肝细胞生长因子诱导敏感非小细胞肺癌细胞对厄洛替尼耐药及机制研究
2017-04-01崔青松朴红梅崔晶刚王富佳安昌善
崔青松,朴红梅,崔晶刚,王富佳,安昌善
·论著·
肝细胞生长因子诱导敏感非小细胞肺癌细胞对厄洛替尼耐药及机制研究
崔青松,朴红梅,崔晶刚,王富佳,安昌善*
目的 研究肝细胞生长因子(HGF)诱导敏感非小细胞肺癌(NSCLC)细胞对厄洛替尼耐药的机制,观察c-Met及其下游信号通道蛋白是否参与HGF诱导不同基因型NSCLC细胞对厄洛替尼耐药。方法 2014年1月—2015年1月,选择人NSCLC细胞株PC-9〔表皮生长因子受体(EGFR)突变型,敏感株〕、H292(EGFR野生型,敏感株)及人胚肺成纤维细胞MRC-5细胞,通过ELISA法检测PC-9、H292、MRC-5细胞培养上清液中HGF水平。用MRC-5细胞培养上清液诱导PC-9、H292细胞,采用Western blotting法检测c-Met及其下游通道蛋白表达情况。将56只雌性、SPF级BALB/c裸鼠随机分为8组,每组7只。在PC-9细胞诱导模型中,对照组(C组)和厄洛替尼处理组(E组)裸鼠皮下接种PC-9细胞悬液,MRC-5诱导组(H组)、MRC-5和厄洛替尼处理组(HE组)裸鼠皮下接种PC-9+MRC-5细胞悬液;当移植瘤直径达到4 mm时,C组和H组采用0.9%氯化钠溶液灌胃,E组和HE组采用厄洛替尼灌胃。在H292细胞诱导模型中,C组、E组裸鼠皮下接种H292细胞悬液,H组、HE组裸鼠皮下接种H292+MRC-5细胞悬液;模型建立后灌胃方式同PC-9细胞诱导模型。给药结束后处死裸鼠,比较PC-9、H292细胞诱导模型中各组移植瘤重量。采用免疫组化法检测裸鼠移植瘤组织中c-Met及其下游通道蛋白表达水平。结果 PC-9、H292细胞培养上清液中均未检测到HGF,MRC-5细胞培养上清液中HGF水平为(1 262±90)pg/ml。Western blotting法结果显示,MRC-5细胞培养上清液中HGF能活化PC-9、H292细胞中p-Met、p-Akt、p-Stat3、磷酸化细胞外调节蛋白激酶1/2(p-Erk1/2)活性。PC-9细胞诱导模型中:E组移植瘤重量小于C组(P<0.05);HE组移植瘤重量小于H组,大于E组(P<0.05)。H292细胞诱导模型中:E组移植瘤重量小于C组(P<0.05);HE组移植瘤重量小于H组,大于E组(P<0.05)。c-Met、p-Met分别定位于细胞膜和细胞质。在PC-9、H292细胞诱导模型中:C组、H组、E组、HE组c-Met表达水平比较,差异均无统计学意义(P0.05);H组、HE组p-Met表达水平分别高于C组、E组(P<0.05)。Stat3定位于细胞质,p-Stat3定位于细胞核。在PC-9、H292细胞诱导模型中:C组、H组、E组、HE组Stat3表达水平比较,差异均无统计学意义(P0.05);H组、HE组p-Stat3表达水平分别高于C组、E组(P<0.05)。Akt、p-Akt均定位于细胞质。在PC-9、H292细胞诱导模型中:C组、H组、E组、HE组Akt表达水平比较,差异均无统计学意义(P0.05);H组、HE组p-Akt表达水平分别高于C组、E组(P<0.05)。Erk1/2定位于细胞质,p-Erk1/2定位于细胞核。在PC-9、H292细胞诱导模型中:C组、H组、E组、HE组Erk1/2表达水平比较,差异均无统计学意义(P0.05);H组、HE组p-Erk1/2表达水平分别高于C组、E组(P<0.05)。结论 MRC-5细胞分泌的HGF能够在裸鼠体内诱导敏感NSCLC细胞PC-9、H292对厄洛替尼耐药,HGF通过激活c-Met及其下游通道蛋白的磷酸化可能是不同基因型NSCLC细胞对厄洛替尼耐药的重要机制。
癌,非小细胞肺;肝细胞生长因子;抗药性,肿瘤
崔青松,朴红梅,崔晶刚,等.肝细胞生长因子诱导敏感非小细胞肺癌细胞对厄洛替尼耐药及机制研究[J].中国全科医学,2017,20(9):1076-1083.[www.chinagp.net]
CUI Q S,PIAO H M,CUI J G,et al.Mechanism of erlotinib resistance induced by hepatocyte growth factor in sensitive non-small-cell lung cancer cells in vivo[J].Chinese General Practice,2017,20(9):1076-1083.
厄洛替尼是表皮生长因子受体酪氨酸激酶抑制剂(epidermal growth factor receptor-tyrosine kinase inhibitor,EGFR-TKI),是目前治疗表皮生长因子受体(epidermal growth factor receptor,EGFR)敏感突变的非小细胞肺癌(non-small cell lung cancer,NSCLC)最常用的分子靶向药物,是治疗NSCLC的一线药物[1-3]。临床研究显示,厄洛替尼对部分EGFR野生状态NSCLC细胞亦有一定的抑制作用[4-5]。由于厄洛替尼存在原发性耐药或获得性耐药,仅对部分有特殊基因突变或肿瘤标志物表达的NSCLC有效[6]。c-Met是肝细胞生长因子(hepatocyte growth factor,HGF)的受体,在肿瘤增殖、侵袭过程中起重要作用。有研究显示,在体外HGF诱导不同基因型NSCLC细胞对EGFR-TKI耐药,可能与其诱导c-Met磷酸化活化有关[7-8]。在肺癌组织细胞微环境中,成纤维细胞普遍存在,可表达、分泌大量与肿瘤细胞增殖、存活相关的细胞因子及基质成分。本研究选择EGFR不同基因型人NSCLC敏感细胞株(EGFR突变型和EGFR野生型),并采用人胚肺成纤维细胞MRC-5细胞建立HGF诱导,采用Western blotting法检测c-Met及其下游通道蛋白的表达情况,将MRC-5细胞分别与PC-9、H292细胞混合种植于裸鼠右侧肋下皮下,建立PC-9、H292细胞的裸鼠移植瘤模型,观察肿瘤生长情况,并检测裸鼠移植瘤组织中c-Met及其下游通道蛋白的表达情况,旨在探究体内HGF诱导不同基因型NSCLC细胞对厄洛替尼耐药机制。
本研究背景:
本课题组前期研究表明,在体外肝细胞生长因子(HGF)诱导敏感非小细胞肺癌(NSCLC)细胞对表皮生长因子受体酪氨酸激酶抑制剂(EGFR-TKI)厄洛替尼耐药,HGF刺激c-Met及其下游通道蛋白的磷酸化可能是敏感NSCLC细胞对EGFR-TKI耐药的重要机制。
1 材料与方法
1.1 实验材料 人NSCLC细胞株PC-9(EGFR突变型,敏感株)、H292(EGFR野生型,敏感株)由同济大学附属上海肺科医院中心实验室提供;人胚肺成纤维细胞MRC-5细胞购自上海生命科学院细胞库;56只雌性、4周龄、SPF级BALB/c裸鼠购自上海市斯莱克实验动物有限公司,许可证号SCXK(沪)2003-0003,放在标准塑料盒中由专职饲养员负责饲养;盐酸厄洛替尼原料购自大连美仑生物技术有限公司;Human HGF ELISA试剂盒购自美国R&D Systems公司,兔抗人磷酸化c-Met(p-Met)(Tyr1234/1235,145 kDa)、c-Met(140/170 kDa)、磷酸化Akt(p-Akt)(Ser473,60 kDa)、Akt(60 kDa)、磷酸化细胞外调节蛋白激酶1/2(p-Erk1/2)(Tyr202/Y204,42/44 kDa)、Erk1/2(42/44 kDa)购自CST公司,磷酸化Stat3(p-Stat3)(S727,98 kDa)、Stat3(92 kDa)购自Abcam公司。
1.2 细胞培养及药物配制 2014年1月—2015年1月,将人NSCLC细胞株PC-9、H292的液氮冻存管迅速放入37 ℃恒温水浴箱中快速融化,PBS洗涤后,常规培养于含10%胎牛血清的DMEM培养液中,置于37 ℃、5% CO2恒温细胞培养箱中孵育,3~4 d换液传代1次。厄洛替尼原料采用DMSO溶解稀释成浓度为50 μmol/ml的母液,用药时DMSO终质量浓度应小于0.1%,称取150 mg厄洛替尼加入45 ml 0.9%氯化钠溶液中,再加入5 ml Tween-80,避光、4 ℃保存,使用前充分搅拌。
1.3 ELISA法检测细胞上清液中HGF水平 采用胰酶消化细胞,分别将PC-9(2×105个)、H292(2×105个)、MRC-5(2×105个)细胞接种于6孔板,放入细胞培养箱孵育48 h,取细胞培养上清液。在酶标板的包被中加入150 μl试剂稀释液后,每孔加入50 μl标准品、细胞培养上清液,轻晃混匀,用封板膜封板后室温孵育2 h。弃去液体,甩干,冲洗4次,每孔加入200 μl酶标试剂,室温孵育1.75 h。再次弃去液体,甩干,用冲洗液冲洗4次,每孔加入200 μl显色剂,室温孵育30 min。每孔加入50 μl终止液,用酶标仪测量波长450 nm处光密度(OD)值;以标准物的浓度为横坐标,OD值为纵坐标绘制标准曲线,根据细胞培养上清液的OD值由标准曲线查出相应的浓度,再乘稀释倍数即为细胞培养上清液中HGF水平。实验独立重复3次。
1.4 Western blotting法检测c-Met及其下游信号通道蛋白表达情况 取对数生长期的PC-9(5×105个)、H292(5×105个)细胞接种于6孔板,细胞饥饿过夜后加入MRC-5(2×105个)细胞培养48 h,收集上清液1 ml,立即冰上裂解细胞,4 ℃下14 800×g离心30 min,收集蛋白裂解液。采用BCA蛋白定量法进行蛋白定量。取30~40 μg蛋白经8%~10%十二烷基硫酸钠-聚丙烯酰氨凝胶电泳(SDS-PAGE)后,转印至硝酸纤维素(NC)膜上,用5%脱脂奶粉封闭1 h,一抗孵育4 ℃过夜,TBST洗膜(10 min)3次后二抗室温摇床孵育1 h,TBST洗膜(5 min)5次后ECL化学发光试剂显色、曝光成像。
1.5 裸鼠移植瘤模型的建立及药物抑瘤分析 将PC-9细胞、H292细胞和MRC-5细胞分别制成终密度为5×106个/ml、5×106个/ml、5×106个/ml的细胞悬液,而后将PC-9细胞、H292细胞分别与MRC-5细胞混合,制成混悬液PC-9(5×106个/ml)+MRC-5(5×106个/ml)、H292(5×106个/ml)+MRC-5(5×106个/ml);当裸鼠饲养到6周龄时,随机分为8组,每组7只。
在PC-9细胞诱导模型中,对照组(C组)和厄洛替尼处理组(E组)裸鼠于右侧肋下皮下接种PC-9细胞悬液100 μl,MRC-5诱导组(H组)、MRC-5和厄洛替尼处理组(HE组)裸鼠右侧肋下皮下接种PC-9+MRC-5细胞悬液100 μl;当移植瘤直径达到4 mm时,C组和H组采用含1%Tween-80的0.9%氯化钠溶液灌胃,E组和HE组采用已配制好的厄洛替尼25 mg·kg-1·d-1灌胃,每周给药5 d,连续4周。
按照上述实验步骤及方法,在H292细胞诱导模型中,C组、E组裸鼠于右侧肋下皮下接种H292细胞悬液100 μl,H组、HE组裸鼠右侧肋下皮下接种H292+MRC-5细胞悬液100 μl;模型建立后灌胃方式亦同上。测量8组裸鼠第4、7、11、14、18、21、25天移植瘤体积(移植瘤体积=移植瘤长径×移植瘤短径2/2),并绘制移植瘤生长曲线。给药结束后处死裸鼠,剥离移植瘤组织后称重。
1.6 免疫组化法检测移植瘤组织中c-Met及其下游信号通道蛋白表达水平 将剥离的移植瘤组织固定、包埋,制成石蜡切片,脱蜡、水化,PBS洗涤3次,3 min/次。用含有3%过氧化氢的PBS孵育切片30 min,PBS洗涤3次,3 min/次。用微波法进行抗原修复,PBS洗涤3次,3 min/次,将切片取出,加入5%羊血清,室温孵育30 min。弃去切片上的羊血清,用PBS洗涤3次,5 min/次,加入已稀释的一抗后,放入湿盒中室温静置1 h,然后4 ℃过夜。将一抗弃去并用PBS洗涤5次,5 min/次;加入二抗后室温孵育2 h,PBS洗涤5次,5 min/次;快速加入DAB,孵育约5 min;PBS洗涤3次,3 min/次;加上苏木素染液,再用PBS返蓝5 min。系列梯度乙醇脱水,透明,中性树胶封固。将所有切片照相,每组选取10张照片,所有照片用IPP 6.0图像分析软件进行定量分析。
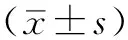
2 结果
2.1 ELISA法检测细胞上清液中HGF水平 PC-9、H292细胞培养上清液中均未检测到HGF,MRC-5细胞培养上清液中HGF水平为(1 262±90) pg/ml。
2.2 Western blotting法检测c-Met及其下游信号通道蛋白表达情况 MRC-5细胞培养上清液中HGF能活化PC-9、H292细胞中p-Met、p-Stat3、p-Akt、p-Erk1/2活性(见图1)。
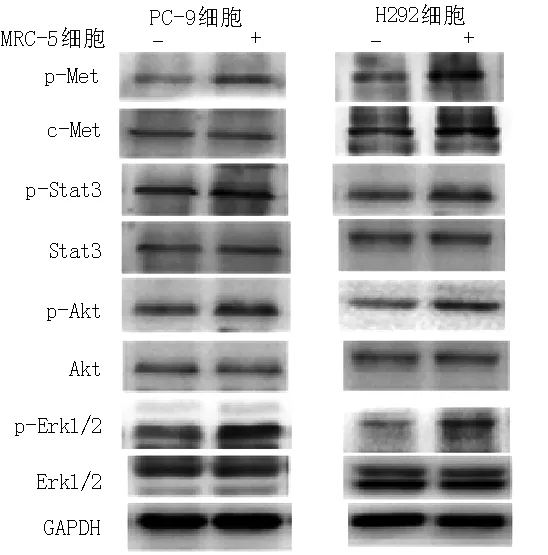
注:p-Met=磷酸化c-Met,p-Stat3=磷酸化Stat3,p-Akt=磷酸化Akt,p-Erk1/2=磷酸化细胞外调节蛋白激酶1/2
图1 Western blotting法检测c-Met及其下游信号通道蛋白表达情况
Figure 1 Expressions of c-Met and its downstream signaling pathway proteins examined by Western blotting
2.3 PC-9、H292细胞诱导模型中各组不同时间点移植瘤体积比较 PC-9细胞诱导模型中:4组第4、7天移植瘤体积比较,差异无统计学意义(P0.05);4组第11、14、18、21、25天移植瘤体积比较,差异有统计学意义(P<0.05);其中E组第11、14、18、21、25天移植瘤体积小于C组,差异有统计学意义(P<0.05);HE组第18、21、25天移植瘤体积小于H组,差异有统计学意义(P<0.05);HE组第11、14、18、21、25天移植瘤体积大于E组,差异有统计学意义(P<0.05,见表1)。
H292细胞诱导模型中:4组第4、7天移植瘤体积比较,差异无统计学意义(P0.05);4组第11、14、18、21、25天移植瘤体积比较,差异有统计学意义(P<0.05);其中E组第11、14、18、21、25天移植瘤体积小于C组,差异有统计学意义(P<0.05);HE组第11、14、18、21、25天移植瘤体积小于H组,差异有统计学意义(P<0.05);HE组第11、14、18、21、25天移植瘤体积大于E组,差异有统计学意义(P<0.05,见表2)。
2.4 给药结束后PC-9、H292细胞诱导模型中各组移植瘤重量比较 PC-9细胞诱导模型中:C组、H组、E组、HE组移植瘤重量分别为(680±373)、(805±492)、(109±84)、(361±172)mg;4组移植瘤重量比较,差异有统计学意义(F=6.628,P=0.002);其中E组移植瘤重量小于C组,差异有统计学意义(P<0.05);HE组移植瘤重量小于H组,大于E组,差异有统计学意义(P<0.05)。H292细胞诱导模型中:C组、H组、E组、HE组移植瘤重量分别为(920±122)、(979±176)、(389±109)、(564±176)mg;4组移植瘤重量比较,差异有统计学意义(F=25.454,P<0.001);其中E组移植瘤重量小于C组,差异有统计学意义(P<0.05);HE组移植瘤重量小于H组,大于E组,差异有统计学意义(P<0.05)。
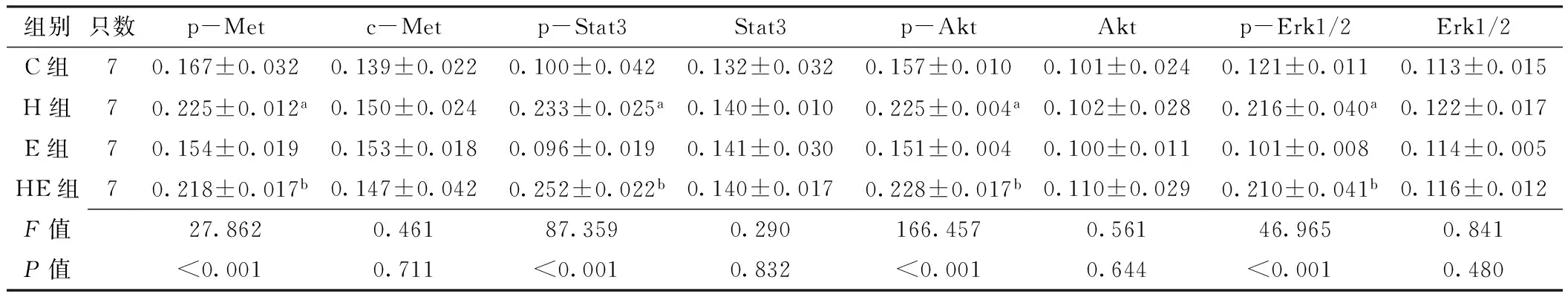
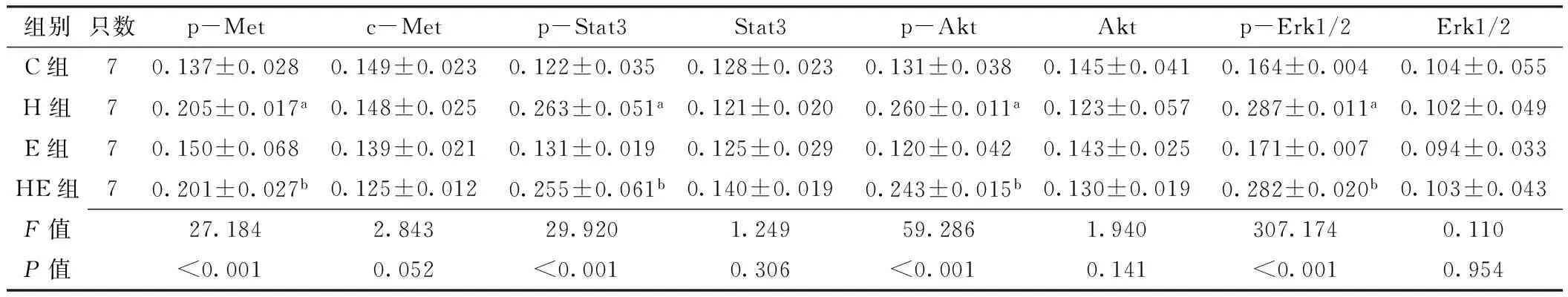
2.5 免疫组化法检测移植瘤组织中c-Met及其下游信号通道蛋白表达水平 c-Met、p-Met分别定位于细胞膜和细胞质(见图2~3,本文图2~3彩图见本刊官网www.chinagp.net电子期刊相应文章附件)。在PC-9、H292细胞诱导模型中:各组c-Met表达水平比较,差异无统计学意义(P0.05);各组p-Met表达水平比较,差异有统计学意义(P<0.05);其中H组、HE组p-Met表达水平分别高于C组、E组,差异有统计学意义(P<0.05,见表3~4)。
Stat3定位于细胞质,p-Stat3定位于细胞核(见图2~3)。在PC-9、H292细胞诱导模型中:各组Stat3
达水平比较,差异无统计学意义(P0.05);各组p-Stat3表达水平比较,差异有统计学意义(P<0.05);其中H组、HE组p-Stat3表达水平分别高于C组、E组,差异有统计学意义(P<0.05,见表3~4)。
Akt、p-Akt均定位于细胞质(见图2~3)。在PC-9、H292细胞诱导模型中:各组Akt表达水平比较,差异均无统计学意义(P0.05)。各组p-Akt表达水平比较,差异均有统计学意义(P<0.05);其中H组、HE组p-Akt表达水平分别高于C组、E组,差异有统计学意义(P<0.05,见表3~4)。
Erk1/2定位于细胞质,p-Erk1/2定位于细胞核(见图2~3)。在PC-9、H292细胞诱导模型中:各组Erk1/2表达水平比较,差异均无统计学意义(P0.05);各组p-Erk1/2表达水平比较,差异均有统计学意义(P<0.05);其中H组、HE组p-Erk1/2表达水平分别高于C组、E组,差异有统计学意义(P<0.05,见表3~4)。
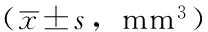
表1 PC-9细胞诱导模型中各组不同时间点移植瘤体积比较
注:PC-9细胞诱导模型中,C组=对照组,H组=MRC-5诱导组,E组=厄洛替尼处理组,HE组=MRC-5和厄洛替尼处理组;与C组比较,aP<0.05;与H组比较,bP<0.05;与E组比较,cP<0.05
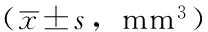
表2 H292细胞诱导模型中各组不同时间点移植瘤体积比较
注:H292细胞诱导模型中,C组=对照组,H组=MRC-5诱导组,E组=厄洛替尼处理组,HE组=MRC-5和厄洛替尼处理组;与C组比较,aP<0.05;与H组比较,bP<0.05;与E组比较,cP<0.05

Table 3 Comparison of expressions of c-Met and its downstream signaling pathway proteins in transplanted tumor tissues in groups of C,H,E and HE in PC-9 cell-induced model
组别只数p-Metc-Metp-Stat3Stat3p-AktAktp-Erk1/2Erk1/2C组70.167±0.0320.139±0.0220.100±0.0420.132±0.0320.157±0.0100.101±0.0240.121±0.0110.113±0.015H组70.225±0.012a0.150±0.0240.233±0.025a0.140±0.0100.225±0.004a0.102±0.0280.216±0.040a0.122±0.017E组70.154±0.0190.153±0.0180.096±0.0190.141±0.0300.151±0.0040.100±0.0110.101±0.0080.114±0.005HE组70.218±0.017b0.147±0.0420.252±0.022b0.140±0.0170.228±0.017b0.110±0.0290.210±0.041b0.116±0.012F值27.8620.46187.3590.290166.4570.56146.9650.841P值<0.0010.711<0.0010.832<0.0010.644<0.0010.480
注:p-Met=磷酸化c-Met,p-Stat3=磷酸化Stat3,p-Akt=磷酸化Akt,p-Erk1/2=磷酸化细胞外调节蛋白激酶1/2;与C组比较,aP<0.05;与E组比较,bP<0.05

Table 4 Comparison of expressions of c-Met and its downstream signaling pathway proteins in transplanted tumor tissues in groups of C,H,E and HE in H292 cell-induced model
组别只数p-Metc-Metp-Stat3Stat3p-AktAktp-Erk1/2Erk1/2C组70.137±0.0280.149±0.0230.122±0.0350.128±0.0230.131±0.0380.145±0.0410.164±0.0040.104±0.055H组70.205±0.017a0.148±0.0250.263±0.051a0.121±0.0200.260±0.011a0.123±0.0570.287±0.011a0.102±0.049E组70.150±0.0680.139±0.0210.131±0.0190.125±0.0290.120±0.0420.143±0.0250.171±0.0070.094±0.033HE组70.201±0.027b0.125±0.0120.255±0.061b0.140±0.0190.243±0.015b0.130±0.0190.282±0.020b0.103±0.043F值27.1842.84329.9201.24959.2861.940307.1740.110P值<0.0010.052<0.0010.306<0.0010.141<0.0010.954
注:与C组比较,aP<0.05;与E组比较,bP<0.05

注:PC-9细胞诱导模型中,C组=对照组,H组=MRC-5诱导组,E组=厄洛替尼处理组,HE组=MRC-5和厄洛替尼处理组
图2 PC-9细胞诱导模型中各组移植瘤组织中c-Met及其下游信号通道蛋白表达(免疫组化染色,×200)
Figure 2 Expressions of c-Met and its downstream signaling pathway proteins in transplanted tumor tissues in groups of C,H,E and HE in PC-9 cell-induced model stained by immunohistochemistry
3 讨论
肺癌已经成为发病率和病死率最高的癌症。由于肺癌的临床症状隐蔽,早期不易筛查出来,约75%的NSCLC患者发现时已处于疾病晚期,而常规化疗后肺癌患者的中位生存期仅为8~10个月[9]。EGFR-TKI在改善晚期NSCLC患者的近、远期疗效与生活质量中的作用显著,已成为晚期NSCLC的有效治疗手段,但临床治疗时仍存在EGFR-TKI原发、继发耐药问题。EGFR突变与EGFR-TKI治疗有效率间存在较高相关性[10]。目前EGFR-TKI获得性耐药公认的两大主要机制为EGFR二次突变(T790M)和MET基因扩增,其他可能的机制有胰岛素样生长因子1受体(IGF-1R)过表达[11]、蛋白酪氨酸磷酸酶基因(phosphatase and tensinhomolog deleted on chromosome ten,PTEN)缺失[12]、BIM下调[13]、整合素β1上调[14]、HGF高表达[15]等。2007年,ENGELMAN等[16]首次发现c-Met基因扩增引起了EGFR-TKI耐药。SENGUTA等[17]研究发现,部分肺癌患者血清中HGF水平明显升高,而这些患者均属于高侵袭型肺癌。HGF也被称为分散因子(scatter facter,SF),是成纤维细胞的衍生因子,由间质细胞(如成纤维细胞、巨噬细胞)产生,具有多种生物学功能的生物信号分子,HGF与其特异性受体c-Met结合,使c-Met磷酸化为p-Met,激活下游通道,包括MAPKs、PI13K/Akt、c-Src/FAK、STAT信号通路,这些信号通路中部分蛋白与EGFR下游通道蛋白重叠,因此,虽然EGFR-TKI有效阻断了EGFR及其下游通道,但是当患者血清中HGF水平升高,并激活c-Met及其下游通道蛋白,便可以绕过EGFR通路,仍然能够引起肿瘤细胞增殖、血管生成并转移。

注:H292细胞诱导模型中,C组=对照组,H组=MRC-5诱导组,E组=厄洛替尼处理组,HE组=MRC-5和厄洛替尼处理组
图3 H292细胞诱导模型中各组移植瘤组织中c-Met及其下游信号通道蛋白表达(免疫组化染色,×200)
Figure 3 Expressions of c-Met and its downstream signaling pathway proteins in transplanted tumor tissues in groups of C,H,E and HE in H292 cell-induced model stained by immunohistochemistry
本课题组前期研究测得厄洛替尼在体外抑制PC-9、H292细胞增殖的作用均呈浓度依赖性,HGF诱导后IC50值显著升高,说明HGF增加了细胞耐药性,其耐药机制与活化的c-Met相关[7]。
肿瘤微环境由肿瘤细胞和宿主基质细胞组成,可促进肿瘤细胞的生长和转移,并影响肿瘤细胞对抗肿瘤药物的敏感性[18-20]。有研究结果显示,在肿瘤微环境中,由基质成纤维细胞分泌的HGF可降低多种肿瘤对靶向药物的敏感性,如PLX4720(RAF抑制剂)[21]、Lapatinib(EGFR及EGFR-2抑制剂)[22]等。MRC-5细胞是人胚肺成纤维细胞,有研究表明其能够分泌HGF[23],本研究中通过ELISA法检测到PC-9、H292细胞不产生HGF,而MRC-5细胞分泌HGF,其水平为(1 262±90)pg/ml。本研究选取PC-9细胞(EGFR突变型,敏感株)和H292细胞(EGFR野生型,敏感株),将PC-9、H292细胞与MRC-5细胞培养上清液混合培养后,MRC-5细胞培养上清液中的HGF能够活化PC-9、H292细胞中p-Met、p-Stat3、p-Akt、p-Erk1/2的活性;表明MRC-5细胞能够分泌高水平HGF,并且其分泌的HGF能使PC-9、H292细胞中c-Met及其下游蛋白磷酸化。因此,本研究将MRC-5细胞分别与PC-9、H292细胞混合种植于裸鼠右侧肋下皮下,建立HGF诱导PC-9、H292细胞耐药模型,使PC-9、H292细胞在裸鼠体内能够持续受到HGF影响。通过对移植瘤生长的观察以及重量的称取,发现厄洛替尼能够抑制PC-9、H292细胞诱导模型E组、HE组肿瘤的生长,但是厄洛替尼对PC-9、H292细胞诱导模型E组肿瘤的抑制作用更加明显,说明MRC-5细胞分泌的HGF能够降低厄洛替尼对PC-9、H292细胞的抑制作用。免疫组化法检测结果显示,PC-9、H292细胞诱导模型中H组、HE组中p-Met、p-Stat3、p-Akt、p-Erk1/2表达水平均增高,与体外实验一致[8]。
综上所述,本研究进一步在裸鼠体内证实HGF通过使PC-9、H292细胞中c-Met及其下游蛋白磷酸化,诱导PC-9、H292细胞对厄洛替尼耐药,并且得出其耐药机制与活化的c-Met及其下游信号通道蛋白相关。
作者贡献:崔青松进行实验设计与实施、资料收集整理、撰写论文并对文章负责;朴红梅、崔晶刚、王福佳进行实验实施、评估、资料收集;安昌善进行质量控制及审校。
本文无利益冲突。
[1]JACKMAN D M,MILLER V A,CIOFFREDI L A,et al.Impact of epidermal growth factor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients:Results of an online tumor registry of clinical trials[J].Clin Cancer Res,2009,15(16):5267-5273.DOI:10.1158/1078-0432.CCR-09-0888.
[2]SEQUIST L V,JOSHI V A,JNNE P A,et al.Response to treatment and survival of patients with non-small cell lung cancer undergoing somatic EGFR mutation testing[J].Oncologist,2007,12(1):90-98.
[3]INOUE A,KOBAYASHI K,USUI K,et al.First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy[J].J Clin Oncol,2009,27(9):1394-1400.DOI:10.1200/JCO. .18.7658.
[4]ZHU C Q,DA CUNHA SANTOS G,DING K,et al.Role of KRAS and EGFR asbiomarkers of response to erlotinib in National Cancer Institute of CanadaClinical Trials Group Study BR.21[J].J Clin Oncol,2008,26(26):4268-4275.
[5]CAREY K D,GARTON A J,ROMERO M S,et al.Kinetic analysis of epidermalgrowth factor receptor somatic matant proteins shows increased sensitivityto the epidermal growth factor receptor tyrosine kinase inhibitor,erlotinib[J].Cancer Res,2006,66(16):8163-8171.
[6]FUKUOKA B M,YANO S,GIACCONE G,et al.Multi-institutional randomized phase Ⅱ trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer(The IDEAL 1 Trial)[corrected][J].J Clin Oncol,2003,21(12):2237-2246.
[7]玄香兰,张佳,安昌善.HGF诱导不同EGFR基因型非小细胞肺癌细胞对厄洛替尼的耐药[J].中国肿瘤生物治疗杂志,2014,21(4):413-418.DOI:10.3872/j.issn.1007-385X.2014.04.010. XUAN X L,ZHANG J,AN C S.HGF-induced resistance to erlotinib in EGFR-mutated and EGFR wildtype nonsmall lung cancer cells in vitro[J].Chinese Journal of Cancer Biotherapy,2014,21(4):413-418.DOI:10.3872/j.issn.1007-385X.2014.04.010.
[8]玄香兰,安昌善,周彩存.c-Met信号通道参与HGF诱导不同基因型非小细胞肺癌细胞株对吉非替尼耐药[J].中国肺癌杂志,2013,16(9):464-469.DOI:10.3779/j.issn.1009-3419.2013.09.05. XUAN X L,AN C S,ZHOU C C.c-Met Signaling pathway participating in the geiftinib resistance of different gene types of non-small cell lung cancer cells induced by HGF in vitro[J].Chinese Journal of Lung Cancer,2013,16(9):464-469.DOI:10.3779/j.issn.1009-3419.2013.09.05.
[9]白春学.肺癌筛查现状与争议[J].中华医学信息导报,2013,28(17):15. BAI C X.Current status and controversy of lung cancer screening[J].China Medical News,2013,28(17):15.
[10]MITSUDOMI T,YATABE Y.Mutations of the epidermal growth factor receptorgene and related genes as determinants of epidermal growth factor receptortyrosine kinase inhibitors sensitivity in lung cancer[J].Cancer Sci,2007,98(12):1817-1824.
[11]GUALBERTO A,POLLAK M.Emerging role of insulin-like growth factor receptor inhibitors in oncology:early clinical trial results and future directions[J].Oncogene,2009,28(34):3009-3021.DOI:10.1038/onc.2009.172.
[12]BIANCO R,SHIN I,RITTER C A,et al.Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors[J].Oncogene,2003,22(18):2812- 2822.
[13]LI Z,ZHOU S,ZHANG L,et al.BIM induction of apoptosis triggered by EGFR-sensitive and resistance cell lines of non-small-cell lung cancer[J].Med Oncol,2011,28(2):572-577.DOI:10.1007/s12032-010-9470-y.
[14]JU L,ZHOU C,LI W,et al.Integrin beta1 over-expression associates with resistance to tyrosine kinase inhibitor gefitinib in non-small cell lung cancer[J].J Cell Biochem,2010,111(6):1565-1574.
[15]WANG W,LI Q,YAMADA T,et al.Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors[J].Clin Cancer Res,2009,15(21):6630- 6638.DOI:10.1158/1078-0432.CCR-09-1001.
[16]ENGELMAN J A,ZEJNULLAHU K,MITSUDOMI T,et al.MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling[J].Science,2007,316(5827):1039-1043.
[17]SENGUTA S,GHERARDI E J,SELLERS L A,et al.Hepatocyte growth factor/scater factor can induce angiogenesis independently of vascular endothelial growth factor[J].Arterioscler Thromb Vasc Bio1,2003,23(1):69-75.
[18]JOYCE J A,POLLARD J W.Microenvironmental regulation of metastasis[J].Nat Rev Cancer,2009,9(4):239-252.DOI:10.1038/nrc2618.
[19]KALLURI R,ZEISBERG M.Fibroblasts in cancer[J].Nat Rev Cancer,2006,6(5):392-401.
[20]MCMILLIN D W,DELMORE J,WEISBERG E,et al.Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity[J].Nat Med,2010,16(4):483-489.DOI:10.1038/nm.2112.
[21]STRAUSSMAN R,MORIKAWA T,SHEE K,et al.Tumor micro-environment elicits innate resistance to RAF inhibitors through HGF secretion[J].Nature,2012,487(408):500-504.DOI:10.1038/nature11183.
[22]CHEN C T,KIM H,LISKA D,et al.MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells[J].Mol Cancer Ther,2012,11(3):660-669.DOI:10.1158/1535-7163.MCT-11-0754.
[23]康小红,王立芳,曹飞,等.肿瘤微环境中肝细胞生长因子介导H1975肺癌细胞对afatinib产生原发耐药[J].中华肿瘤杂志,2013,35(10):732-736.DOI:10.3760/cma.j.issn.0253-3766.2013.10.003. KANG X H,WANG L F,CAO F,et al.Tumor microenvironment elicits primary resistance to afatinib through HGF secretion[J].Chinese Journal of Oncology,2013,35(10):732-736.DOI:10.3760/cma.j.issn.0253-3766.2013.10.003.
(本文编辑:毛亚敏)
Mechanism of Erlotinib Resistance Induced by Hepatocyte Growth Factor in Sensitive Non-small-cell Lung Cancer Cells in Vivo
CUIQing-song,PIAOHong-mei,CUIJing-gang,WANGFu-jia,ANChang-shan*
DepartmentofRespiratoryMedicine,YanbianUniversityHospital,Yanji133000,China
*Correspondingauthor:ANChang-shan,Chiefphysician;E-mail:cs_an2003@aliyun.com
Objective To study the mechanism of erlotinib resistance induced by hepatocyte growth factor(HGF) in non-small-cell lung cancer(NSCLC) cells and to investigate whether c-Met and its downstream signaling pathway proteins participate in the HGF-induced erlotinib resistance of NSCLC cells with different genetypes in vivo.Methods This study was conducted from January 2014 to January 2015.We selected NSCLC cell lines with different EGFR genes(PC-9:EGFR-mutation type,sensitive;H292:EGFR-wild type,sensitive) and human embryonic lung fibroblasts MRC-5.The HGF level in cell culture supernatants secreted by PC-9,H292 and MRC-5 cells was quantified by ELISA.PC-9 and H292 cells were induced by MRC-5 cell culture supernatant;the expressions of c-Met and its downstream signaling pathway proteins were examined by Western blotting.Fifty-six female and SPF BALB/c nude mice were randomly divided into 8 groups,with each group containing 7.In building the PC-9 cell-induced model,the control group(group C) and erlotinib treatment group(group E) were subcutaneously inoculated PC-9 cell suspension and MRC-5 induced group(group H),MRC-5 and erlotinib treatment group(group HE) were inoculated PC-9+MRC-5 cell suspension subcutaneously.In establishing the H292 cell-induced model,group C and group E were inoculated H292 cell suspension subcutaneously while group H and group HE were inoculated H292+MRC-5 cell suspension subcutaneously.When the tumor diameter reached 4 mm in all groups,group C and group H were lavaged with 0.9% sodium chloride solution while group E and group HE were lavaged with erlotinib,respectively,in both built models.Mice were euthanized at after dosing.Comparisons of the weight of transplanted tumor were made between all groups in models induced by PC-9 and H292 cells.The expressions of c-Met and its downstream signaling pathway proteins in transplanted tumor tissues in nude mice were determined via immunohistochemistry.Results The concentration of HGF was not detected in PC-9 and H292 cell culture supernatants,while that in the supernatant of MRC-5 cells was(1 262 ±90) pg/ml.Western blotting results showed that HGF in the MRC-5 cell culture supernatant could stimulate the expression of p-Met,p-Akt,p-Stat3 and phosphorylated extracellular regulated protein kinase 1/2(p-Erk1/2) in PC-9 and H292 cells.The weight of transplanted tumor in group E was less than that in group C(P<0.05) in PC-9 cell-induced model;the weight of transplanted tumor in group HE was less than that in group H,but was greater than that in group E(P<0.05) in PC-9 cell-induced model.The weight of transplanted tumor in group E was less than in group C(P<0.05) in H292 cell-induced model;the weight of transplanted tumor in group HE was less than that in group H,whereas it was greater than that in group E(P<0.05) in H292 cell-induced model.c-Met and p-Met were localized in the cell membrane and cytoplasm,respectively.In both models,expressions of c-Met did not differ significantly between groups of C,H,E,and HE(P0.05);group H had higher expressions of p-Met than group C(P<0.05),and group HE had higher expressions of p-Met than group E(P<0.05).Stat3 was localized in cytoplasm and p-Stat3 in nucleus.In both models,no distinct differences in the expressions of Stat3 were noted between groups of C,H,E and HE(P0.05);group H had higher expressions of Stat3 than group C(P<0.05),and group HE showed higher expressions of Stat3 than group E(P<0.05).Akt and p-Akt were located in cytoplasm.In both models,the differences in the expressions of Akt were not prominent between groups of C,H,E and HE(P0.05);expressions of p-Akt in group H were higher than those in group C(P<0.05);expressions of p-Akt were found to be higher in group HE than in group E(P<0.05).Erk1/2 was localized in cytoplasm and p-Erk1/2 was localized in nucleus.In both models,expressions of Erk1/2 demonstrated no significant differences between groups of C,H,E and HE(P0.05);the expressions of p-Erk1/2 in H group were higher than those in group C(P<0.05);expressions of p-Erk1/2 in group HE were higher than those in group E(P<0.05).Conclusion HGF secreted by MRC-5 cell induces Erlotinib resistance in PC-9 and H292 cells in sensitive NSCLC cells.The HGF-induced activation of c-Met and its downstream signaling pathway proteins phosphorylation may be an important mechanism of Erlotinib resistance in sensitive NSCLC cells with different genetypes.
Carcinoma,non-small-cell lung;Hepatocyte growth factor;Drug resistance,neoplasm
国家自然科学基金资助项目(81160291)
R 730.26
A
10.3969/j.issn.1007-9572.2017.09.011
2016-10-21;
2017-01-27)
133000吉林省延吉市,延边大学附属医院呼吸内科
*通信作者:安昌善,主任医师;E-mail:cs_an2003@aliyun.com
