强还原处理所使用有机物料与其杀菌效果的相互关系
2017-03-29刘亮亮崔慧灵孔继婕张金波蔡祖聪黄新琦
刘亮亮, 崔慧灵, 孔继婕, 张金波,2,3,4,5, 蔡祖聪,2,3,4,5, 黄新琦,2,3,4,5*
(1.南京师范大学地理科学学院, 南京 210023; 2. 虚拟地理环境教育部重点实验室(南京师范大学),南京 210023; 3. 江苏省地理环境演化国家重点实验室培育建设点, 南京 210023; 4. 江苏省物质循环与污染控制重点实验室, 南京 210023; 5. 江苏省地理信息资源开发与利用协同创新中心, 南京 210023)
强还原处理所使用有机物料与其杀菌效果的相互关系
刘亮亮1, 崔慧灵1, 孔继婕1, 张金波1,2,3,4,5, 蔡祖聪1,2,3,4,5, 黄新琦1,2,3,4,5*
(1.南京师范大学地理科学学院, 南京 210023; 2. 虚拟地理环境教育部重点实验室(南京师范大学),南京 210023; 3. 江苏省地理环境演化国家重点实验室培育建设点, 南京 210023; 4. 江苏省物质循环与污染控制重点实验室, 南京 210023; 5. 江苏省地理信息资源开发与利用协同创新中心, 南京 210023)
强还原处理; 杀菌效果; 有机物料; 细菌多样性
随着农业集约化种植模式的不断发展,单一作物长期连作引起的土传病害问题日显突出。目前采取较多的防治措施主要有物理防治[1](土壤暴晒)、化学防治[1](杀菌剂)和生物防治[2](生防菌),虽然取得一定的成效,但均有一定的局限性,如土壤暴晒受气候条件限制,化学防治破坏生态环境,生物防治见效慢和效果不稳定等[3]。2000年,荷兰Blok等[4]和日本Shinmura[5]相继发现的土壤强还原法(reductive soil disinfestation,RSD)能够有效地抑制植物病原菌引起的土传病害,此方法是由向土壤中添加易降解有机碳源并灌溉至田间最大持水量以及覆盖塑料薄膜维持土壤厌氧状态组成。RSD过程中的极端厌氧环境[4]、微生物分解有机碳源产生的有毒物质[6-8](有机酸、氨、硫化氢以及锰和铁等低价金属离子)及微生物群落结构的变化[9]是其主要杀菌机理。诸多研究[10-12]表明RSD具有杀菌广谱性和环保的特征,目前广泛应用于荷兰、日本及美国等国家。近几年,蔡祖聪等[13]研究发现RSD还可以有效改良因连作障碍引起的设施蔬菜地退化土壤,改善土壤理化性质和恢复土壤种植生产力。
现阶段应用于RSD处理中的有机碳源主要包括两种:1)以乙醇[14]和糖浆[15]为主的易降解液体碳源;2)以纤维素、木质素和半纤维素为主的农业固体有机废弃物,如玉米秸秆、水稻秸秆和小麦秸秆等[16]。前者应用成本较高,后者在我国的处理方法仍以焚烧为主,这不仅浪费资源、造成巨大的经济损失,还严重地污染大气环境,如何处理大量的农业固体有机废弃物一直是个难题[17],RSD则可以高效利用这些有机废弃物,变废为宝。然而,目前还少有RSD过程中不同有机碳源与其杀菌效果之间相互关系的研究[18]。所以,本试验在此基础上通过收集多种不同农业固体有机废弃物作为RSD的有机碳源,比较它们之间杀菌效果的差异性并探讨其理化性质与杀菌效果之间的关系,为RSD物料的选择提供科学依据。
1 材料与方法
1.1 供试土壤和有机物料
试验土壤采自云南省石屏县(102.34°E,23.50°N)两块多年种植洋桔梗的田地(云南-1和云南-2);供试有机物料:稻壳、麦麸、芦苇和甘蔗渣采自当地并用粉碎机粉碎(粒径<0.5 cm),葡萄糖和纤维素购自南京聚康医药化工有限公司。土壤和物料风干后过100目筛并分别测定其理化性质(表 1~2)。
表1 土壤理化性质
Table 1 Physicochemical properties of the soil
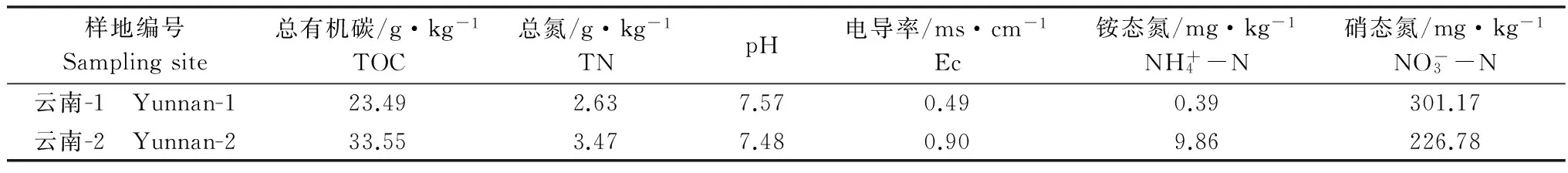
样地编号Samplingsite总有机碳/g·kg-1TOC总氮/g·kg-1TNpH电导率/ms·cm-1Ec铵态氮/mg·kg-1NH+4-N硝态氮/mg·kg-1NO-3-N云南-1 Yunnan-123.492.637.570.490.39301.17云南-2 Yunnan-233.553.477.480.909.86226.78
表2 有机物料理化性质1)
Table 2 Physicochemical properties of the organic matters

有机物料Organicmatter总有机碳/g·kg-1TOC易氧化有机碳/g·kg-1EOC粒径<2mm有机碳/g·kg-1POC水溶性有机碳/g·kg-1WSOC总氮/g·kg-1TN碳/氮C/N稻壳 Ricehull406.081.8381.65.74.5988.45麦麸 Wheatbran439.3110.0437.838.613.0133.76芦苇 Reed427.5102.5359.949.26.8062.86甘蔗渣 Sugarcaneresidue442.484.2442.428.14.6694.93葡萄糖 Glucose385.0276.7385.0381.90.00-纤维素 Cellulose432.37.1432.30.960.00-
1) “-”:代表该物料C/N无法计算。 “-”: Unable to be calculated.
1.2 试验设计
两种试验土壤各设置5个处理,云南-1:原位土壤(CK1)、土壤灌溉至最大田间持水量(45%V/W)并密封(CK2)、向土壤中分别添加2%(W/W)稻壳、麦麸和芦苇后灌溉至最大田间持水量并密封(RSD1、RSD2和RSD3);云南-2:原位土壤(CK3)、土壤灌溉至最大田间持水量(45%V/W)并密封(CK4)、 向土壤中分别添加2%(W/W)甘蔗渣、葡萄糖和纤维素后灌溉至最大田间持水量并密封(RSD4、RSD5和RSD6)。每个处理3个重复,每个重复200 g土,处理周期14 d,处理期间控温35℃,处理结束后取样测定其理化性质和生物学性质。
1.3 测定方法
1.3.1 土壤和物料理化性质的测定
1.3.2 土壤DNA的提取和尖孢镰刀菌、细菌及真菌的定量
土壤DNA的提取采用试剂盒Power SoilTMDNA Isolation Kit(MO BIO Laboratories Inc.,USA),DNA中的尖孢镰刀菌、细菌及真菌的定量通过相应的特异性引物在定量PCR仪CFX96TMReal-Time System(Bio-Rad Laboratories Inc.,Hercules,CA,USA)上进行扩增。反应体系为:2 μL DNA模板,10 μL SYBR Green premixExTaq(2×,TaKaRa,Japan),正反特异性引物各1 μL(尖孢镰刀菌:ITS1-F和AFP308;细菌:Eub338和Eub518;真菌:ITS1-f和5.8 S,表3)及6 μL无菌水。尖孢镰刀菌定量的反应条件:95℃ 2 min预变性;95℃ 10 s解链,58℃ 15 s退火,72℃ 20 s延伸,40个循环;细菌和真菌的定量反应条件:95℃ 2 min预变性;95℃ 10 s解链,53℃ 20 s退火,72℃ 30 s延伸,40个循环。在每个循环的延伸阶段采集荧光信号,反应结束后绘制熔解曲线。尖孢镰刀菌、细菌及真菌的标准曲线参照López-Mondéjar等的方法构建[21],斜率分别为-3.358、-3.368和-3.003。
表3 定量PCR和细菌PCR-DGGE引物列表
Table 3 Primers used in quantitative real-time PCR and bacterial PCR-DGGE

引物Primers序列Sequence(5'-3')参考文献ReferenceITS1-F(F)CTTGGTCATTTAGAGGAAGTAA[22]AFR308(R)CGAATTAACGCGAGTCCCAAC[23]Eub338(F)ACTCCTACGGGAGGCAGCAG[24]Eub518(R)ATTACCGCGGCTGCTGG[25]ITS1-f(F)TCCGTAGGTGAACCTGCGG[26]5.8S(R)CGCTGCGTTCTTCATCG[27]GC-U968(F)L1401(R)GC-AACGCGAAGAACCTTACGCGTGTGTACAAGACCC[28][28]GC(Bacteria)CGCCCGGGGCGCGCCCCGGGCGGGGCGGGGGCACGGGGGG[29]
1.3.3 变性梯度凝胶电泳检测土壤细菌微生物多样性
细菌PCR扩增所使用的通用引物为GC-U968(40 bp GC夹子)和L1401(表 3);反应体系为:1 μL DNA模板,12.5 μL MixTaq(2×),正反引物各1 μL,9.5 μL无菌水;反应条件:94℃ 5 min预变性;94℃ 10 s解链,52℃ 20 s退火,72℃ 30 s延伸,32个循环后72℃ 10 min再延伸,扩增效果通过琼脂糖凝胶电泳检测。
采用D-GENE System(Bio-Rad Laboratories Inc.,Hercules,CA,USA)进行变性梯度凝胶电泳(Denaturing gradient gel electrophoresis,DGGE)。在梯度为40%~60%的聚丙烯酰胺凝胶[6% (W/V),40% acrylamide/bis-acrylamide, 37.5:1,Bio-Rad]中加入PCR产物,然后以60℃、80 V电泳16 h。结束后通过成像仪检测电泳效果,并使用Quantity One 4.6.3 软件对采集图像进行多样性分析。
1.4 数据分析
采用SPSS 19.0(SPSS Inc.,Chicago,USA)中的单因素方差、Excel 2013和Origin 9.0统计软件对土壤和物料的理化性质与生物学性质进行处理分析,并使用LSD最小显著差数法检验各处理间的差异显著性(P<0.05);采用SPSS 19.0统计软件中的双变量分析法分别检测物料理化性质与杀菌效果、细菌数量及细菌群落结构之间的相关性。
2 结果与分析
处理结束后,稻壳、麦麸和芦苇RSD处理土壤pH与CK1相比差异不显著(P>0.05),CK2土壤pH显著低于CK1和稻壳、麦麸及芦苇RSD处理(P<0.05),稻壳RSD处理土壤pH显著高于麦麸RSD处理;CK4和甘蔗渣RSD处理土壤pH与CK3相比差异不显著,但葡萄糖和纤维素RSD处理土壤pH较CK3显著下降,分别下降了0.63和0.26。
CK2和稻壳、麦麸及芦苇RSD处理土壤电导率与CK1相比均显著下降,且RSD处理土壤电导率显著低于CK2;CK4和甘蔗渣、葡萄糖及纤维素RSD处理土壤电导率与CK3相比均显著下降,且葡萄糖RSD处理土壤电导率显著高于CK4和甘蔗渣及纤维素RSD处理(表4)。
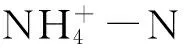
表4 各处理结束后土壤理化性质分析表1)
Table 4 Physicochemical properties of the soil at the end of treatments
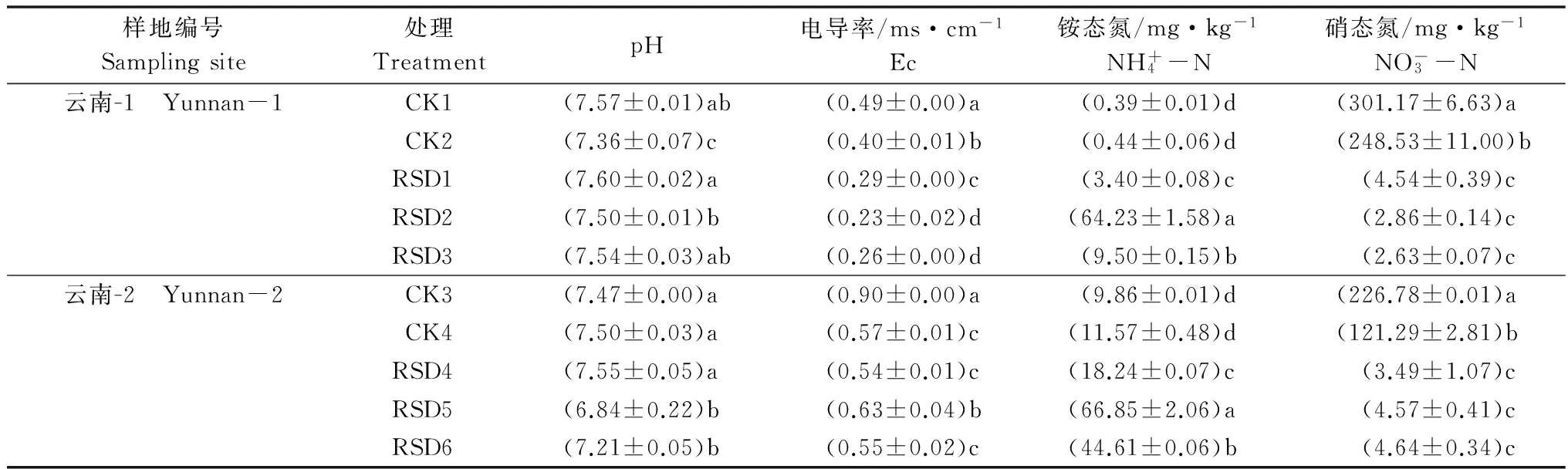
样地编号Samplingsite处理TreatmentpH电导率/ms·cm-1Ec铵态氮/mg·kg-1NH+4-N硝态氮/mg·kg-1NO-3-N云南-1 Yunnan-1CK1(7.57±0.01)ab(0.49±0.00)a(0.39±0.01)d(301.17±6.63)aCK2(7.36±0.07)c(0.40±0.01)b(0.44±0.06)d(248.53±11.00)bRSD1(7.60±0.02)a(0.29±0.00)c(3.40±0.08)c(4.54±0.39)cRSD2(7.50±0.01)b(0.23±0.02)d(64.23±1.58)a(2.86±0.14)cRSD3(7.54±0.03)ab(0.26±0.00)d(9.50±0.15)b(2.63±0.07)c云南-2 Yunnan-2CK3(7.47±0.00)a(0.90±0.00)a(9.86±0.01)d(226.78±0.01)aCK4(7.50±0.03)a(0.57±0.01)c(11.57±0.48)d(121.29±2.81)bRSD4(7.55±0.05)a(0.54±0.01)c(18.24±0.07)c(3.49±1.07)cRSD5(6.84±0.22)b(0.63±0.04)b(66.85±2.06)a(4.57±0.41)cRSD6(7.21±0.05)b(0.55±0.02)c(44.61±0.06)b(4.64±0.34)c
1) CK1和CK3为原位土壤;CK2和CK4为土壤灌溉至田间最大持水量(45%V/W)并密封;RSD1~RSD6: 200 g土壤中分别添加2%(W/W)有机物后灌溉至田间最大持水量(45%V/W)并密封,RSD1: 稻壳; RSD2: 麦麸; RSD3: 芦苇; RSD4: 甘蔗渣; RSD5: 葡萄糖; RSD6: 纤维素。表中同列数据后不同字母表示相同采样点不同处理经LSD最小显著差数法检验差异显著(P<0.05),下同。 CK1 and CK3: Untreated soil; CK2 and CK4: 200 g of soil flooded (45%) to saturate and sealed with valve bag;RSD1-RSD6: 200 g of soil incorporated with 2% (W/W) organic matter and flooded to saturate following sealed with valve bag. RSD1: Rice hull, RSD2: Wheat bran, RSD3: Reed, RSD4: Sugarcane residue, RSD5: Glucose, RSD6: Cellulose. Different letters are statistically different between the different treatments of the same sampling site following LSD tests (P<0.05); The same below.
2.2 土壤尖孢镰刀菌、细菌和真菌数量变化
处理结束后,CK2土壤尖孢镰刀菌数量(1.86×107copies/g)与CK1(2.73×107copies/g)相比差异不显著(P>0.05),但稻壳(3.39×106copies/g)、麦麸(2.75×106copies/g)及芦苇 (4.20×106copies/g)RSD处理尖孢镰刀菌数量与CK1相比分别显著下降了88.60%、90.76%及85.91% (P<0.05);CK4(1.64×107copies/g)和纤维素(1.61×107copies/g)RSD处理土壤尖孢镰刀菌数量与CK3(2.32×107copies/g)相比分别下降了36.7%和30.65%,但差异不显著,而甘蔗渣(6.42×106copies/g)和葡萄糖(3.29×106copies/g)RSD处理土壤尖孢镰刀菌数量与CK3相比分别显著下降了72.3%和85.82%,并且甘蔗渣、葡萄糖和纤维素RSD处理间的杀菌效果差异显著(图1)。
处理结束后,CK2土壤细菌数量(6.18×1010copies/g)降低至CK1(8.22×1010copies/g)的75%,稻壳(9.58×1010copies/g)和芦苇(1.07×1011copies/g)土壤细菌数量分别增加至CK1的1.16和1.30倍,但差异不显著(P>0.05),而麦麸(1.35×1011copies/g)RSD处理土壤细菌数量显著增加至CK1的1.64倍;CK4(2.30×1011copies/g)和甘蔗渣(2.67×1011copies/g)、葡萄糖(2.67×1011copies/g)及纤维素(1.42×1011copies/g)RSD处理土壤细菌数量分别显著增加至CK3(7.69×1010copies/g)的2.99、3.47、3.48和1.84倍(图1)。

图1 不同有机物料强还原处理对土壤微生物数量的影响Fig.1 Effects of various organic matters on soil microbial quantity during reductive soil disinfestation
CK2土壤真菌数量(8.50×108copies/g)与CK1 (8.57×108copies/g)差异不显著(P>0.05),但稻壳(1.33×109copies/g)、麦麸(2.24×109copies/g)和芦苇(1.29×108copies/g)RSD处理分别显著增加至CK1的1.55、2.61和1.50倍,且麦麸RSD土壤真菌数量显著高于其他厌氧处理;除纤维素(1.51×109copies/g)RSD处理土壤真菌数量显著增加至CK3(1.14×109copies/g)的1.03倍,其他厌氧处理土壤真菌数量较CK3差异不显著(图1)。
2.3 细菌多样性变化
通过Quantity one 软件对图谱进行分析,选用香农-维纳指数(Shannon-Wiener index,H′)和丰富度(Abundance,S)评价各处理间细菌群落结构的变化。从图2细菌DGGE图谱的条带亮度、位置及数量可见各处理间细菌多样性差异较明显。CK2细菌多样性与CK1相比,H′和S均显著下降(P<0.05),稻壳RSD处理的H′与S与CK1相比差异不显著,麦麸和芦苇RSD处理的H′与S均显著高于CK1,且麦麸RSD处理的H′与S值最高,表明添加麦麸的RSD处理细菌多样性最丰富。CK4和甘蔗渣、葡萄糖及纤维素RSD处理与CK3相比,H′均显著下降,且葡萄糖和纤维素RSD处理的H′显著低于CK4和甘蔗渣RSD处理;CK4的S值与CK3相比差异不显著,甘蔗渣RSD处理的S值显著低于CK3,但葡萄糖和纤维素RSD处理的S值显著低于CK3、CK4和甘蔗渣RSD处理,表明添加葡萄糖和纤维素的RSD处理细菌多样性最低(表 5)。
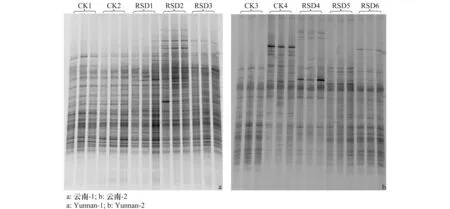
图2 处理结束后细菌DGGE图Fig.2 DGGE profiles of bacteria at the end of treatments
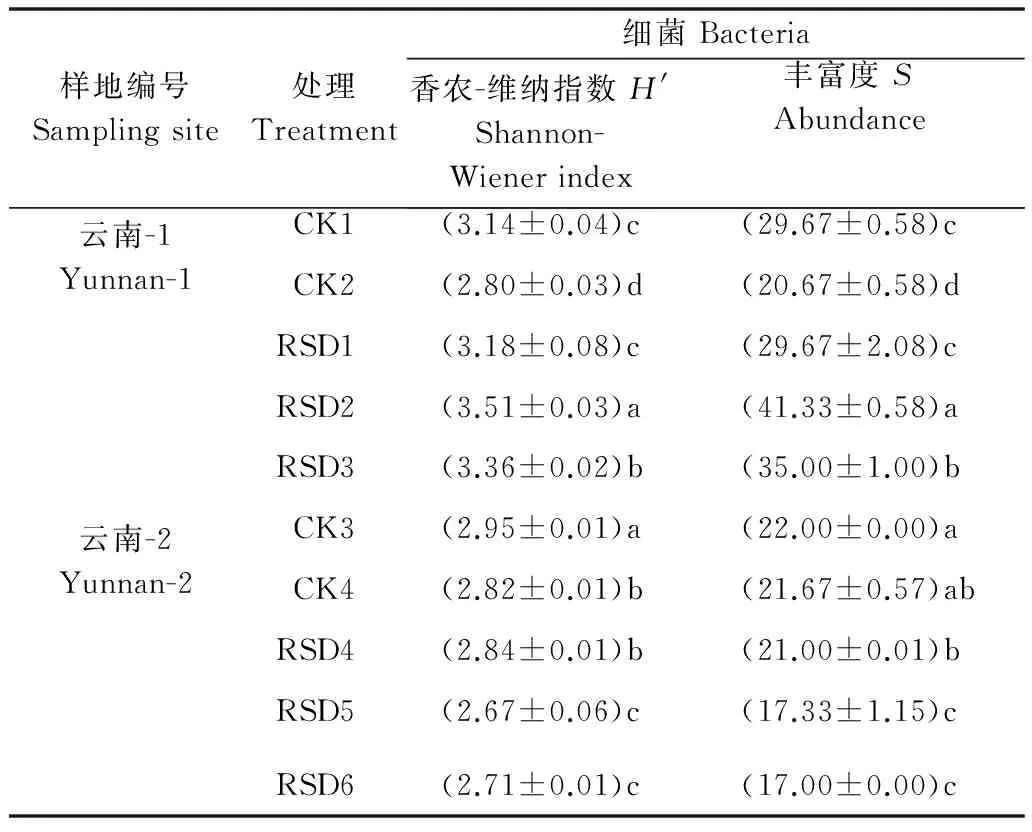
样地编号Samplingsite处理Treatment细菌Bacteria香农-维纳指数H'Shannon-Wienerindex丰富度SAbundance云南-1Yunnan-1CK1(3.14±0.04)c(29.67±0.58)cCK2(2.80±0.03)d(20.67±0.58)dRSD1(3.18±0.08)c(29.67±2.08)cRSD2(3.51±0.03)a(41.33±0.58)aRSD3(3.36±0.02)b(35.00±1.00)b云南-2Yunnan-2CK3(2.95±0.01)a(22.00±0.00)aCK4(2.82±0.01)b(21.67±0.57)abRSD4(2.84±0.01)b(21.00±0.01)bRSD5(2.67±0.06)c(17.33±1.15)cRSD6(2.71±0.01)c(17.00±0.00)c
2.4 物料理化性质与杀菌效果、细菌数量及细菌多样性间相关性分析
当双变量检验不包含CK2和CK4时,有机物料的TOC、EOC、POC(<2 mm)和TN与杀菌效果呈极显著正相关性(P<0.01),C/N与杀菌效果呈显著负相关性(P<0.05);包含CK2和CK4时,TOC和POC(<2 mm)与杀菌效果无显著相关性(P>0.05),而EOC和TN仍与杀菌效果呈显著正相关以及C/N与杀菌效果呈显著负相关。C/N始终与细菌数量呈极显著正相关性,EOC在无CK2和CK4时与其呈显著正相关,而其他理化性质与细菌数量都无显著相关性。无论是否包含CK2和CK4,物料理化性质中的TN始终与细菌H′和S呈极显著正相关性,C/N始终与H′和S呈显著负相关(表6)。
表6 物料理化性质与杀菌效果、细菌数量及细菌多样性相关性1)
Table 6 Correlations between physicochemical properties of the types of organic matter and disinfestation effects,bacterial populations and bacterial diversities
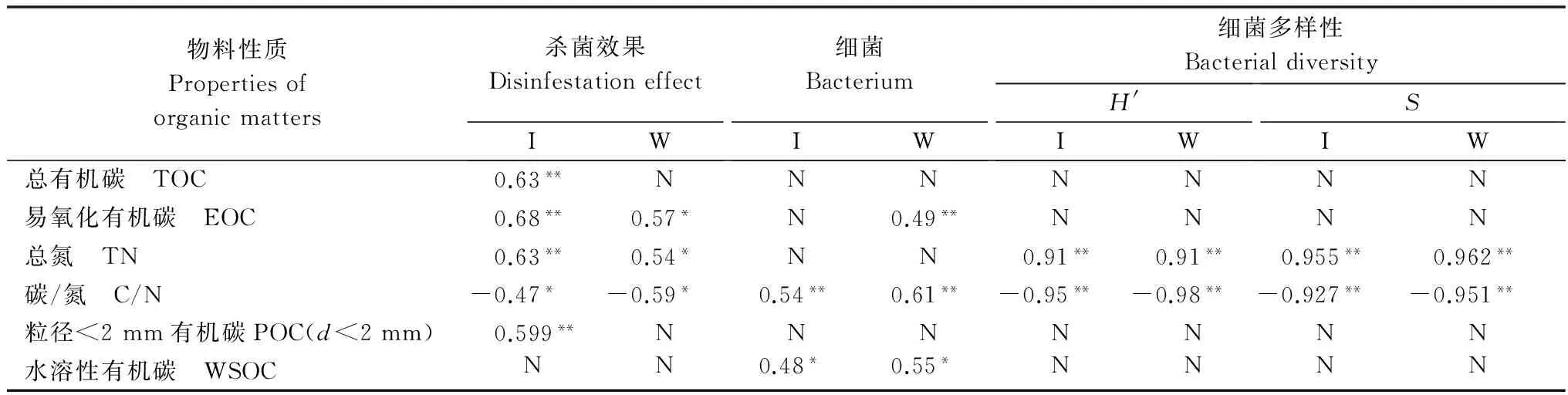
物料性质Propertiesoforganicmatters杀菌效果DisinfestationeffectIW细菌BacteriumIW细菌多样性BacterialdiversityH'IWSIW总有机碳 TOC0.63**NNNNNNN易氧化有机碳 EOC0.68**0.57*N0.49**NNNN总氮 TN0.63**0.54*NN0.91**0.91**0.955**0.962**碳/氮 C/N-0.47*-0.59*0.54**0.61**-0.95**-0.98**-0.927**-0.951**粒径<2mm有机碳POC(d<2mm)0.599**NNNNNNN水溶性有机碳 WSOCNN0.48*0.55*NNNN
1) C/N=(土壤TOC+2%物料TOC)/(土壤TN+2%物料TN); “I” 和“W”分别表示包含CK2和CK4与不包含CK2和CK4,“*”、“**”和“N”分别表示物料性质与杀菌效果间的相关性经双变量检验为显著、极显著和无相关性。 C/N was calculated using the following formula: (soil TOC+2% organic matter TOC)/(soil TN+2% organic matter TN). I and W: the treatments with and without CK2 and CK4, respectively;*,**and N represent significant correlation, highly significant correlation, and no correlation following bivariate test.
3 讨论
已有的研究表明,添加不同有机物料的RSD处理杀菌效果存在差异性[16],本试验中虽然大部分RSD处理都取得了较好的杀菌效果,但也呈现出显著差异性,尤其是添加纤维素RSD处理的杀菌效果仅为30.65%(图1),所以研究物料理化性质与杀菌效果间的关系至关重要。
黄新琦等[6]的研究结果表明:单独厌氧处理并不能取得较好的杀菌效果,有机碳源的添加是RSD取得成功的关键所在,这与本试验物料TOC与杀菌效果间的显著正相关(含CK2和CK4)结果一致。但在不包含CK2和CK4时TOC与杀菌效果无显著相关性,主要是由于各物料中的TOC含量大致相同(40%左右)。物料中的TOC可大致分为易降解(如EOC)和难降解(纤维素、木质素等)两种形态[30-31],Mowlick等[9]通过PCR-DGGE及克隆文库发现RSD处理前期厚壁菌门Firmicutes利用有机质大量繁殖并成为优势种群,其中优势属中碳源分解者瘤胃球菌属Ruminococcus和产酸菌(如Clostridia和Coprococcus)[32]居多,且Rui 等[33]推测RSD处理前期碳源分解者能够快速分解物料中的易降解有机碳并协同产酸菌产生有机酸,后期有机质中剩余部分为难降解有机碳源,微生物分解缓慢,产酸能力也较弱,从而有机酸迅速下降,上述RSD杀菌机理中有机酸(乙酸、丙酸、丁酸和异戊酸)的杀菌作用也最为关键;此外,RSD处理过程中产生的有益微生物,如芽胞杆菌Bacillus可利用易降解有机碳源大量繁殖,这些有益微生物的拮抗作用、竞争作用及寄生作用能够较好地抑制病原菌[2];本试验中物料添加以EOC为主的葡萄糖RSD处理的杀菌效果(85.82%)显著高于添加纤维素处理的杀菌效果(30.65%),推测EOC与细菌及杀菌效果间的显著正相关性可能与生防菌的繁殖及有机酸的产生有关。
本试验除了物料的碳源(TOC和EOC)与杀菌效果呈显著正相关外,TN也与杀菌效果呈显著正相关,换言之,在物料TOC大致相同的情况下,TN越高,C/N越低,杀菌效果越好。Rodriguez-Kabana[34]和Oka[35]等的研究结果也表明物料中的C/N与对根结线虫的抑制呈负相关,这与本试验的结果一致。主要原因可能与氨化作用有关,即微生物分解有机氮过程中产生的有毒物质氨具有杀菌作用,TN越高氨化作用强度越大,产生的氨也越多,从而杀菌效果也越好[36];Shrestha等[37]认为物料C/N还与RSD处理过程中的厌氧条件呈显著负相关,即RSD处理中添加的物料C/N越低,厌氧强度越剧烈,而诸多研究表明RSD过程中的厌氧条件也是杀菌机理之一[4-5];此外,可能与本试验中C/N和细菌数量及细菌多样性分别呈显著正相关和负相关有关,由于RSD过程中优势种群厚壁菌门大量繁殖,导致细菌多样性减少,而低C/N有利于刺激细菌的繁殖和提高它们的活性,从而利于抑制病原菌[38]。
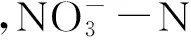
总之,RSD方法不仅可以有效杀灭土传病原菌,改善土壤理化性质,修复退化设施蔬菜地土壤,恢复土壤种植生产力,还可为高效利用农业固体有机废弃物提供有效途径。本研究结果表明,高EOC和低C/N的农业固体废弃有机物所参与的RSD处理杀菌效果更好。
[1] Gamliel A, Austerweil M, Kritzman G. Non-chemical approach to soilborne pest management-organic amendments [J]. Crop Protection, 2000, 19(8): 847-853.
[2] 殷晓敏, 陈弟, 郑服丛. 尖镰孢枯萎病生物防治研究进展[J]. 广西农业科学, 2008, 39(2): 172-178.
[3] Katan J. Physical and cultural methods for the management of soil-borne pathogens [J]. Crop Protection, 2000, 19(8): 725-731.
[4] Blok W J, Lamers J G, Termorshuizen A J, et al. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping [J]. Phytopathology, 2000, 90(3): 253-259.
[5] Shinmura A. Causal agent and control of root rot of welsh onion[R]∥PSJ Soilborne Disease Workshop Report, 2000: 133-143.
[6] 黄新琦, 温腾, 孟磊, 等. 土壤快速强烈还原对于尖孢镰刀菌的抑制作用[J]. 生态学报, 2014, 34(16):4527-4533.
[7] Momma N, Kobara Y, Momma M. Fe2+and Mn2+, potential agents to induce suppression ofFusariumoxysporumfor biological soil disinfestation [J]. Journal of General Plant Pathology, 2011, 77(6): 331-335.
[8] 黄新琦, 温腾, 孟磊, 等. 土壤强还原过程产生的有机酸对土传病原菌的抑制作用[J]. 植物保护, 2015, 41(6):38-43.
[9] Mowlick S, Hirota K, Takehara T, et al. Development of anaerobic bacterial community consisted of diverse clostridial species during biological soil disinfestation amended with plant biomass [J]. Soil Science and Plant Nutrition, 2012, 58(3): 273-287.
[10]Goud J K C, Termorshuizen A J, Blok W J, et al. Long-term effect of biological soil disinfestation onVerticilliumwilt [J]. Plant Disease, 2004, 88(7): 688-694.
[11]Momma N, Kobara Y, Uematsu S, et al. Development of biological soil disinfestations in Japan[J]. Applied Microbiology and Biotechnology, 2013, 97(9): 3801-3809.
[12]Hewavitharana S S, Ruddell D, Mazzola M. Carbon source-dependent antifungal and nematicidal volatiles derived during anaerobic soil disinfestation[J]. European Journal of Plant Pathology, 2014, 140(1): 39-52.
[13]蔡祖聪, 张金波, 黄新琦, 等. 强还原土壤灭菌防控作物土传病的应用研究[J]. 土壤学报, 2015, 52(3): 469-476.
[14]Momma N, Momma M, Kobara Y. Biological soil disinfestation using ethanol: effect onFusariumoxysporumf.sp.lycopersiciand soil microorganisms[J]. Journal of General Plant Pathology, 2010, 76(5): 336-344.
[15]Butler D M, Rosskopf E N, Kokalis-Burelle N, et al. Exploring warm-season cover crops as carbon sources for anaerobic soil disinfestation (ASD)[J]. Plant and Soil, 2012, 355(1/2): 149-165.
[16]Wen Teng, Huang Xinqi, Zhang Jinbo, et al. Effects of water regime, crop residues, and application rates on control ofFusariumoxysporumf.sp.cubense[J]. Journal of Environmental Sciences, 2015, 31: 30-37.
[17]鞠昌华. 我国农作物秸秆处理的困境与对策[J]. 贵州农业科学, 2011, 39(6): 221-224.
[18]Strauss S, Kluepfel D. Anaerobic soil disinfestation: A chemical-independent approach to pre-plant control of plant pathogens [J]. Journal of Integrative Agriculture, 2015, 14(11): 2309-2318.
[19]鲁如坤. 土壤农业化学分析方法[M].北京: 中国农业科技出版社, 2000.
[20]Blair G J, Lefroy R D, Lisle L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems [J]. Crop and Pasture Science, 1995, 46(7): 1459-1466.
[21]López-Mondéjar R, Antón A, Raidl S, et al. Quantification of the biocontrol agentTrichodermaharzianumwith real-time TaqMan PCR and its potential extrapolation to the hyphal biomass[J]. Bioresource Technology, 2010, 101(8): 2888-2891.
[22]Gardes M, Bruns T D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts [J]. Molecular Ecology, 1993, 2(2): 113-118.
[23]Lievens B, Brouwer M, Vanachter A, et al. Quantitative assessment of phytopathogenic fungi in various substrates using a DNA macroarray[J]. Environmental Microbiology, 2005, 7(11): 1698-1710.
[24]Dorsch M, Lane D, Stackebrandt E. Towards a phylogeny of the genusVibriobased on 16S rRNA sequences [J]. International Journal of Systematic Bacteriology, 1992, 42(1): 58-63.[25]Muyzer G, De Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA [J]. Applied and Environmental Microbiology, 1993, 59(3): 695-700.
[26]Fierer N, Jackson J A, Vilgalys R, et al. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays [J]. Applied and Environmental Microbiology, 2005, 71(7): 4117-4120.
[27]Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from severalCryptococcusspecies [J]. Journal of Bacteriology, 1990, 172(8): 4238-4246.
[28]Zoetendal E G, Akkermans A D L, De Vos W M. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria [J]. Applied and Environmental Microbiology, 1998, 64(10): 3854-3859.
[29]Nübel U, Engelen B, Felske A, et al. Sequence heterogeneities of genes encoding 16S rRNAs inPaenibacilluspolymyxadetected by temperature gradient gel electrophoresis [J]. Journal of Bacteriology, 1996, 178(19): 5636-5643.
[30]Heal O W, Anderson J M, Swift M J. Plant litter quality and decomposition: an historical overview[C]∥Cadisch G,Giller K E.Driven by nature: plant litter quality and decomposition,1997:3-30.
[31]Puget P, Drinkwater L. Short-term dynamics of root-and shoot-derived carbon from a Leguminous green manure [J].Soil Science Society of America Journal,2001,65(3):771-779.
[32]Huang Xinqi, Liu Liangliang, Wen Teng, et al. Illumina MiSeq investigations on the changes of microbial community in theFusariumoxysporumf.sp.cubenseinfected soil during and after reductive soil disinfestation [J]. Microbiological Research, 2015, 181: 33-42.
[33]Rui Junpeng, Peng Jingjing, Lu Yahai. Succession of bacterial populations during plant residue decomposition in rice field soil[J]. Applied and Environmental Microbiology, 2009, 75(14): 4879-4886.
[34]Rodriguez-Kabana R, Morgan-Jones G, Chet I. Biological control of nematodes: Soil amendments and microbial antagonists[J]. Plant and Soil, 1987, 100(1/3): 237-247.
[35]Oka Y, Shapira N, Fine P.Control of root-knot nematodes in organic farming systems by organic amendments and soil solarization [J]. Crop Protection, 2007, 26(10): 1556-1565.
[36]Tenuta M, Lazarovits G. Ammonia and nitrous acid from nitrogenous amendments kill the microsclerotia ofVerticilliumdahliae[J]. Phytopathology, 2002, 92: 255-264.
[37]Shrestha U, Ownley B H, Rosskopf E N, et al. Optimization of amendment C∶N ratio in anaerobic soil disinfestation for control ofSclerotiumrolfsii[C]∥Proceedings of the international research conference on methyl bromide alternatives and emissions reductions. San Diego, California, 2013: 4-6.
[38]Rousk J, Bååth E. Fungal and bacterial growth in soil with plant materials of different C/N ratios [J]. FEMS Microbiology Ecology, 2007, 62(3): 258-267.
[39]朱同彬, 孟天竹, 张金波, 等. 强还原方法对退化设施蔬菜地土壤的修复[J]. 应用生态学报, 2013, 24(9): 2619-2624.
[40]Zhu Tongbin, Zhang Jinbo, Yang Wenyan, et al. Effects of organic material amendment and water content on NO, N2O, and N2emissions in a nitrate-rich vegetable soil [J]. Biology and Fertility of Soils, 2013, 49(2): 153-163.
[41]Meng Tianzhu, Zhu Tongbin, Zhang Jinbo, et al. Effect of liming on sulfate transformation and sulfur gas emissions in degraded vegetable soil treated by reductive soil disinfestation[J]. Journal of Environmental Sciences, 2015, 36: 112-120.
(责任编辑:田 喆)
Relationships between different types of organic matters and disinfestation effects during reductive soil disinfestation
Liu Liangliang1, Cui Huiling1, Kong Jijie1, Zhang Jinbo1,2,3,4,5, Cai Zucong1,2,3,4,5, Huang Xinqi1,2,3,4,5
(1.CollegeofGeography,NanjingNormalUniversity,Nanjing210023,China; 2.KeyLaboratoryofVirtualGeographicEnvironment(NanjingNormalUniversity),MinistryofEducation,Nanjing210023,China; 3.StateKeyLaboratoryCultivationBaseofGeographicalEnvironmentalEvolution(JiangsuProvince),Nanjing210023,China; 4.JiangsuProvincialKeyLaboratoryofMaterialCyclingandPollutionControl,Nanjing210023,China; 5.JiangsuCenterforCollaborativeInnovationinGeographicalInformationResourceDevelopmentandApplication,Nanjing210023,China)
Although reductive soil disinfestation (RSD) through creating anaerobic condition and incorporating soil with large amounts of organic matters is an effective method for soil disinfestation, there is limited information about the relationships between the type of organic matter and disinfestation effects (DEs). Hence, in this study, various RSD treatments incorporated with rice hull (RSD1), wheat bran (RSD2), and reed (RSD3), sugarcane residue (RSD4), glucose (RSD5), and cellulose (RSD6) were respectively conducted, and quantitative real-time PCR and denaturing gradient gel electrophoresis (DGGE) were performed to investigate the relationships between organic matters and DEs. The results showed that the content of soil NO-3-N and electrical conductivity (Ec) significantly decreased in the RSD treatments, whereas the content of NH+4-N significantly increased. The DEs of these RSD treatments (RSD1-6) were 88.60%, 90.76%, 85.91%, 72.3%, 85.87%, and 30.65%, respectively. Total organic carbon (TOC), easily oxidized organic carbon (EOC), and total nitrogen (TN) in the organic matter had a significant positive correlation with DE and bacterial population (P<0.05). Besides, the initial soil C/N after incorporating the organic matters had a significant negative correlation with DE, and had a significant positive correlation with bacterial population. Therefore, the RSD treatments incorporated with the organic matters with higher EOC, TN and lower C/N would obtain a better disinfestation effect.
reductive soil disinfestation; disinfestation effect; organic matter; bacterial diversity
2016-04-14
2016-05-25
国家自然科学基金(41301335)
S 432.4
A
10.3969/j.issn.0529-1542.2017.02.012
* 通信作者 E-mail:xqhuang@njnu.edu.cn
