大环内酯类抗菌药物介导的药物相互作用评估
2017-03-17吕秋菊蒲强红乐山市人民医院内分泌科四川乐山614000乐山市人民医院药学部四川乐山614000
吕秋菊,蒲强红(1.乐山市人民医院内分泌科,四川乐山 614000;2.乐山市人民医院药学部,四川乐山614000)
大环内酯类抗菌药物介导的药物相互作用评估
吕秋菊1*,蒲强红2#(1.乐山市人民医院内分泌科,四川乐山 614000;2.乐山市人民医院药学部,四川乐山614000)
目的:评估大环内酯类抗菌药物红霉素、克拉霉素与阿奇霉素介导的药物相互作用,为临床合理用药提供参考。方法:检索PubMed、中国知网(CNKI)、万方等数据库中关于红霉素、克拉霉素与阿奇霉素介导的药物相互作用的临床试验文献,并搜集其药品说明书,采用美国食品与药物管理局(FDA)推荐的药物相互作用标准进行统计分析。结果:临床试验发现有20种药物与红霉素存在药物相互作用可能性,有强、中度和弱相互作用的分别为3、6、11种;22种药物与克拉霉素存在药物相互作用可能性,有强、中度或弱相互作用的分别为2、11、9种;5种药物与阿齐霉素存在药物相互作用可能性,且全部为弱相互作用。以咪达唑仑为CYP3A4底物评估三者介导的药物相互作用强度,发现克拉霉素、红霉素和阿奇霉素分别引起强、中度和弱相互作用。结论:红霉素和克拉霉素发生药物相互作用的可能性较阿奇霉素大,可能原因是红霉素和克拉霉素对CYP3A4抑制强度远大于阿奇霉素。
药物相互作用;CYP3A4;红霉素;克拉霉素;阿齐霉素
大环内酯类(Macrolides)是一类具有14、15、16元大环内酯环基本结构与相似抗菌谱的抗菌药物。大环内酯类抗菌药物常用于治疗大多数革兰氏阳性菌、部分革兰氏阴性菌(如奈瑟菌、军团菌、嗜血杆菌等)及非典型病原体感染。临床上大多数药物相互作用是由药物代谢酶或药物转运体介导[1-4]。大环内酯类抗菌药物为主要的药物代谢酶CYP3A4与药物转运体P糖蛋白(P-glycoprotein,P-GP)抑制剂,且不同大环内酯类药物对上述药物代谢酶和转运体的抑制强度有所差别,故不同大环内酯类药物介导的药物相互作用是否亦有差别尚不清楚。因此,本研究采用检索临床文献方法来评估常用大环内酯类抗菌药物红霉素(Erythromycin)、克拉霉素(Clarithromycin)与阿奇霉素(Azithromycin)介导的药物相互作用,为临床合理用药提供参考。
1 资料与方法
1.1 资料来源
考察红霉素、克拉霉素与阿奇霉素所介导的药物相互作用的曲线下面积比值(AUCR=AUC有抑制剂/AUC无抑制剂)。纳入标准:红霉素、克拉霉素与阿奇霉素介导的药物相互作用的临床研究,且有AUCR报告。排除标准:红霉素、克拉霉素与阿奇霉素介导的药物相互作用的非临床研究,或者无AUCR报告的临床研究。所有数据来源于PubMed、中国知网(CNKI)、万方等数据库及美国食品与药物管理局(FDA)网站。
1.2 方法
在CNKI、万方等国内数据库采用“红霉素”“克拉霉素”“阿奇霉素”与“曲线下面积”“AUC”等检索词检索药物相互作用有关文献。在PubMed数据库则采用“Erythromycin”“Clarithromycin”“Azithromycin”与“AUC”“area under curve”“Area under curves”“Area under the concentration-time curve”“Area under the plasma concentration-time curve”“Area under the serum concentration time curves”等检索词检索文献。在FDA网站(www. FDA.org)检索红霉素、克拉霉素与阿奇霉素的药品说明书。检索时间限定为数据库建库到2016年6月10日。首先排除非临床试验与无法提供AUC的文献,保留临床试验文献。然后逐一阅读文献与药品说明书,提取剂量、底物、AUCR等数据。如有相同底物被同一抑制剂的药物相互作用被多篇文献报道,则估算AUCR平均值纳入最终分析。药物相互作用风险程度评估采用美国FDA推荐标准——弱相互作用:1.25≤AUCR<2.0;中度相互作用:2.0≤AUCR<5.0;强相互作用:AUCR≥5.0。
2 结果
2.1 红霉素介导的药物相互作用
红霉素介导的药物相互作用见表1。如表1所示,临床试验共测试红霉素与35种药物的相互作用,其中利多卡因又分口服和静脉两种给药途径,共计有36个药物相互作用结果。研究发现与红霉素可能有强相互作用的3种药物为双氢麦角隐亭、辛伐他汀和丁螺环酮;有中度相互作用的6种药物为依维莫司、咪达唑仑、乌利司他、西地那非、利多卡因(口服)和非洛地平;有弱相互作用的11种药物为沙奎那韦、佐匹克隆、希美加群、罗氟司特、非索非那定、伏立康唑、氯雷他定、阿托伐他汀、雌二醇、洛沙坦和喹硫平。从药物类别来看,红霉素与羟甲基戊二酰辅酶A(HMG-CoA)还原酶抑制剂或H1受体拮抗药的相互作用研究较多,分别有4种药物。

表1 红霉素介导的药物相互作用
2.2 克拉霉素介导的药物相互作用
克拉霉素介导的药物相互作用见表2。如表2所示,临床试验共测试克拉霉素与36种药物的相互作用。研究发现与克拉霉素可能有强相互作用的2种药物为辛伐他汀和咪达唑仑;有中度相互作用的11种药物为秋水仙碱、他克莫司、西布曲明、卡麦角林、氯胺酮、阿托伐他汀、孟鲁司特、西地那非、奥美拉唑、普伐他汀和羟考酮;有弱相互作用的9种药物:曲唑酮、利福布丁、利奈唑胺、埃索美拉唑、兰索拉唑、地高辛、达比加群、茚地那韦和安贝生坦。从药物类别来看,克拉霉素与质子泵抑制剂、抗HIV药或HMG-CoA还原酶抑制剂的相互作用研究较多,分别有6、4、3种药物。
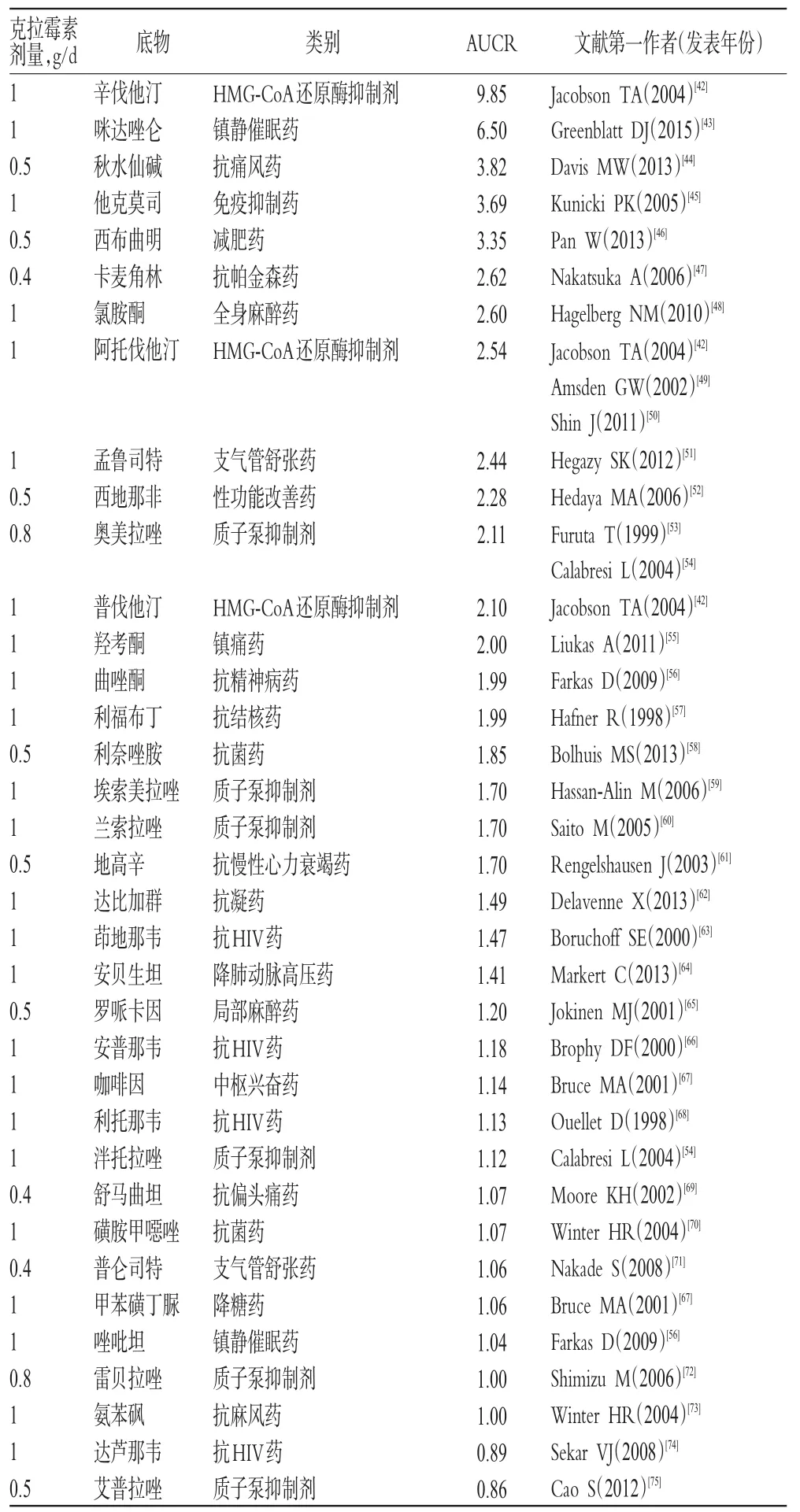
表2 克拉霉素介导的药物相互作用
2.3 阿奇霉素介导的药物相互作用
阿奇霉素介导的药物相互作用见表3。如表3所示,临床试验共测试阿奇霉素与22种药物的相互作用。研究发现,与阿奇霉素可能有弱相互作用的5种药物为非索非那定、希美加群、秋水仙碱、伊维菌素和咪达唑仑;无强或中度相互作用的药物。从药物类别来看,阿奇霉素霉素与H1受体阻滞药或抗HIV药的相互作用研究较多,分别有4、3种药物。
2.4 3种药物介导的药物相互作用类别比较
3种药物介导的药物相互作用类别比较见表4。如表4所示,红霉素与克拉霉素引起的药物相互作用远多于阿奇霉素,分别为20、22、5个。药物相互作用中,阿奇霉素仅有弱相互作用的临床报道,而红霉素和克拉霉素3种强度的相互作用均有报道。选用咪达唑仑(Midazolam)为CYP3A4底物来分析三者引起的药物相互作用强度,克拉霉素、红霉素与阿奇霉素分别引起强、中度、弱相互作用(AUCR分别为6.50、3.81、1.26),见图1A。由此可见,克拉霉素为CYP3A4的强抑制剂,红霉素为CYP3A4的中度抑制剂,而阿奇霉素为CYP3A4的弱抑制剂。选用地高辛或非索非那定为P-GP底物来分析三者引起的药物相互作用强度,克拉霉素、红霉素与阿奇霉素都导致弱相互作用(AUCR分别为1.70、1.61、1.67),表明三者对P-GP的抑制强度可能类似,见图1B。

表3 阿奇霉素介导的药物相互作用
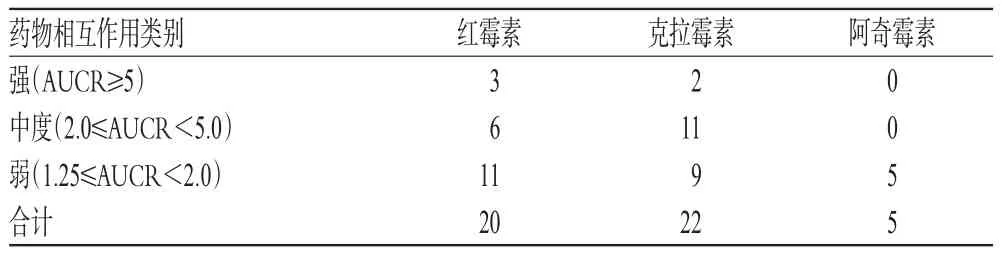
表4 3种药物介导的药物相互作用类别比较

图1 红霉素、克拉霉素和阿奇霉素介导的药物相互作用强度比较
3 讨论
本研究结果表明,红霉素和克拉霉素发生药物相互作用的可能性与强度远大于阿奇霉素。可能原因是三者对药物主要代谢酶CYP3A4活性而非药物转运体PGP活性的抑制强度不同造成的。以咪达唑仑为CYP3A4底物评估三者介导的药物相互作用强度,发现克拉霉素、红霉素和阿奇霉素分别引起强、中度和弱相互作用,因此克拉霉素、红霉素和阿奇霉素分别是CYP3A4的强抑制剂、中度抑制剂和弱抑制剂[9,43-44]。而以地高辛或非索非那定为P-GP底物评估三者介导的药物相互作用强度,发现克拉霉素、红霉素和阿奇霉素都引起弱相互作用,因此3种药物对P-GP的转运活性抑制程度可能相当[19,61,76]。
从药物类别来看,研究者主要关注3种大环内酯类抗菌药物与HMG-CoA还原酶抑制剂、质子泵抑制剂、H1受体拮抗药和抗HIV药的药物相互作用。以HMG-CoA还原酶抑制剂为例,红霉素、克拉霉素和阿奇霉素介导的药物相互作用强度还是有所区别。以CYP3A4底物阿托伐他汀为例,克拉霉素、红霉素和阿奇霉素分别介导的阿托伐他汀的AUCR为2.54、1.33和0.98,表明三者分别引起中度、弱和无相互作用[22,42,49-50]。上述差异可能是由于克拉霉素、红霉素和阿奇霉素对CYP3A4活性抑制程度不同造成的。此外,同一大环内酯类抗菌药物对不同HMG-CoA还原酶抑制剂的药物相互作用可能性亦有区别。红霉素介导的辛伐他汀、阿托伐他汀、西立伐他汀(已退市)和瑞舒伐他汀的AUCR分别为6.20、1.33、1.21、1.20,因此红霉素仅与辛伐他汀和阿托伐他汀存在强与弱药物相互作用可能性[6,22,26,28,42,50]。同样,克拉霉素介导的辛伐他汀、阿托伐他汀和普伐他汀的AUCR分别为9.85、2.54、2.10,故克拉霉素与辛伐他汀存在强相互作用可能性,而与阿托伐他汀与普伐他汀存在中度相互作用可能性[42,82]。造成上述药物相互作用差异的主要原因是不同HMG-CoA还原酶抑制剂经CYP3A4的代谢程度有差异。辛伐他汀和阿托伐他汀主要经CYP3A4代谢,而普伐他汀和瑞舒伐他汀则少量经CYP3A4代谢。故红霉素和克拉霉素与辛伐他汀和阿托伐他汀有明显的药物相互作用可能性。因此,对于伴有胃十二指肠溃疡的高胆固醇血症患者,如选用含克拉霉素的幽门螺杆菌根治方案,则降胆固醇药应尽量避免使用辛伐他汀和阿托伐他汀。
综上所述,红霉素和克拉霉素与其他药物发生相互作用的可能性与强度远大于阿奇霉素,可能原因是是红霉素和克拉霉素对CYP3A4抑制强度远大于阿奇霉素。其次,阿奇霉素是大环内酯类抗菌药物中发生药物相互作用较少的品种。
[1] Muller F,Fromm MF.Transporter-mediated drug-drug interactions[J].Pharmacogenomics,2011,12(7):1017-1037.
[2] 徐海燕,刘冬,王文刚,等.他汀类药物与常见心血管药物相互作用的研究进展[J].中国药房,2016,27(11):1582-1584.
[3] Wanwimolruk S,Phopin K,Prachayasittikul V.Cytochrome P450enzyme mediated herbal drug interactions:Part 2[J]. EXCLI J,2014,13:869-896.
[4] Wanwimolruk S,Prachayasittikul V.Cytochrome P450enzyme mediated herbal drug interactions:Part 1[J].EXCLI J,2014,13:347-391.
[5] de Mey C,Althaus M,Ezan E,et al.Erythromycin increases plasma concentrations of alpha-dihydroergocryptine in humans[J].Clin Pharmacol Ther,2001,70(2):142-148.
[6] Kantola T,Kivisto KT,Neuvonen PJ.Erythromycin and verapamil considerably increase serum simvastatin and simvastatin acid concentrations[J].Clin Pharmacol Ther,1998,64(2):177-182.
[7] Kivisto KT,Lamberg TS,Kantola T,et al.Plasma buspirone concentrations are greatly increased by erythromycin and itraconazole[J].Clin Pharmacol Ther,1997,62(3):348-354.
[8] Kovarik JM,Beyer D,Bizot MN,et al.Effect of multipledose erythromycin on everolimus pharmacokinetics[J]. Eur J Clin Pharmacol,2005,61(1):35-38.
[9] Zimmermann T,Yeates RA,Laufen H,et al.Influence of the antibiotics erythromycin and azithromycin on the pharmacokinetics and pharmacodynamics of midazolam[J].Arzneimittelforschung,1996,46(2):213-217.
[10] Pohl O,Osterloh I,Gotteland JP.Effects of erythromycin at steady-state concentrations on the pharmacokinetics of ulipristal acetate[J].J Clinical Pharm Ther,2013,38(6):512-517.
[11] Muirhead GJ,Faulkner S,Harness JA,et al.The effects of steady-state erythromycin and azithromycin on the pharmacokinetics of sildenafil in healthy volunteers[J].Br J Clin Pharmacol,2002,53(Suppl 1):37S-43S.
[12] Isohanni MH,Neuvonen PJ,Olkkola KT.Effect of fluvoxamine and erythromycin on the pharmacokinetics of oral lidocaine[J].Basic Clin Pharmacol Toxicol,2006,99(2):168-172.
[13] Isohanni MH,Neuvonen PJ,Olkkola KT.Effect of erythromycin and itraconazole on the pharmacokinetics of oral lignocaine[J].Pharmacol Toxicol,1999,84(3):143-146.
[14] Bailey DG,Bend JR,Arnold JM,et al.Erythromycin-felodipine interaction:magnitude,mechanism,and comparison with grapefruit juice[J].Clin Pharmacol Ther,1996,60(1):25-33.
[15] Grub S,Bryson H,Goggin T,et al.The interaction of saquinavir(soft gelatin capsule)with ketoconazole,erythromycin and rifampicin:comparison of the effect in healthy volunteers and in HIV-infected patients[J].Eur J Clin Pharmacol,2001,57(2):115-121.
[16] Aranko K,Luurila H,Backman JT,et al.The effect of erythromycin on the pharmacokinetics and pharmacodynamics of zopiclone[J].Br J Clin Pharmacol,1994,38(4):363-367.
[17] Matsson EM,Eriksson UG,Knutson L,et al.Biliary excretion of ximelagatran and its metabolites and the influence of erythromycin following intraintestinal administration to healthy volunteers[J].J Clin Pharmacol,2011,51(5):770-783.
[18] Lahu G,Huennemeyer A,Herzog R,et al.Effect of repeated dose of erythromycin on the pharmacokinetics of roflumilast and roflumilast N-oxide[J].Int J Clin Pharmacol Ther,2009,47(4):236-245.
[19] Petri N,Borga O,Nyberg L,et al.Effect of erythromycin on the absorption of fexofenadine in the jejunum,ileum and colon determined using local intubation in healthy volunteers[J].Int J Clin Pharmacol Ther,2006,44(2):71-79.
[20] Shi HY,Yan J,Zhu WH,et al.Effects of erythromycin on voriconazole pharmacokinetics and association with CYP2C19 polymorphism[J].Eur J Clin Pharmacol,2010,66(11):1131-1136.
[21] Brannan MD,Reidenberg P,Radwanski E,et al.Loratadine administered concomitantly with erythromycin:pharmacokinetic and electrocardiographic evaluations[J].Clin Pharmacol Ther,1995,58(3):269-278.
[22] Siedlik PH,Olson SC,Yang BB,et al.Erythromycin coadministration increases plasma atorvastatin concentrations [J].J Clin Pharmacol,1999,39(5):501-504.
[23] Blode H,Zeun S,Parke S,et al.Evaluation of the effects of rifampicin,ketoconazole and erythromycin on the steady-state pharmacokinetics of the components of a novel oral contraceptive containing estradiol valerate and dienogest in healthy postmenopausal women[J].Contraception,2012,86(4):337-344.
[24] Williamson KM,Patterson JH,McQueen RH,et al.Effects of erythromycin or rifampin on losartan pharmacokinetics in healthy volunteers[J].Clin Pharmacol Ther,1998,63(3):316-323.
[25] Li KY,Li X,Cheng ZN,et al.Effect of erythromycin on metabolism of quetiapine in Chinese suffering from schizophrenia[J].Eur J Clin Pharmacol,2005,60(11):791-795.
[26] Muck W,Ochmann K,Rohde G,et al.Influence of erythromycin pre-and co-treatment on single-dose pharmacokinetics of the HMG-CoA reductase inhibitor cerivastatin[J]. Eur J Clin Pharmacol,1998,53(6):469-473.
[27] Jokinen MJ,Ahonen J,Neuvonen PJ,et al.The effect of erythromycin,fluvoxamine,and their combination on the pharmacokinetics of ropivacaine[J].Anesthesia and Analgesia,2000,91(5):1207-1212.
[28] Cooper KJ,Martin PD,Dane AL,et al.The effect of erythromycin on the pharmacokinetics of rosuvastatin[J].Eur J Clin Pharmacol,2003,59(1):51-56.
[29] Nave R,Drollmann A,Steinijans VW,et al.Lack of pharmacokinetic drug-drug interaction between ciclesonide and erythromycin[J].Int J Clin Pharmacol Ther,2005,43(6):264-270.
[30] Luurila H,Olkkola KT,Neuvonen PJ.Interaction between erythromycin and the benzodiazepines diazepam and flunitrazepam[J].Pharmacol Toxicol,1996,78(2):117-122.
[31] Wong SL,Cao G,Mack RJ,et al.The effect of erythromy-cin on the CYP3A component of sertindole clearance in healthy volunteers[J].J Clin Pharmacol,1997,37(11):1056-1061.
[32] Isohanni MH,Neuvonen PJ,Palkama VJ,et al.Effect of erythromycin and itraconazole on the pharmacokinetics of intravenous lignocaine[J].Eur J Clin Pharmacol,1998,54(7):561-565.
[33] Banfield C,Hunt T,Reyderman L,et al.Lack of clinically relevant interaction between desloratadine and erythromycin[J].Clin Pharmacokinet,2002,41(Suppl 1):29-35.
[34] Paulsen O,Hoglund P,Nilsson LG,et al.The interaction of erythromycin with theophylline[J].Eur J Clin Pharmacology,1987,32(5):493-498.
[35] Pesco-Koplowitz L,Hassell A,Lee P,et al.Lack of effect of erythromycin and ketoconazole on the pharmacokinetics and pharmacodynamics of steady-state intranasal levocabastine[J].J Clin Pharmacol,1999,39(1):76-85.
[36] Sachdeo RC,Narang-Sachdeo S,Montgomery PA,et al. Evaluation of the potential interaction between felbamate and erythromycin in patients with epilepsy[J].J Clin Pharmacol,1998,38(2):184-190.
[37] Keranen T,Jolkkonen J,Jensen PK,et al.Absence of interaction between oxcarbazepine and erythromycin[J].Acta Neurol Scand,1992,86(2):120-123.
[38] Purkins L,Wood N,Ghahramani P,et al.No clinically significant effect of erythromycin or azithromycin on the pharmacokinetics of voriconazole in healthy male volunteers[J].Br J Clin Pharmacol,2003,56(Suppl 1):30-36.
[39] Tran JQ,Di Cicco RA,Sheth SB,et al.Assessment of the potential pharmacokinetic and pharmacodynamic interactions between erythromycin and argatroban[J].J Clin Pharmacol,1999,39(5):513-519.
[40] Thomsen MS,Groes L,Agerso H,et al.Lack of pharmacokinetic interaction between tiagabine and erythromycin [J].J Clin Pharmacol,1998,38(11):1051-1056.
[41] Hagg S,Spigset O,Mjorndal T,et al.Absence of interaction between erythromycin and a single dose of clozapine [J].Eur J Clin Pharmacol,1999,55(3):221-226.
[42] Jacobson TA.Comparative pharmacokinetic interaction profiles of pravastatin,simvastatin,and atorvastatin when coadministered with cytochrome P450inhibitors[J].American J Cardiol,2004,94(9):1140-1146.
[43] Greenblatt DJ,Harmatz JS.Ritonavir is the best alternative to ketoconazole as an index inhibitor of cytochrome P450-3A in drug-drug interaction studies[J].Br J Clin Pharmacol,2015,80(3):342-350.
[44] Davis MW,Wason S,Digiacinto JL.Colchicine-antimicrobial drug interactions:what pharmacists need to know in treating gout[J].Consult Pharm,2013,28(3):176-183.
[45] Kunicki PK,Sobieszczanska-Malek M.Pharmacokinetic interaction between tacrolimus and clarithromycin in a heart transplant patient[J].Ther Drug Monit,2005,27(1):107-108.
[46] Pan W,Bae SK,Shim EJ,et al.Effects of clopidogrel and clarithromycin on the disposition of sibutramine and its active metabolites M1 and M2 in relation to CYP2B6*6 polymorphism[J].Xenobiotica,2013,43(2):211-218.
[47] Nakatsuka A,Nagai M,Yabe H,et al.Effect of clarithromycin on the pharmacokinetics of cabergoline in healthy controls and in patients with Parkinson’s disease[J].J Pharmacol Sci,2006,100(1):59-64.
[48] Hagelberg NM,Peltoniemi MA,Saari TI,et al.Clarithromycin,a potent inhibitor of CYP3A,greatly increases exposure to oral S-ketamine[J].Eur J Pain,2010,14(6):625-629.
[49] Amsden GW,Kuye O,Wei GC.A study of the interaction potential of azithromycin and clarithromycin with atorvastatin in healthy volunteers[J].J Clin Pharmacol,2002,42(4):444-449.
[50] Shin J,Pauly DF,Pacanowski MA,et al.Effect of cytochrome P4503A5 genotype on atorvastatin pharmacokinetics and its interaction with clarithromycin[J].Pharmacotherapy,2011,31(10):942-950.
[51] Hegazy SK,Mabrouk MM,Elsisi AE,et al.Effect of clarithromycin and fluconazole on the pharmacokinetics of montelukast in human volunteers[J].Eur J Clin Pharmacol,2012,68(9):1275-1280.
[52] Hedaya MA,El-Afify DR,El-Maghraby GM.The effect of ciprofloxacin and clarithromycin on sildenafil oral bioavailability in human volunteers[J].Biopharm Drug Dispos,2006,27(2):103-110.
[53] Furuta T,Ohashi K,Kobayashi K,et al.Effects of clarithromycin on the metabolism of omeprazole in relation to CYP2C19 genotype status in humans[J].Clin Pharmacol Ther,1999,66(3):265-274.
[54] Calabresi L,Pazzucconi F,Ferrara S,et al.Pharmacokinetic interactions between omeprazole/pantoprazole and clarithromycin in health volunteers[J].Pharmacol Res,2004,49(5):493-499.
[55] Liukas A,Hagelberg NM,Kuusniemi K,et al.Inhibition of cytochrome P4503A by clarithromycin uniformly affects the pharmacokinetics and pharmacodynamics of oxycodone in young and elderly volunteers[J].J Clin Psychopharmacol,2011,31(3):302-308.
[56] Farkas D,Volak LP,Harmatz JS,et al.Short-term clarithromycin administration impairs clearance and enhances pharmacodynamic effects of trazodone but not of zolpidem [J].Clin Pharmacol Ther,2009,85(6):644-650.
[57] Hafner R,Bethel J,Power M,et al.Tolerance and pharmacokinetic interactions of rifabutin and clarithromycin in human immunodeficiency virus-infected volunteers[J].Antimicrob Agents Chemother,1998,42(3):631-639.
[58] Bolhuis MS,van Altena R,van Soolingen D,et al.Clarithromycin increases linezolid exposure in multidrug-re-sistant tuberculosis patients[J].Eur Respir J,2013,42(6):1614-1621.
[59] Hassan-Alin M,Andersson T,Niazi M,et al.Studies on drug interactions between esomeprazole,amoxicillin and clarithromycin in healthy subjects[J].Int J Clin Pharmacol Ther,2006,44(3):119-127.
[60] Saito M,Yasui-Furukori N,Uno T,et al.Effects of clarithromycin on lansoprazole pharmacokinetics between CYP2C19 genotypes[J].Br J Clin Pharmacol,2005,59(3):302-309.
[61] Rengelshausen J,Goggelmann C,Burhenne J,et al.Contribution of increased oral bioavailability and reduced nonglomerular renal clearance of digoxin to the digoxin-clarithromycin interaction[J].Br J Clin Pharmacol,2003,56(1):32-38.
[62] Delavenne X,Ollier E,Basset T,et al.A semi-mechanistic absorption model to evaluate drug-drug interaction with dabigatran:application with clarithromycin[J].Br J Clin Pharmacol,2013,76(1):107-113.
[63] Boruchoff SE,Sturgill MG,Grasing KW,et al.The steady-state disposition of indinavir is not altered by the concomitant administration of clarithromycin[J].Clin Pharmacol Ther,2000,67(4):351-359.
[64] Markert C,Hellwig R,Burhenne J,et al.Interaction of ambrisentan with clarithromycin and its modulation by polymorphic SLCO1B1[J].Eur J Clin Pharmacol,2013,69(10):1785-1793.
[65] Jokinen MJ,Ahonen J,Neuvonen PJ,et al.Effect of clarithromycin and itraconazole on the pharmacokinetics of ropivacaine[J].Pharmacol Toxicol,2001,88(4):187-191.
[66] Brophy DF,Israel DS,Pastor A,et al.Pharmacokinetic interaction between amprenavirand clarithromycin in healthy male volunteers[J].Antimicrob Agents Chemother,2000,44(4):978-984.
[67] Bruce MA,Hall SD,Haehner-Daniels BD,et al.In vivo effect of clarithromycin on multiple cytochrome P450[J]. Drug Metab Dispos,2001,29(7):1023-1028.
[68] Ouellet D,Hsu A,Granneman GR,et al.Pharmacokinetic interaction between ritonavir and clarithromycin[J].Clin Pharmacol Ther,1998,64(4):355-362.
[69] Moore KH,Leese PT,McNeal S,et al.The pharmacokinetics of sumatriptan when administered with clarithromycin in healthy volunteers[J].Clin Ther,2002,24(4):583-594.
[70] Winter HR,Trapnell CB,Slattery JT,et al.The effect of clarithromycin,fluconazole,and rifabutin on sulfamethoxazole hydroxylamine formation in individuals with human immunodeficiency virus infection(AACTG 283)[J].Clin Pharmacol Ther,2004,76(4):313-322.
[71] Nakade S,Yamauchi A,Komaba J,et al.Effect of clarithromycin on the pharmacokinetics of pranlukast in healthy volunteers[J].Drug Metab Pharmacokinet,2008, 23(6):428-433.
[72] Shimizu M,Uno T,Yasui-Furukori N,et al.Effects of clarithromycin and verapamil on rabeprazole pharmacokinetics between CYP2C19 genotypes[J].Eur J Clinical Pharmacol,2006,62(8):597-603.
[73] Winter HR,Trapnell CB,Slattery JT,et al.The effect of clarithromycin,fluconazole,and rifabutin on dapsone hydroxylamine formation in individuals with human immunodeficiency virus infection(AACTG 283)[J].Clin Pharmacol Ther,2004,76(6):579-587.
[74] Sekar VJ,Spinosa-Guzman S,De Paepe E,et al.Darunavir/ritonavir pharmacokinetics following coadministration with clarithromycin in healthy volunteers[J].J Clin Pharmacol,2008,48(1):60-65.
[75] Cao S,Zhou G,Ou-Yang DS,et al.Pharmacokinetic interactions between ilaprazole and clarithromycin following ilaprazole,clarithromycin and amoxicillin triple therapy [J].Acta Pharmacol Sin,2012,33(8):1095-1100.
[76] Gupta S,Banfield C,Kantesaria B,et al.Pharmacokinetic and safety profile of desloratadine and fexofenadine when coadministered with azithromycin:a randomized,placebo-controlled,parallel-group study[J].Clin Ther,2001,23(3):451-466.
[77] Dorani H,Schutzer KM,Sarich TC,et al.Pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor ximelagatran co-administered with different classes of antibiotics in healthy volunteers[J].Eur J Clin Pharmacol,2007,63(6):571-581.
[78]Amsden GW,Gregory TB,Michalak CA,et al.Pharmacokinetics of azithromycin and the combination of ivermectin and albendazole when administered alone and concurrently in healthy volunteers[J].Am J Trop Med Hyg,2007,76(6):1153-1157.
[79] Bachmann K,Jauregui L,Chandra R,et al.Influence of a 3-day regimen of azithromycin on the disposition kinetics of cyclosporine A in stable renal transplant patients[J]. Pharmacol Res,2003,47(6):549-554.
[80] Solans A,Izquierdo I,Donado E,et al.Pharmacokinetic and safety profile of rupatadine when coadministered with azithromycin at steady-state levels:a randomized,openlabel,two-way,crossover,phase②study[J].Clin Ther,2008,30(9):1639-1650.
[81] Cook JA,Randinitis EJ,Bramson CR,et al.Lack of a pharmacokinetic interaction between azithromycin and chloroquine[J].Am J Trop Med Hyg,2006,74(3):407-412.
[82] Methaneethorn J,Chaiwong K,Pongpanich K,et al.A pharmacokinetic drug-drug interaction model of simvastatin and clarithromycin in humans[J].Conf Proc IEEE Eng MedBiolSoc,2014,doi:10.1109/EMBC.2014.6944922.
(编辑:晏 妮)
R969.3
A
1001-0408(2017)05-0715-06
2016-06-19
2016-07-20)
*主治医师,硕士。研究方向:内分泌与代谢病学。电话:0833-2119335。E-mail:22641201@qq.com
#通信作者:主管药师,副教授,博士。研究方向:临床药学。电话:0833-2119382。E-mail:243937683@qq.com
DOI10.6039/j.issn.1001-0408.2017.05.38
