Comparison of two care schedules for monitoring of cardiotoxicity in patients receiving trastuzumab-based therapy for early-stage breast cancer: study protocol for a randomized controlled non-inferiority trial
2017-04-09OlexiyAseyevCarolStoberJeffreySulpherMarkClemonsChristopherJohnsonDeanFergussonLisaVandermeerSashaMazzarelloSusanDent
Olexiy Aseyev, Carol Stober, Jeffrey Sulpher Mark Clemons Christopher Johnson Dean Fergusson,Lisa Vandermeer, Sasha Mazzarello, Susan Dent
1 Department of Medicine, Division of Medical Oncology, The Ottawa Hospital, University of Ottawa, Ottawa, ON, Canada
2 Ottawa Hospital Research Institute, University of Ottawa, Ottawa, ON, Canada
3 Clinical Epidemiology Program, The Ottawa Hospital Research Institute, Ottawa, ON, Canada
4 Department of Clinical Sciences, The Thunder Bay Regional Health Sciences Centre and Northern Ontario School of Medicine,Thunder Bay, ON, Canada
INRTODUCTION
Breast cancer is the most common malignancy diagnosed in Canadian women, with approximately 22,900 cases reported annually.1-3Approximately 20-25% of breast cancer tumors overexpress Her2-neu, a pro-oncogenic surface receptor of the epidermal growth factor receptor family.4-6
One year of trastuzumab-based therapy is widely accepted as the standard of care for women with early-stage HER2-positive breast cancer. However, retrospective analyses of adjuvant trials have demonstrated an increased risk of cardiotoxicity (heart failure in 4-8% of NSABP B-31) in a small number of patients3(Figure 1). Currently, there are no validated tools to predict which patients will develop cardiacrelated complications from trastuzumab-based therapy.Left ventricular ejection fraction (LVEF), measured with transthoracicechocardiography or multiple gated acquisition scan (MUGA)4, has traditionally been used to assess cardiac function in patients receiving potentially cardiotoxic systemic therapy, however, neither the optimal type of cardiac imaging or its frequency is known. While national and international treatment guidelines recommend cardiac imaging every 36,7or 45months during adjuvant trastuzumab therapy, there is a lack of evidence supporting a defined cardiac monitoring schedule.
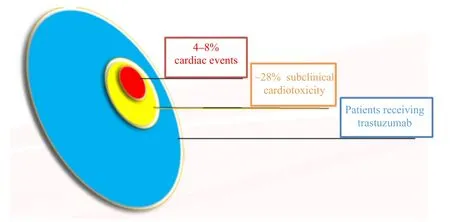
Figure 1: Cardiotoxicity rates among breast cancer patients receiving trastuzumab-based therapy.
To the best of our knowledge, no clinical trial has prospectively compared the two most commonly used frequencies of cardiac monitoring (i.e., 3 vs. 4 months). Determining the optimal imaging strategy could streamline the management of patients receiving potentially cardiotoxic therapy,improve patient’s quality of life, and potentially reduce cost to the health care system without compromising safety or efficacy of cancer treatment.
Objectives
The primary objective of the current study is to demonstrate that cardiac monitoring (echocardiogram(ECHO)/MUGA)every 4 months is not inferior to every 3 months in the detection of cardiac dysfunction (decrease in LVEF) in patients receiving trastuzumab-based therapy for early-stage breast cancer (Figure 2). Secondary objectives include changes in LVEF, delay or discontinuation of trastuzumab therapy,referrals to cardiology and feasibility of study physicians using an integrated consent model to enter patients in this study.
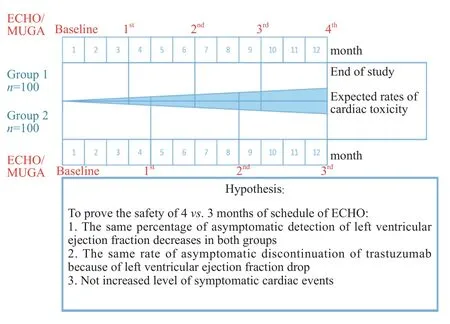
Figure 2: Timeline of cardiac monitoring schedules with expected rates of cardiotoxicity over time.
METHODS/DESIGN
The ReThinking Clinical Trials (REaCT) Program
The development of the REaCT program for comparing standard of care interventions is outlined elsewhere8. Essentially the key components include: selection of clinically relevant and practical questions; demonstration of clinical equipoise through surveys of knowledge users and completion of systematic reviews; simply defined study endpoints and avoidance of superfluous data collection; use of an integrated consent model (ICM) incorporating oral consent9;efficient REB approval10; web-based randomization in the clinic and the use of real-time electronic data capture.
Trial design
This is a prospective single-center randomized clinical trial (React-EF study), which focuses on optimization of cardiac monitoring once schedule. This study is designed to include 200 patients with stage I-III breast cancer receiving (neo)adjuvant trastuzumab-based therapy at The Ottawa Hospital Cancer Centre between June 2016 and July 2018. The main objective of this study is to demonstrate the safety of cardiac monitoring every 4 months versus every 3 months. The rates of changes in LVEF diagnosed by ECHO or MUGA throughout the course of trastuzumabbased therapy in both groups will be the primary clinical outcome for this study. The results of this study will be used to define optimal cardiac monitoring strategies for this patient population.
This study will use a novel method to allow comparisons of established standards of care for cardiac monitoring using the “integrated consent model” as part of a pragmatic clinical trial.9By integrating medical and clinical practices,physicians will be able to inform their patients about the randomized clinical trial, akin to a typical conversation between the physician and patient, without written informed consent.This clinical interaction would then be documented, as ordinarily done in clinical practice.
This study protocol was approved by the Ottawa Health Science Network Research Ethics Board (approval number:OHSN-REB 20150777-01H). The study will be performed in accordance with the Declaration of Helsinki, and informed consent will be collected from each study participant prior to enrollment. Paper writing and modification comply with the Guidance for Protocols of Clinical Trials (SPIRIT checklist).
Study population
Patients with histologically confirmed early-stage HER2-positive breast cancer receiving trastuzumab-based therapy for early-stage breast cancer, who are over 18 years of age,have a normal baseline LVEF (> 53%) and are able to provide verbal consent (Additional file 1) will be included in this study. Patients will be excluded if they have any contraindication to transthoracic echocardiography or MUGA.
Participants will be withdrawn from the study if any of the following occurs:
· Intercurrent illnesses which would, in the judgment of the investigator, affect assessment of clinical status to a significant degree, and require discontinuation of the assigned cardiac monitoring schedule
· Request by the participant or their legally authorized representative (consent withdrawal)
· Non-compliance to the study protocol or logistic consideration
· Participant is lost to follow-up
Recruitment
The Ottawa Hospital Cancer Centre sees about 1,000 patients per year with stage I-III breast cancer and about 10 to 15 patients per month would meet the study inclusion criteria for this study. We plan to recruit 200 patients over 18 to 24 months, or approximately 10 to 12 patients per month.
Randomization
Eligible and consented patients will be randomized using a permuted block design to receive cardiac evaluation (by either transthoracic ECHO or MUGA) every 3 months, or every 4 months while on treatment. We will randomize participants using a web-based system developed by the Methods Centre at The Ottawa Hospital. Each site investigator will have the web-based randomization system short-cut uploaded to their device in order to randomize participants.The physician must dictate that the verbal consent and eligibility review has taken place prior to randomization.The investigators will then forward the randomization confirmation email indicating the selected cardiac monitoring schedule to the local study clinical research associate. Stratification will be performed for anthracycline and non-anthracycline-based chemotherapy. Screening and randomization logs will be kept on file.
Outcomes
We anticipate this study will demonstrate the non-inferiority of 4 versus 3 months of LVEF monitoring in two independent groups with our non-inferiority margin of 4%. The primary clinical outcome for this study will be the rates of changes in LVEF diagnosed by ECHO or MUGA throughout the course of trastuzumab-based therapy in both groups.Based on calculated sample size we expect to enroll 100 patients in each group (Figure 3).
Secondary clinical outcomes include: 1. Rates of trastuzumab delay/discontinuation; 2. referral to cardiology;3. rate of cardiac events.
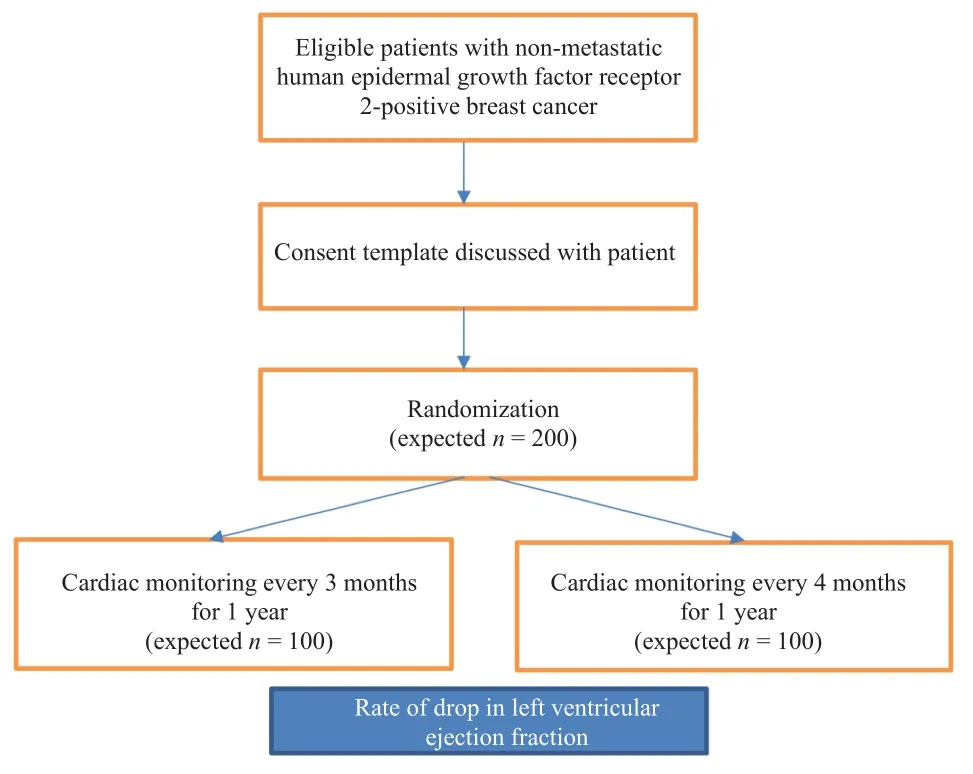
Figure 3: Study flow diagram.
Feasibility of performing this study will be measured with a combination of endpoints:
1. Percentage of patients who receive trastuzumab therapy for early-stage breast cancer compared to the number of participants who agree to randomization. The total number of patients receiving adjuvant trastuzumab at each site will be provided by pharmacy.
2. Percentage of participants who complete their assigned cardiac monitoring schedule compared to the percentage who discontinue their cardiac monitoring schedule while on study (compliance) will be calculated using the medical record.
3. Percentage of medical oncologists who attend the site initiation visit and agree to participate in the trial compared to the percentage of medical oncologists that actually approach patients regarding the trial. At the Ottawa Hospital Cancer Centre, patients are assigned to a primary medical oncologist. Therefore, all approached patients will have their primary oncologist documented on the medical record, which will be compared to the list of all oncologists agreeing to participate in the trial.
Participant timeline
Recruitment will take place from June 2016 to July 2018.Each participant will be enrolled in the study for approximately 13 months in total (screening, 12-month treatment,neither observational period nor post-treatment follow up is required). The timeline with details of the cardiac monitoring schedules is summarized in Figure 2.
Sample size calculation
As per protocol, one of the outcome points will be change in LVEF throughout the course of trastuzumab-based therapy(> 4%) in both groups. It is known that normal LVEF is> 53% (average - 61% with standard deviation 8%). In adjuvant clinical trials, many patients during trastuzumabbased treatment experienced a reversible decrease in LVEF of between 4-6%. As a criterion of non-inferiority, the mean of last measured LVEF while on trastuzumab treatment can be used to prove the same safety and sensitivity of both schedules. Our non-inferiority margin between groups is 4% (MLVEF3months- MLVEF4months< ± 4%) with a standard deviation of the mean change of 8%. To demonstrate noninferiority of 4 versus 3 months of monitoring mean LVEF in two independent groups with t tests (Means: Difference between two independent means) and our non-inferiority margin of 4%, we require 87 patients in each group - total 174 +10% (drop rate) = 200 (Figure 2). We assumed a significance level α = 0.05, power (1-β) of 95%, and effect size d = 0.5 (G*Power 3.1.9.2.).
Data collection
Participant data to becollected over the treatment period include the following:
· Past medical history (history of cardiovascular comorbidities)
· Type of cardiac monitoring-ECHO or MUGA
· Schedule of cardiac monitoring (3 vs. 4 months)
· Baseline and follow-up LVEF (%)
· Rate of treatment delay/discontinuation
· Referral to cardiology
All data will be collected using a specially developed web-based secure electronic case report form system.
Data analysis
Participants will be randomized using a web-based system developed by the Methods Centre at The Ottawa Hospital,Canada. Stratification will be performed for anthracycline and non-anthracycline-based chemotherapy.
Descriptive analysis: Baseline characteristics and management data will be presented with means (continuous measures) or proportions (categorical or ordinal data) with 95% confidence intervals.
Intention-to-treat analysis: In both studies, all statistical analyses of the data will be based on an intentionto-treat approach (based on subjects who underwent randomization). In addition, a treatment received as per protocol analysis of the primary outcome measure will be conducted.
Analysis of the primary outcome (LVEF): The primary outcome (LVEF) will be measured at baseline and every 3 or 4 months with the primary (and common) time point measure at 1 year. As the primary outcome is a continuous variable, the mean difference between both treatment groups at 1 year of follow up and the last measured LVEF while on trastuzumab treatment will be assessed using repeated-measures analysis of variance, which control for study sites (random effect) and eventually control for differences in confounding variables, should any arise despite randomization. The purpose of our model is not inferential, but rather to estimate the expected change in treatment effect and confidence interval, i.e., whether or not the 4 monthly group is found to be inferior by noninferiority by a margin of 4%.
Analysis of secondary feasibility and clinical outcomes:Secondary feasibility outcomes will be reported using descriptive statistics. Protocol adherence will be reported as a proportion. Dichotomous clinical outcomes will be compared using a one-sided Z-test while continuous variables will be compared using a one-sided t-test in an analogous manner. Relative risk (dichotomous) and mean differences(continuous) along with their 95% confidence intervals will also be calculated and presented.
Secondary analyses will be considered to better understand the influence of compliance, and losses to follow-up on the robustness of the intention-to-treat analysis. The general approaches will include an analysis of primary and secondary outcomes including only participants who completed the study as per their assigned diagnostic schedule protocol.patients (> 65 years old) with early-stage breast cancer treated with trastuzumab received cardiac monitoring according to the NCCN guidelines.11Cardiac imaging significantly increases cost to the health care system and can reduce the patient’s quality of life as it requires repeat visits to the cancer centre.This pragmatic clinical trial will assess the non-inferiority of cardiac monitoring every 4 months versus every 3 months in patients with early breast cancer receiving trastuzumab based therapy. Future trials will explore cardiac monitoring strategies using composite data including baseline cardiac risk factors,baseline cardiac imaging and cardiac biomarkers. Health care economics will be explored.
TRIAL STATUS
This study is currently recruiting participants.
Additional file
Additional file 1: Consent Script.
Criteria for feasibility success
The following criteria were established a priori and needed to be met to deem this feasibility trial successful:
1) Over 50% of appropriate patients approached agree to participate in the randomized controlled trial,
2) Over 50% of physicians who agree at study commencement to participate in the study do indeed approach patients for the study. Prior to study commencement and at study closure, all of the medical oncologists who treat breast cancer patients will be sent an email requesting feedback on their enthusiasm for the study and how they feel optimal methods of patient accrual can be incorporated into the feasibility of this study.
DISCUSSION
The majority of patients receiving trastuzumab-based therapy for early breast cancer will not experience clinically significant cardiotoxicity (i.e., heart failure). However,national and international treatment guidelines recommend cardiac imaging every 36,7or 45months during adjuvant trastuzumab therapy.
Published guidelines acknowledge a lack of evidence supporting a defined cardiac monitoring schedule. Interestingly, a recent study, using SEER data, showed that only 36% of older
We are grateful for patients and their families for their assistance with this study, as well as physicians for approaching patients.
Author contributions
This study was conceived and designed in collaboration between SD, JS, OA, MC, DF, CS, CJ, LV, and SM. The paper was drafted by OA. All authors participated in revision of the paper and approved the final version of this paper prior to publication.
Conflicts of interest
None declared.
Research ethics
This study protocol was approved by the Ottawa Health Science Network Research Ethics Board (OHSN-REB 20150777-01H).The study will be performed in accordance with the Declaration of Helsinki, and informed consent will be collected from each study participant prior to enrollment. Paper writing and modification comply with the Guidance for Protocols of Clinical Trials(SPIRIT checklist).
Registration
ClinicalTrials.gov identifier: NCT02696707; registered on February 18, 2016.
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms. In the form, the patients will give their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Open access statement
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Contributor agreement
A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check
This paper has been checked twice with duplication-checking software iThenticate.
Peer review
A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
1. Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer:NSABP B-31. J Clin Oncol. 2005;23:7811-7819.
2. Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer.N Engl J Med. 2005;353:1673-1684.
3. Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol.2002;20:1215-1221.
4. Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging.J Am Soc Echocardiogr.2014;27:911-939.
5. Jones AL, Barlow M, Barrett-Lee PJ, et al. Management of cardiac health in trastuzumab-treated patients with breast cancer:updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer. 2009;100:684-692.
6. Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy:ESMO Clinical Practice Guidelines.Ann Oncol. 2012;23 Suppl 7:vii155-166.
7. Network NCC. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)®: Breast Cancer Version 2.2015. National Comprehensive Cancer Network, Fort Washington Accessed. 2015;11.
8. Hilton J, Mazzarello S, Fergusson D, et al. Novel methodology for comparing standard-of-care interventions in patients with cancer. J Oncol Pract. 2016;12:e1016-1024.
9. Kim SY, Miller FG. Informed consent for pragmatic trials--the integrated consent model. N Engl J Med. 2014;370:769-772.
10. Sugarman J, Califf RM. Ethics and regulatory complexities for pragmatic clinical trials.JAMA. 2014;311:2381-2382.
11. Chavez-MacGregor M, Niu J, Zhang N, et al. Cardiac monitoring during adjuvant trastuzumab-based chemotherapy among older patients with breast cancer.J Clin Oncol. 2015;33:2176-2183.
