inhibits tumor growth and metastasis in mice. Cancer Lett 2013;335:463-471.
25 Oshikawa K, Yanagisawa K, Tominaga Si, Sugiyama Y. ST2 protein induced by inflammatory stimuli can modulate acute lung inflammation. Biochem Biophys Res Commun 2002;299:18-24.
26 Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 2005;5:446-458.
27 Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology 2011;53:774-780.
28 Wang J, Cai Y, Ji H, Feng J, Ayana DA, Niu J, et al. Serum IL-33 levels are associated with liver damage in patients with chronic hepatitis B. J Interferon Cytokine Res 2012;32:248-253.
29 Bonilla WV, Fröhlich A, Senn K, Kallert S, Fernandez M, Johnson S, et al. The alarmin interleukin-33 drives protective antiviral CD8+T cell responses. Science 2012;335:984-989.
30 Miyagaki T, Sugaya M, Yokobayashi H, Kato T, Ohmatsu H, Fujita H, et al. High levels of soluble ST2 and low levels of IL-33 in sera of patients with HIV infection. J Invest Dermatol 2011;131:794-796.
Received January 15, 2016
Accepted after revision October 19, 2016
Author Affiliations: Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200025, China (Jiang SW, Wang P, Xiang XG, Mo RD, Lin LY, Lu J and Xie Q); Discipline of Pathology, School of Medical Sciences and Bosch Institute, University of Sydney, Australia (Bao SS)
Qing Xie, MD, Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200025, China (Tel: +86-21-64370045ext680403; Email: xieqingrjh@163.com)
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(16)60185-6
Published online March 3, 2017.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Pancreaticoduodenectomy for borderline resectable pancreatic head cancer with a modified artery-first approach technique
- Long-term outcome of patients with chronic pancreatitis treated with micronutrient antioxidant therapy
- High-grade pancreatic intraepithelial lesions: prevalence and implications in pancreatic neoplasia
- HIDA scan for functional gallbladder disorder: ensure that you know how the scan was done
- Novel HBV mutations and their value in predicting efficacy of conventional interferon
- The association of non-alcoholic fatty liver disease and metabolic syndrome in a Chinese population
