Synthesis of Triethylenetetramine Functionalized Mesoporous ZrO2 Adsorbents for CO2 Capture
2017-02-28CHENShengYANGFanmingCHENLang
CHEN Sheng,YANG Fan-ming,CHEN Lang
(1.College of Chemistry and Chemical Engineering,Provincial Hunan Key Laboratory for Cost-Effective Utilization of Fossil Fuel Aimed at Reducing Carbon-dioxide Emissions,Hunan University,Changsha 410082,China;2.Yali Middle School,Changsha 410007,China)
Synthesis of Triethylenetetramine Functionalized Mesoporous ZrO2Adsorbents for CO2Capture
CHEN Sheng2,YANG Fan-ming1,CHEN Lang1
(1.College of Chemistry and Chemical Engineering,Provincial Hunan Key Laboratory for Cost-Effective Utilization of Fossil Fuel Aimed at Reducing Carbon-dioxide Emissions,Hunan University,Changsha 410082,China;2.Yali Middle School,Changsha 410007,China)
Mesoporous ZrO2was prepared and functionalized with triethylenetetramine (TETA).The as-synthesized materials were characterized by powder X-ray diffraction,N2adsorption-desorption,Fourier transform infrared spectroscopy,X-ray photoelectron spectroscopy,dispersive spectroscopy,thermogravimetric analysis,and CO2temperature-programmed desorption.The CO2adsorption performance of the adsorbents was tested in a stream of 5% CO2.The results show that TETA-functionalized mesoporous ZrO2is a good agent for CO2adsorption.A proper increase of the gas flow rate shows a positive impact whereas an increase of the adsorption temperature has a negative effect on CO2adsorption.At a TETA loading of 200 mg and a CO2flow rate of 20 cm3·min-1,the adsorbent exhibits a maximum adsorption capacity of 4.16 mmol·g-1at 75 ℃.In addition,the adsorbent exhibits excellent reusability.The remarkable adsorption capacity and recyclability suggest that the synthesized adsorbents are promising for CO2capture.
triethylenetetramine; zirconium oxide; adsorbent; carbon dioxide
With the growth of world economy,the emission of carbon dioxide (CO2) into the atmosphere leads to global warming and climate change[1-3].In order to mitigate CO2emission,carbon capture and sequestration (CCS) has become a global interest.Currently,CO2adsorption with solid adsorbents is considered as an effective method for CCS.For efficient CO2adsorption at a low temperature,solid substances such as zeolites[4],silicas[5],carbons[6-7],metal-organic frameworks (MOFs) mat erials[3,8],superamolecular-organic frameworks (SOFs) materials[9],and covalent-organic frameworks (COFs) materials are employed[10-11].
To enhance CO2adsorption,porous materials functionalized with organic amines were developed[12-26].For instance,polyethyleneimine (PEI) modified MCM-41 (MCM-41-PEI) displays a CO2adsorption capacity of 2.54 mmol·g-1at 75 ℃[13],which is 13 times than that of MCM-41[12].The capacity of ethylenediamine functionalized MOFs material (en-Mg2dobpdc) achieves a CO2uptake of about 1.0 mmol·g-1at 40 ℃,whereas that of Mg2dobpdc alone is only about 0.24 mmol·g-1[3].With the functionalized materials,CO2adsorption capacity is substantially enhanced because of the interaction between the amino-groups and CO2molecules (Scheme 1)[14,17].In addition,with the doping of heteroatoms onto the support,the performance of the adsorbents can be markedly elevated.In these materials,heteroatoms with many electro-holes act as Lewis acid sites and react with the organic amines,leading to the enrichment of CO2-philic sites and better thermal stability of the adsorbents.These prior results inspired us to prepare new solid materials utilizing metal oxides with Lewis acid sites.
It is known that zirconyl chloride octahydrate (ZrOCl2·8H2O) is enriched with electron holes[27-28].It is hence possible to generate an oxide with Lewis acid sites from ZrOCl2·8H2O[29].In this study,we synthesized ZrO2from ZrOCl2·8H2O and NaOH,and used it as the support of triethylenetramine (TETA) that is affluent with N atoms.By exposing the as-generated materials to a stream of 5% CO2,we evaluated their CO2adsorption performance at 75 ℃.In addition,we investigated the effect of TETA amount,adsorption temperature,and gas flow rate on the adsorption performance.Finally,we assessed the recyclability of the adsorbents.
Where R1,R2,R3and R4represent alkyl groups
Scheme 1 Route for chemical adsorption of CO2over organic-amine functionalized solid materials
1 Experimental
1.1 Synthetic procedures
ZrO2was synthesized according to the following procedure.At first,1.5 g of polyethylene glycol (PEG,MW=2000) and 0.9 g of NaOH were dissolved in 80 ml of deionized water.With the addition of 3.22 g of ZrOCl2·8H2O,the mixture was stirred at room temperature (RT) for 4 h.Then,the mixture was transferred to an autoclave and hydrothermally treated in an oven at 110 ℃ for 24 h under static conditions.The resulted product was separated by centrifugation,washed thoroughly with deionized water,and dried at 120 ℃ for 24 h.The as-obtained product was collected for this study(99.4% yield based on Zr).
The TETA-modified ZrO2adsorbents were synthesized according to the method reported by Xu et al.[12].A designated amount of TETA was dissolved in 10 g of ethanol,followed by the addition of 0.5 g of the synthesized ZrO2.The mixture was stirred vigorously until the majority of solvent was evaporated.Then the product was dried at 80 ℃ for 24 h and at 100 ℃ for another 6 h.The as-prepared adsorbents are named herein as ZrO2-TETA-n,wherenstands for the nominal weight (mg) of TETA.
1.2 Characterization
Powder X-ray diffraction (XRD) patterns of the adsorbents were collected on a Shimadzu XD-5A diffractometer with monochromatized Cu Kαradiation (λ=0.154 06 nm) at a setting of 30 kV and 20 mA.N2adsorption-desorption performance tests were performed at 77 K over a Quantachrome Nova-Win2 instrument.The Fourier transform infrared spectroscopy (FT-IR) measurements were conducted using a Nicolet 6 700 FT-IR spectrometer.X-ray photoelectron spectroscopy (XPS) characterization was performed using an ESCALAB 250Xi Spectrometer.Energy dispersive spectroscopy (EDS) was performed on a FEI QuANTA 200 instrument.Thermogravimetric analysis (TGA) was conducted over thermal analyzer TA-60WS with the sample heated (in N2) from 35 to 700 ℃ (heating rate:10 ℃·min-1).Temperature-programmed desorption of CO2(CO2-TPD) was carried out over a Micromeritics 2 920 apparatus equipped with a thermal conductivity detector (TCD).
1.3 CO2adsorption and adsorbent regeneration
CO2adsorption experiments were carried out over a Micromeritics AutoChem II 2920 Chemisorption Analyzer.The method was reported elsewhere[19].About 100 mg of sample was used and degassed at 100 ℃ for 60 min in an argon flow (80 cm3·min-1).Then,the sample was exposed to a flow of 5% CO2for 10 min at a desired temperature.After CO2adsorption,the sample temperature was raised to 100 ℃ and kept at this temperature for 60 min in an argon flow (80 cm3·min-1).The CO2adsorption capacity of an adsorbent was estimated by integrating the resulted breakthrough curve.
For regeneration,a spent material was heated to 100 ℃ and kept at this temperature for 60 min in an argon flow (80 cm3·min-1).To assess the reusability of the samples,the procedure of CO2adsorption-desorption was repeated 10 times using ZrO2-TETA-200 as a model.
2 Results and Discussion
2.1 XRD
The XRD patterns of ZrO2and ZrO2-TETA-nare displayed in Fig.1.The diffraction pattern of the as-prepared ZrO2matches well with that of standard ZrO2(PDF card:50-1089),indicating that the ZrO2was successfully obtained.Since there is no detection of signals assignable to impurities,it is deduced that pure ZrO2can be synthesized following the above procedure.There is no significant change of ZrO2diffraction pattern after TETA modification,demonstrating that the structure of ZrO2remains largely intact.However,with the introduction of TETA there is a shift of the (011) and (110) peaks from 30.16° and 35.10° to 30.24° and 35.18°,respectively.And there is a decrease of the peak intensity with respect to the increase of TETA amount.These results are similar to those reported by Zhao et al[2].and Xu et al.[12],who ascribed the phenomena to the interaction between N and Zr species and the deterioration of crystallinity.Our results suggest that TETA was successfully introduced to ZrO2.
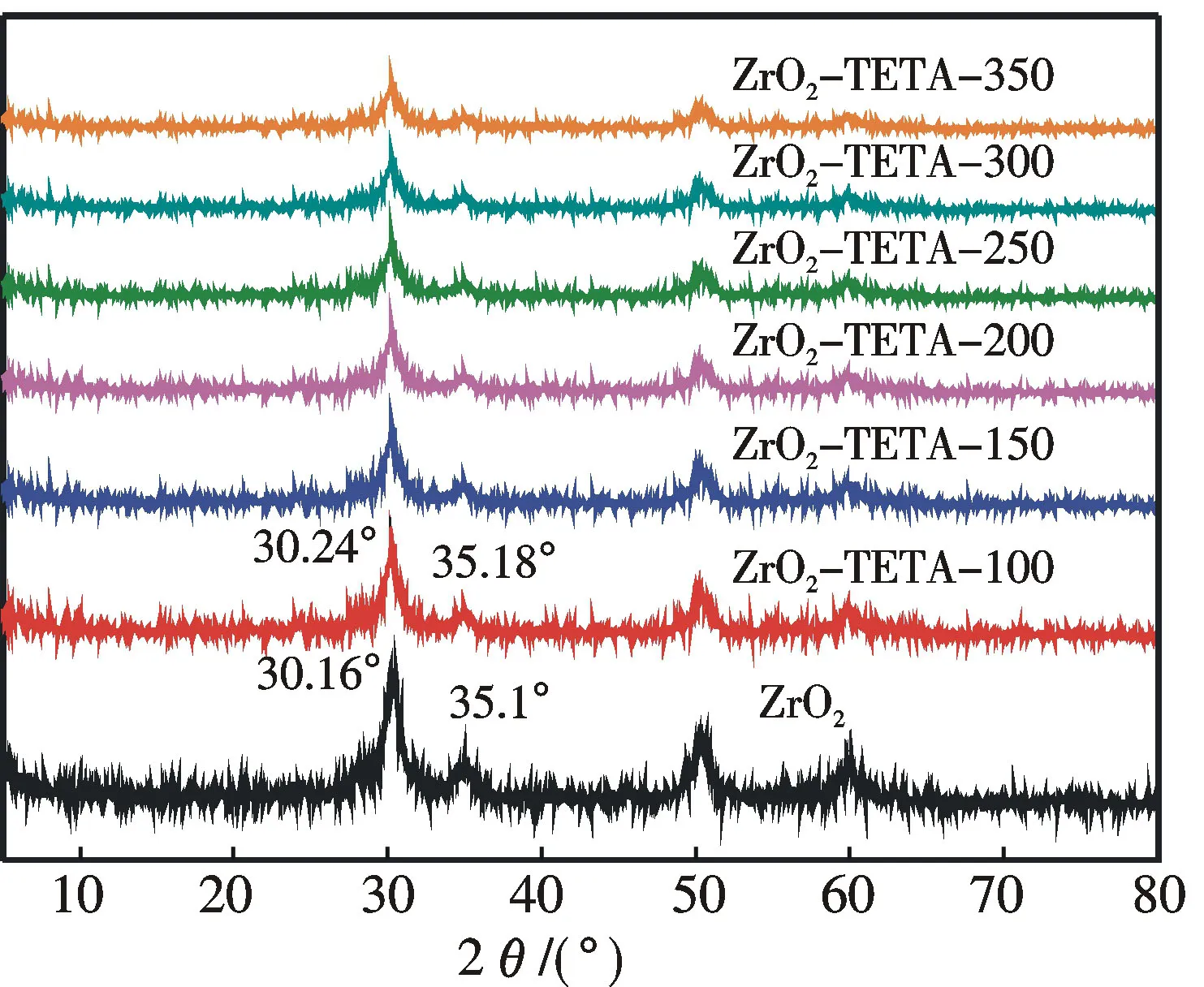
Fig.1 XRD patterns of ZrO2 and ZrO2-TETA-n
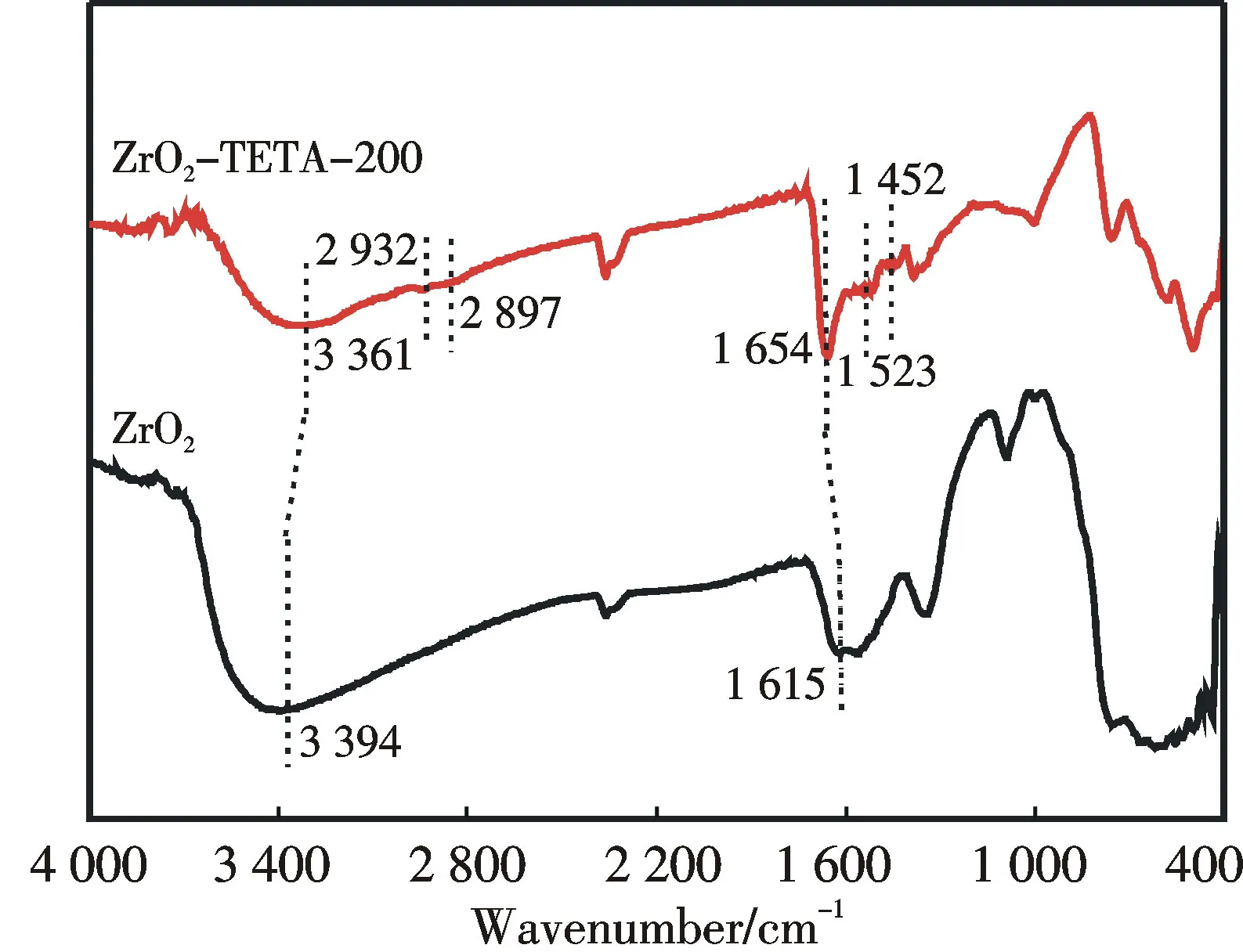
Fig.2 FT-IR spectra of ZrO2 and ZrO2-TETA-200
2.2 N2adsorption-desorption
Our results of N2adsorption-desorption isotherms are illustrated in Tab.1.The BET specific surface area (SBET),pore volume (Vp) and pore diameter (dp) of the synthesized ZrO2are 64 m2·g-1,0.24 cm3·g-1and 15.4 nm,respectively.These results indicate that the ZrO2material is mesoporous.TheSBET,Vpanddpvalues decrease with the rise of TETA amount due to the filling of pore channels.Nonetheless,the ZrO2-TETA-nadsorbents are mesoporous,with pore diameters ranging from 2 nm to 50 nm.
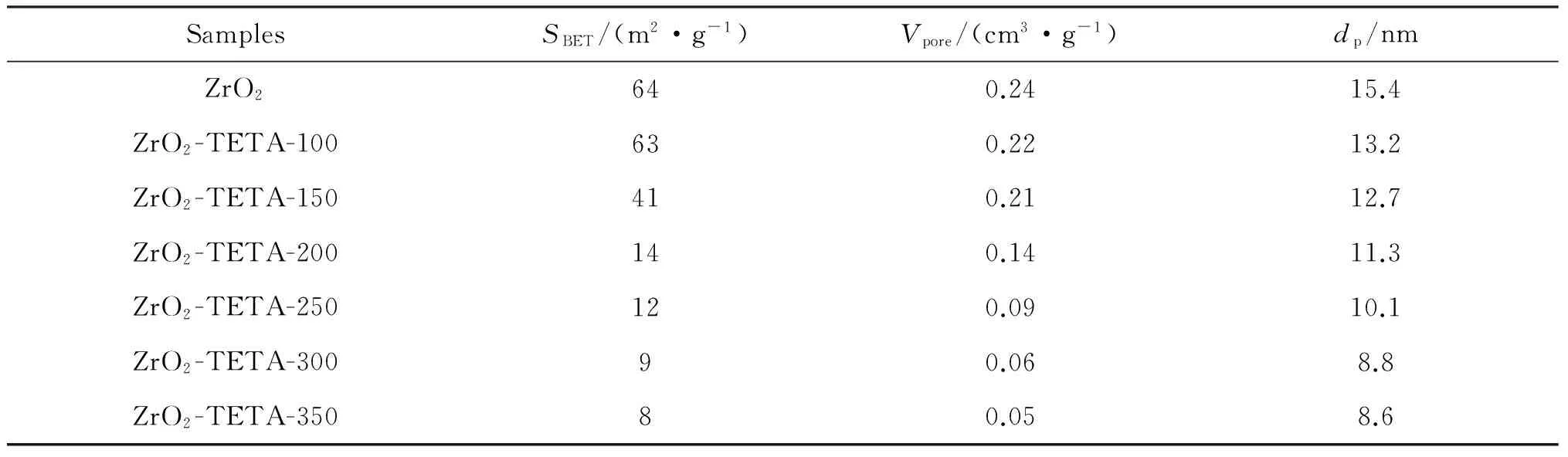
Tab.1 Textural property of ZrO2 and ZrO2-TETA-n
2.3 FT-IR
The FT-IR spectra of ZrO2and ZrO2-TETA-200 are displayed in Fig.2.Similar to the work of Chen et al.[29],there is a peak at 1 615 cm-1in the spectrum of ZrO2,which is attributed to the existence of Lewis acid sites.Compared with ZrO2,ZrO2-TETA-200 shows additional peaks at 1 452,1 523,2 897 and 2 932 cm-1.The peaks at 1 452 and 1 523 cm-1can be attributed to the deformation of C—H and N—H bonds,respectively,whereas those at 2 897 and 2 932 cm-1to C—H stretching vibrations[20-22].Furthermore,the peaks attributable to ZrO2observed over ZrO2-TETA-200 are lower in intensity in comparison with those observed over the as-prepared ZrO2sample,providing further evidence for the successful introduction of TETA onto ZrO2.
2.4 XPS
The XPS results of ZrO2and ZrO2-TETA-200 are displayed in Fig.3.There are apparent Zr(3d) peaks at 182.3 and 184.7 eV detected over ZrO2.The ZrO2-TETA-200 sample shows Zr(3d) peaks at 181.8 and 184.1 eV,which are lower in binding energy than those of ZrO2(Fig.3A).The shift of these peaks is similar to that reported by Martin et al.[30]who ascribed the phenomenon to the interaction between Zr and N species.In addition,the N(1s) profile collected over ZrO2-TETA-200 can be ascribed to N—Zr,N—H and N—C species (Fig.3B)[31].Again,these results indicate that TETA is successfully introduced to ZrO2.
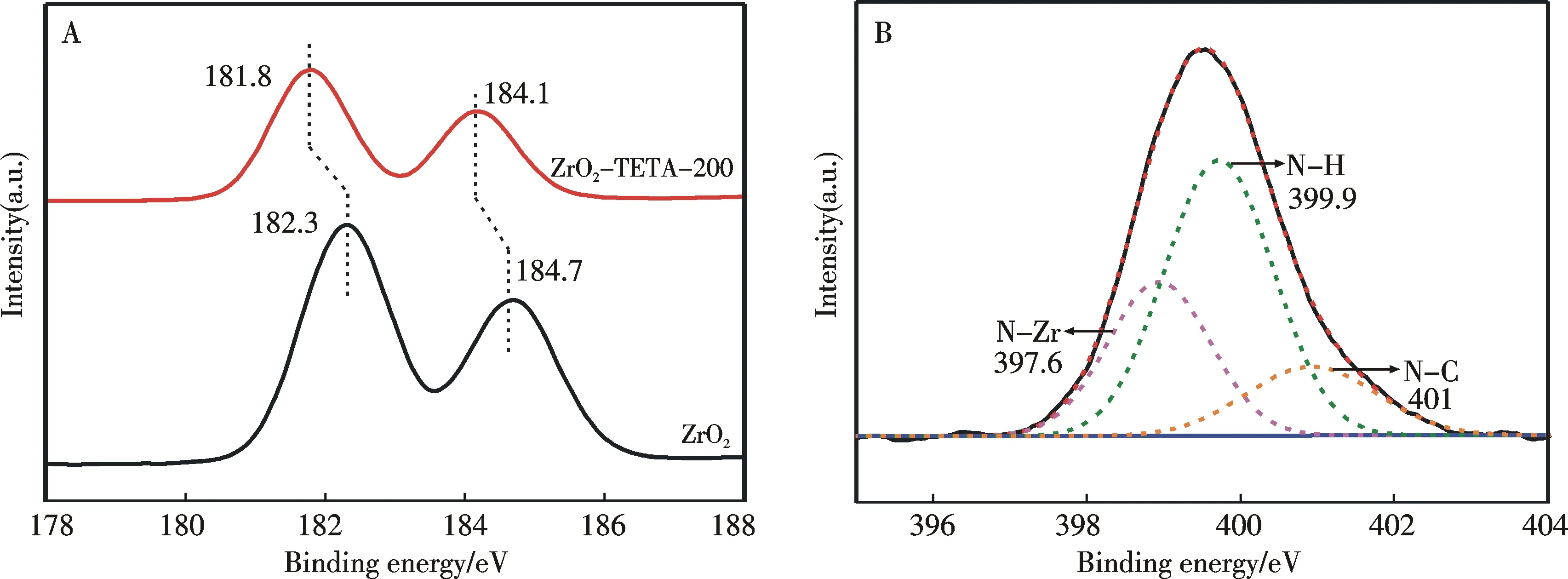
Fig.3 XPS spectra of ZrO2 and ZrO2-TETA-200.(A) Zr(3d) spectra of ZrO2 and ZrO2-TETA-200; (B) N(1s) spectrum of ZrO2-TETA-200
2.5 EDS
The EDS results of ZrO2-TETA-nare compiled in Tab.2.The detections of Zr,C,N and O over ZrO2-TETA-nwere observed.C and N contents increased while Zr and O contents decreased with the increase of TETA amount,confirming for one more time that TETA was successfully introduced to ZrO2.
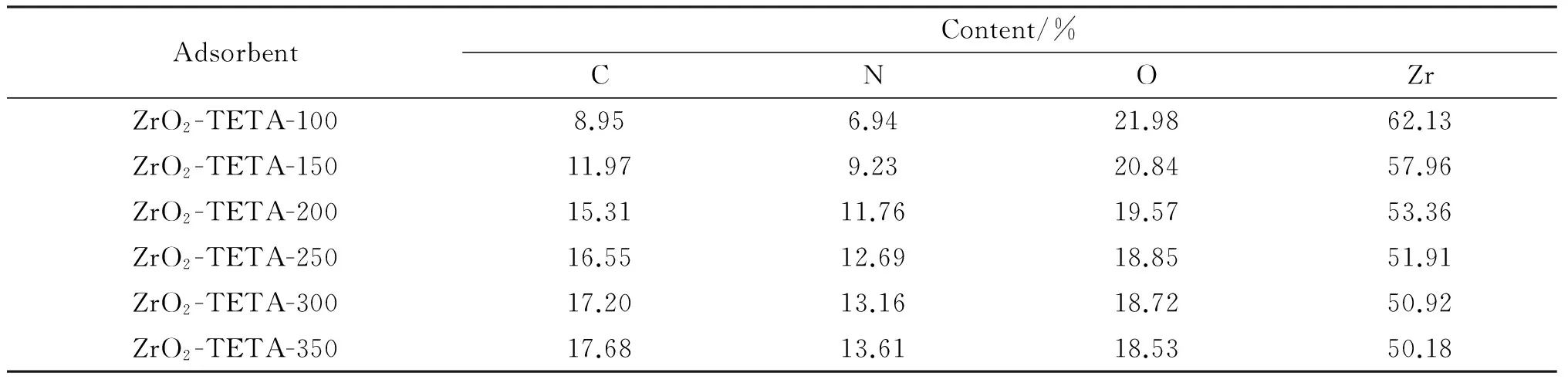
Tab.2 Element content of ZrO2-TETA-n*
*H was too light to be detected.
2.6 TG analysis
TG profiles of ZrO2-TETA-nare illustrated in Fig.4.For ZrO2-TETA-100,ZrO2-TETA-150,and ZrO2-TETA-200,the decomposition temperature is around 190 ℃,which is about 15 ℃ higher than that of ZrO2-TETA-250,ZrO2-TETA-300,and ZrO2-TETA-350.When the TETA amount is below 200 mg,there is strong interaction between TETA and the support because of N—Zr bonding.In excess amount of TETA (above 200 mg),the interaction between TETA and ZrO2is not as strong and there is decrease in decomposition temperature.
2.7 CO2adsorption
According to Xu et al.,solid adsorbents functionalized with organic amine perform excellently for CO2adsorption at 75 ℃[12].In the present investigation we also adopted 75 ℃ as the temperature for CO2adsorption.The breakthrough curves corresponding to CO2adsorption are shown in Fig.5(彩图见封三).It is observed that the CO2concentration returns to the initial level within 10 min.In addition,there is complete CO2desorption when the materials are heated to 100 ℃ (5 ℃·min-1) and remained at 100 ℃ for 60 min in an argon flow (80 cm3·min-1) (Fig.6).These results indicate that the spent adsorbents can be easily regenerated.
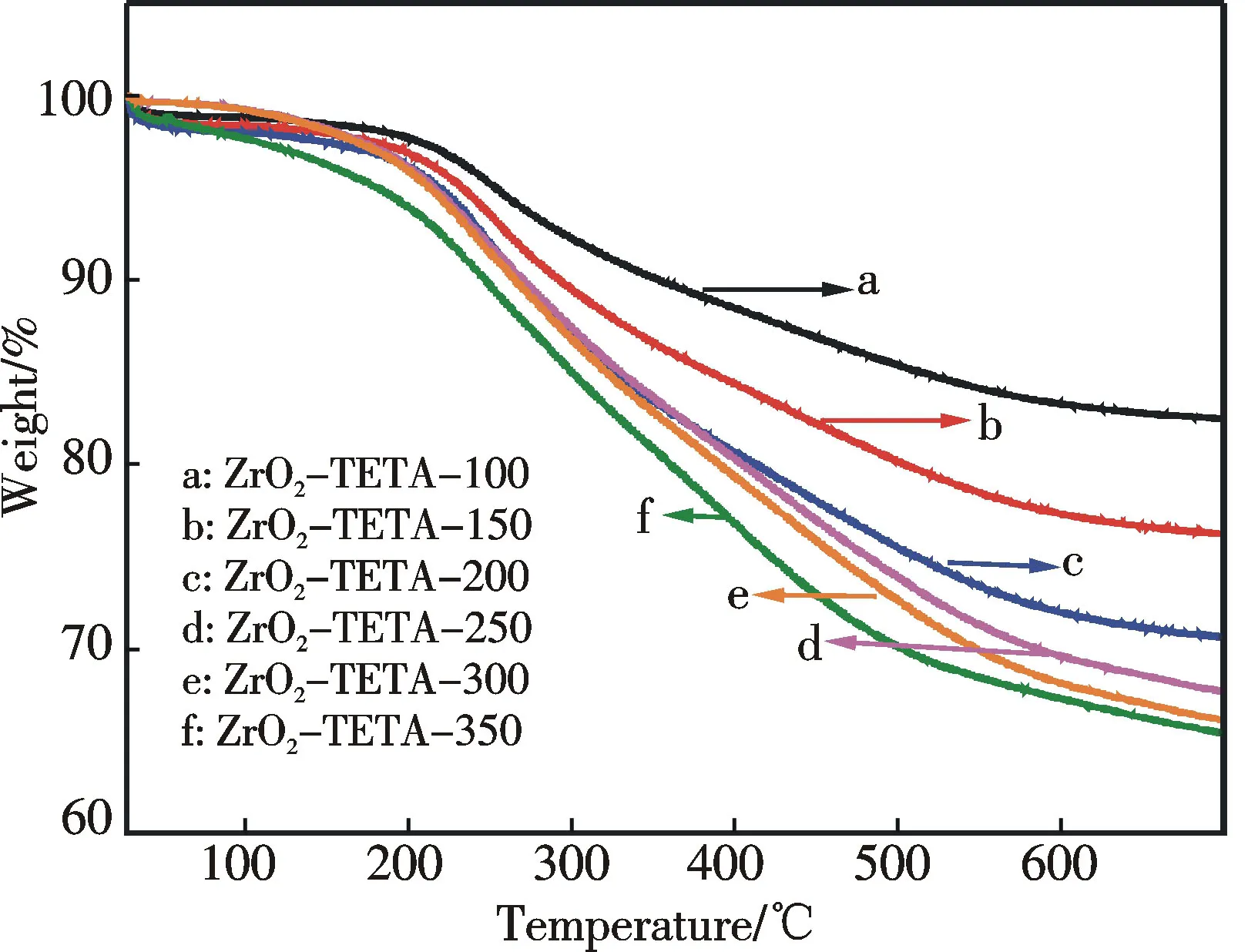
Fig.4 TGA profiles of ZrO2 and ZrO2-TETA-n
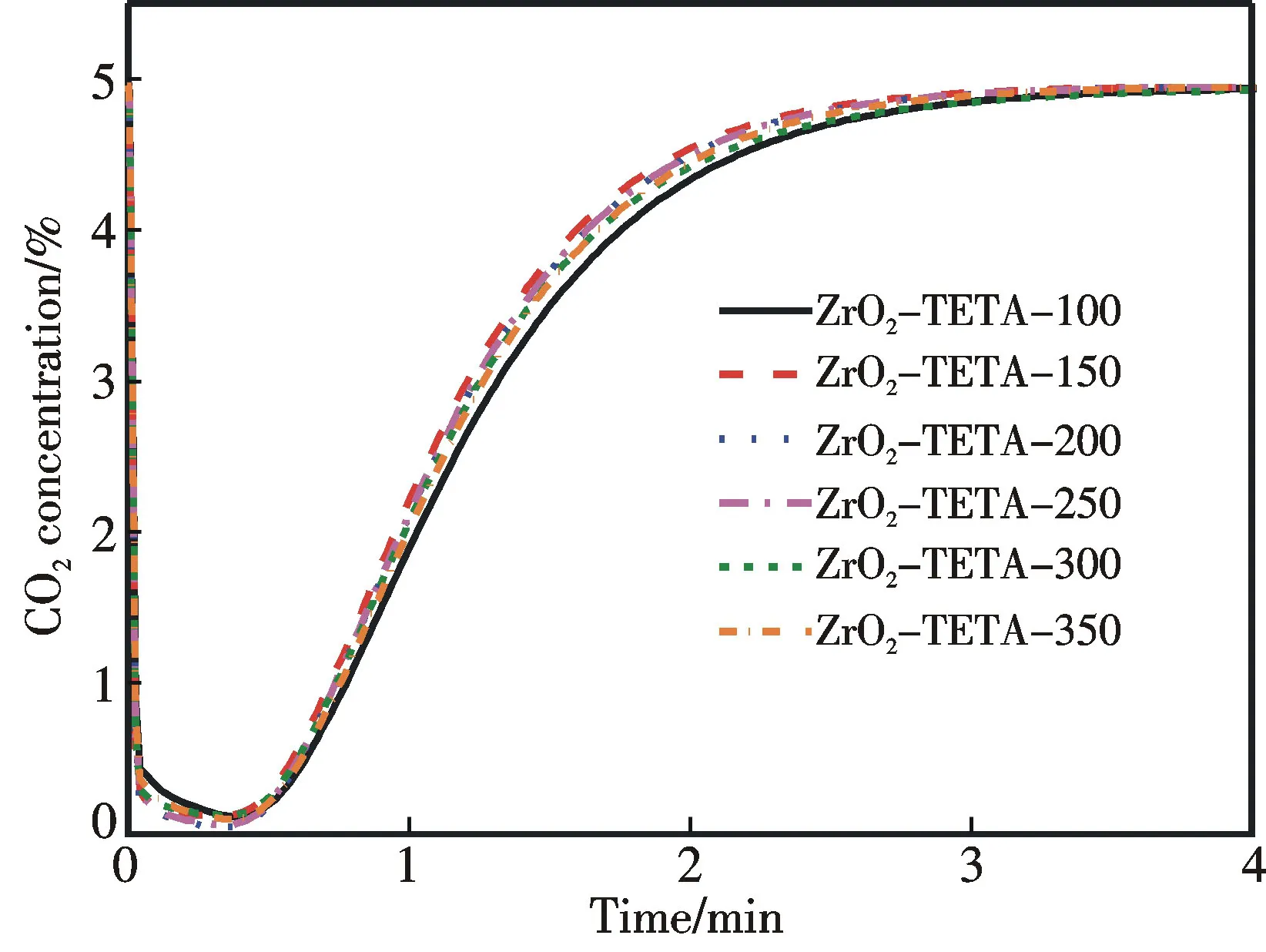
Fig.5 Breakthrough curves of CO2 adsorption on ZrO2-TETA-n in a stream of 5% CO2 at 75 ℃ (within a 5-min period)
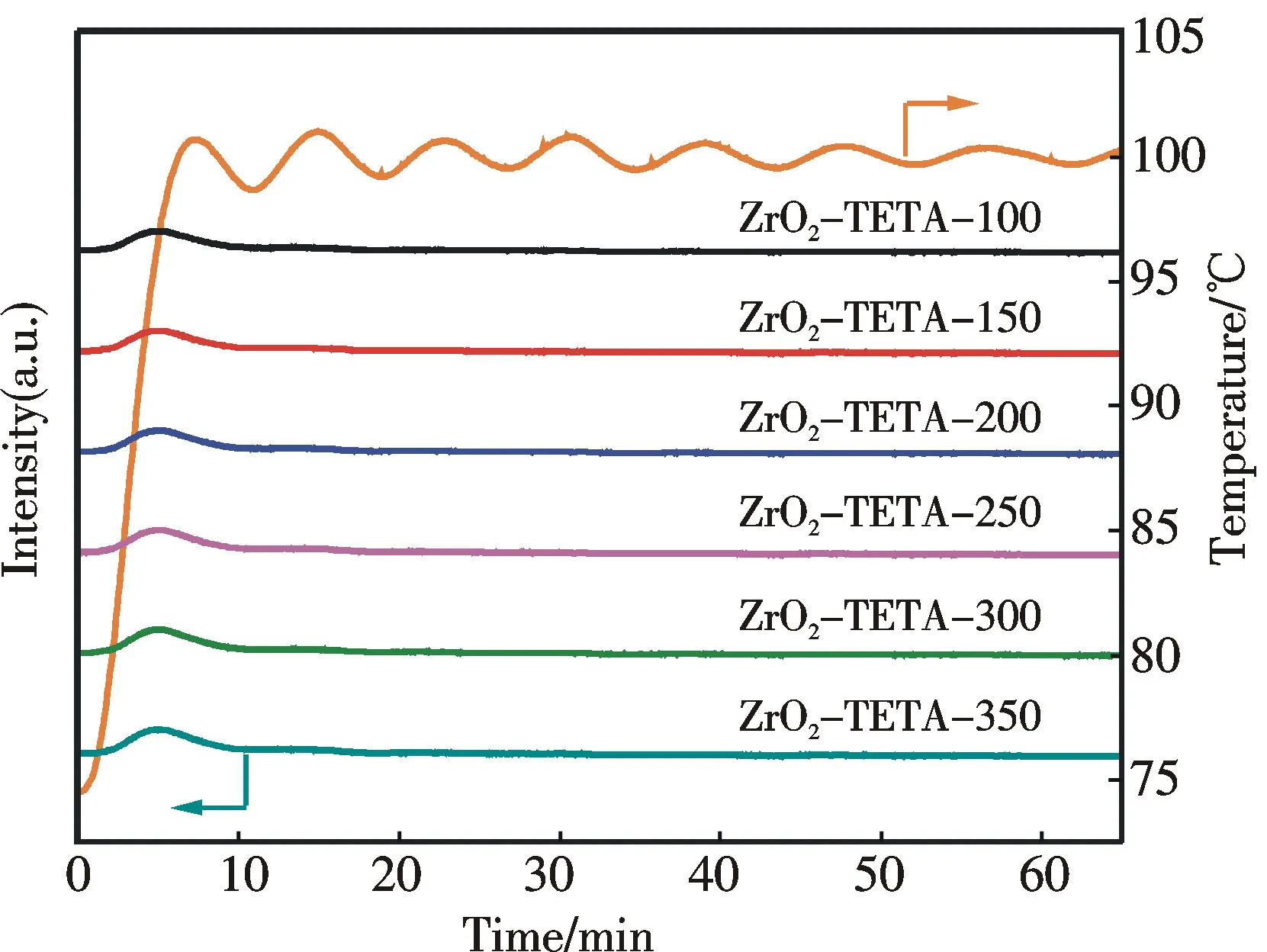
Fig.6 CO2-TPD curves of ZrO2-TETA-n from 75 to 100 ℃ at
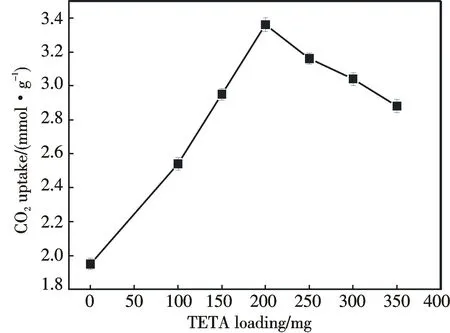
Fig.7 CO2 adsorption capacity of ZrO2 and ZrO2-TETA-n as a a ramping rate of 5 ℃·min-1 function of TETA amount (Conditions:adsorbate=5% CO2,T=75 ℃,gas flow rate=10 cm3·min-1
2.8 Effect of TETA amount
The CO2adsorption capacities of ZrO2and ZrO2-TETA-nat 75 ℃ are displayed in Fig.7.Similar to the phenomena of amine modification[2,3,12-26],the CO2adsorption capacity of ZrO2-TETA-nis larger than that of ZrO2,demonstrating that TETA is a good agent for CO2uptake.The CO2adsorption capacity increases with the increase of TETA amount from 100 to 200 mg as a result of the increase of CO2affinity sites originating from-NH2[2,3,12-26].However,there is a decline of the adsorption capacity when the rise of TETA amount is from 200 to 350 mg,which is in line with the results of previous work when TETA was in excess[12,19].With the surface covered by TETA agglomerates,it is difficult for gaseous CO2molecules to react with the basic sites.Consequently,the excess amount of TETA has a negative effect on CO2adsorption.
2.9 Effect of adsorption temperature
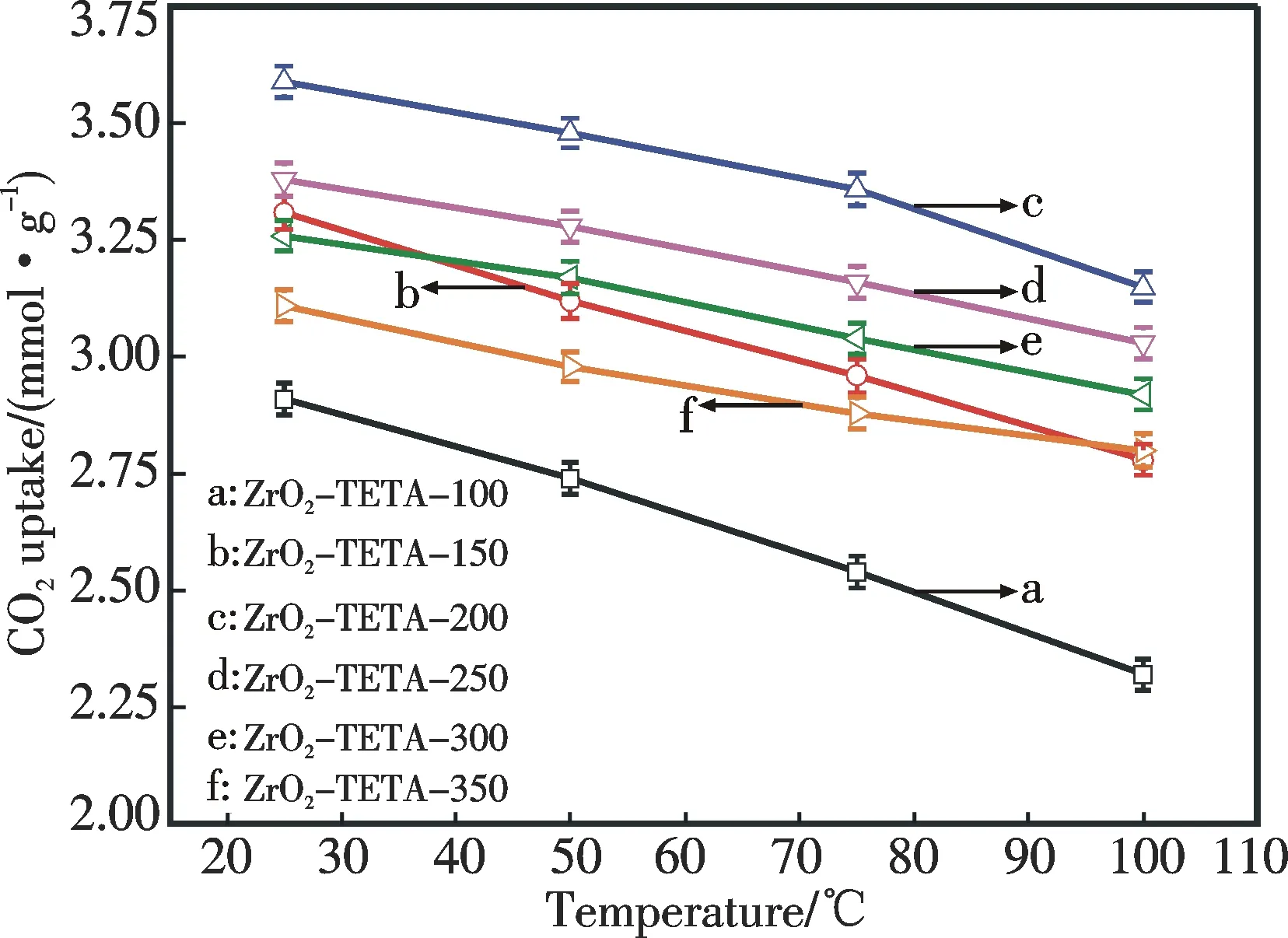
Fig.8 CO2 adsorption capacity of ZrO2-TETA-n as a function of temperature (Conditions:t=10 min,adsorbate=5% CO2,gas flow rate=10 cm3·min-1)
The CO2adsorption performance of organic amine-modified adsorbents varies with adsorption temperature[12,17-20,22].For example,due to both kinetic and thermodynamic factors PEI-modified MCM-41 (MCM-41-PEI) performs the best at 75 ℃.In this study,the influence of adsorption temperature on the adsorption performance of ZrO2-TETA-nwas examined and the results are displayed in Fig.8.It is clear that the adsorption capacity decreases with the increase of the adsorption temperature.It should be pointed out that CO2adsorption over organic amine-modified adsorbents is a reversible and exothermic process[12,17-20,22].With the increase of the adsorption temperature,the adsorption equilibrium (Scheme 1) shifts to the left,leading to the decline of adsorption capacity.
2.10 Effect of gas flow rate
It was reported that the gas flow rate has a strong impact on CO2adsorption[26,35].In the present work,the influence of the CO2flow rate over ZrO2-TETA-200 was investigated,and the results are shown in Fig.9.Similar to the work of Zhao et al.[26],the breakthrough time decreases with the increase of CO2flow rate (Fig.9A).At a low flow rate,CO2adsorption is controlled by CO2diffusion through the gas-film system of surface particles[32].With the increase of the flow rate from 5 to 20 cm3·min-1,CO2adsorption equilibrium (Scheme 1) is shifted to the right due to the higher presence of CO2molecules on the surface,leading to the increase of CO2adsorption capacity (Fig.9B).However,further increase of the flow rate from 20 to 35 cm3·min-1results in a decline in CO2adsorption capacity (Fig.9B).When the flow rate is larger than 20 cm3·min-1,CO2adsorption is governed by the contact time between the gas molecules and basic sites as well as by the change of mass transfer zone.At a large flow rate,CO2diffusion extends beyond the gas-film system and the contact time between them decreases rapidly,leading to the decline of chemical adsorption capacity.Besides,the mass transfer zone increases with the rise of flow rate,leading to the decline of mass transfer coefficient.Consequently,CO2adsorption capacity of ZrO2-TETA-200 declines with further rise of the CO2flow rate.
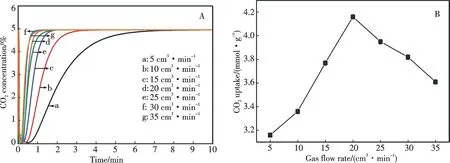
Fig.9 (A) Influence of CO2 flow rate on the adsorption capacity; (B) Breakthrough curves of CO2 adsorption over ZrO2-TETA-200 at different gas flow rates and CO2 adsorption capacity of ZrO2-TETA-200 as a function of gas flow rate
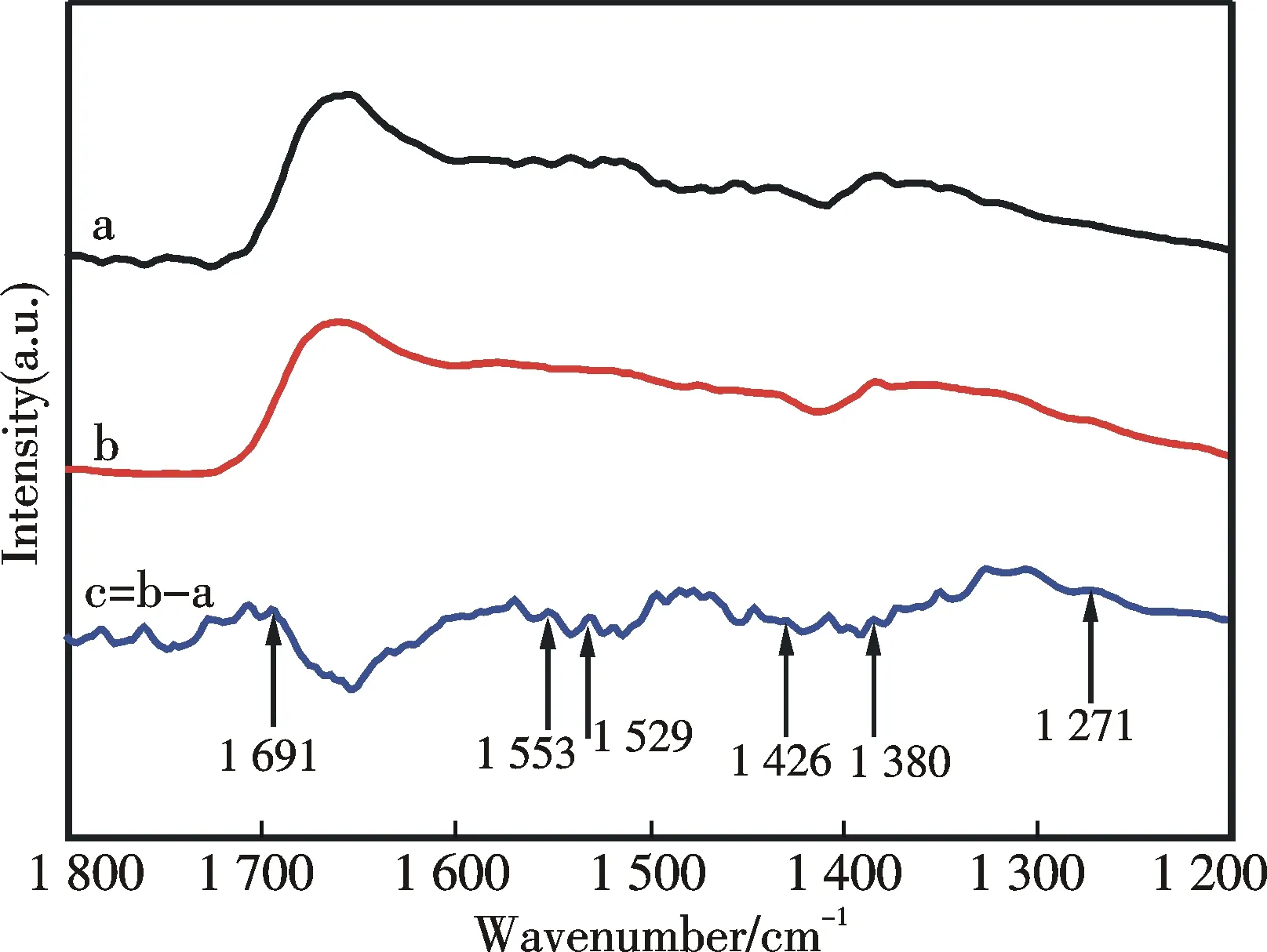
Fig.10 FT-IR spectra of ZrO2-TETA-200.(a):before,(b):after CO2 adsorption,and (c):the result of spectrum (b)-spectrum (a)
Displayed in Tab.3 is a comparison of CO2adsorption performance of ZrO2-TETA-200 with other organic amine-functionalized materials.It is clear that ZrO2-TETA-200 is a good candidate for CO2adsorption.
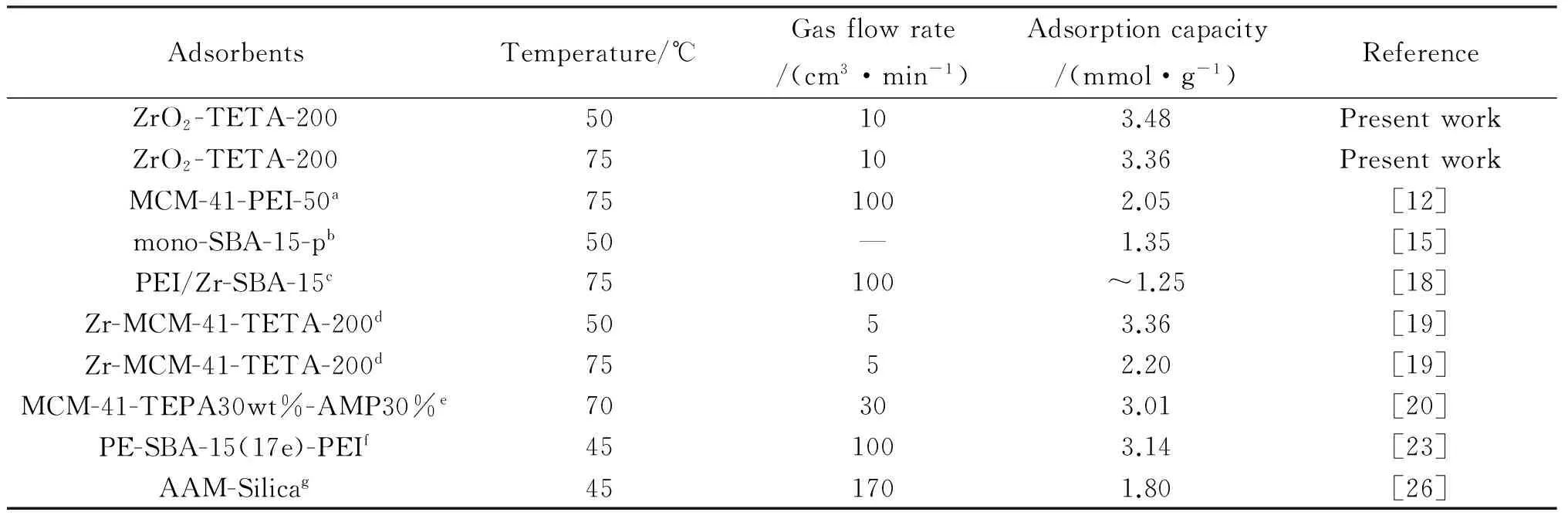
Tab.3 CO2 adsorption performance of ZrO2-TETA-200 and other amine-modified adsorbents
aMCM-41-PEI-50:polyethyleneimine modified MCM-41;bmono-SBA-15-p:3-amino propyltrimethoxysilane modified P123-occluded SBA-15;cPEI/Zr-SBA-15:polyethyleneimine modified Zr-doped SBA-15;dZr-MCM-41-TETA-200:triethylenetetramine functionalized Zr-doped MCM-41;eMCM-41-TEPA30wt%-AMP30%:tetraethylenepentamine and 2-amino-2-methyl-l-propanol modified MCM-41;fPE-SBA-15(17e)-PEI:polyethyleneimine modified pore expanded SBA-15;gAAM-Silica:acrylamide modified silica gel.
2.11 Recyclability performance

Fig.11 Recyclability study of ZrO2-TETA-200 (Conditions:T=75 ℃,t=10 min,5% CO2 in a stream of 10 cm3·min-1 flow rate)
In practical applications,performance stability in cyclic operations is of paramount importance[12-13,16,18-20].The recyclability of ZrO2-TETA-200 for CO2adsorption at 75 ℃ was examined and the test results are shown in Fig.11.One can see that the adsorbent shows excellent recyclability ad reusability with a test of 10 repeating runs.These results suggest that ZrO2-TETA-200 is a promising candidate for the capture of CO2.
3 Conclusions
The adsorbents generated through the introduction of triethylenetetramine to mesoporous ZrO2show excellent CO2adsorption performance in a stream of 5% CO2.At 75 ℃,a maximum CO2uptake of 4.16 mmol·g-1has been achieved over ZrO2-TETA-200 at a CO2flow rate of 20 cm3·g-1.An increase of TETA amount or gas flow rate has a positive impact on its performance whereas an increase in the adsorption temperature has a negative effect on the adsorption.The spent adsorbent can be easily regenerated at 100 ℃ for 60 min in argon.It can be concluded that the adsorbent is suitable for efficient CO2capture.
[1] WANG J,HUANG L,YANG R,etal.Recent advances in solid sorbents for CO2capture and new development trends[J].Energy Environ Sci,2014,7(11):3478-3518.
[2] ZHAO X,HU X,HU G,etal.Enhancement of CO2adsorption and amine efficiency of titania modified by moderate loading of diethylenetriamine[J].J Mater Chem A,2013,1(20):6208-6215.
[3] LEE W R,HWANG S Y,RYU D W,etal.Diamine-functionalized metal-organic framework:exceptionally high CO2capacities from ambient air and flue gas,ultrafast CO2uptake rate,and adsorption mechanism[J].Energy Environ Sci,2014,7(2):744-751.
[4] SIRIWARDANE R V,SHEN M S,AND E P F,etal.Adsorption of CO2on molecular sieves and activated carbon[J].Energy & Fuels,2001,15(2):279-284.
[5] BELMABKHOUT Y,SERNA-GUERRERO R,SAYARI A.Adsorption of CO2from dry gases on MCM-41 silica at ambient temperature and high pressure.1:Pure CO2adsorption[J].Chem Eng Sci,2009,64(17):3721-3728.
[6] DRAGE T C,BLACKMAN J M,PEVIDA C,etal.Evaluation of activated carbon adsorbents for CO2capture in gasification[J].Energy & Fuels,2009,23(5):2790-2796.
[7] LI X,CHENG Y,ZHANG H,etal.Efficient CO2capture by functionalized graphene oxide nanosheets as fillers to fabricate multi-permselective mixed matrix membranes[J].ACS Appl Mater Interfaces,2015,7(9):5528-5537.
[8] AHRENHOLTZ S R,LANDAVERDE-ALVARADO C,WHITTING M,etal.Thermodynamic Study of CO2sorption by polymorphic microporous MOFs with open Zn(II) coordination sites[J].Inorg Chem,2015,54(9):4328-4336.
[9] LU J,PEREZ-KRAP C,SUYETIN M,etal.A robust binary supramolecular organic framework (SOF) with high CO2adsorption and selectivity[J].J Am Chem Soc,2014,136(1):522-526.
[10] DING S Y,WANG W.Covalent organic frameworks (COFs):from design to applications[J].Chem Soc Rev,2013,42(1):538-568.
[11] COTE A P,BENIN A L,OCKWING N W,etal.Porous,crystalline,covalent organic frameworks[J].Science,2005,310:1166-1170.
[12] XU X,SONG C,ANDRESEN J M,etal.Novel polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2capture[J].Energy & Fuels,2002,16(6):1463-1469.
[13] NIGAR H,GARCIA-BANOS B,CATAL-CIVERA J M,etal.Amine-functionalized mesoporous silica.A material capable of CO2adsorption and fast regeneration by microwave heating[J].AIChE J,2015,61(2):547-555.
[14] HAO S,CHANG H,XIAO Q,etal.One-pot synthesis and CO2adsorption properties of ordered mesoporous SBA-15 materials Functionalized with APTMS[J].J Phys Chem C,2011,115(26):12873-12882.
[15] ZHOU L,FAN J,GUI G,etal.Highly efficient and reversible CO2adsorption by amine-grafted platelet SBA-15 with expanded pore diameters and short mesochannels[J].Green Chem,2014,16(8):4009-4016.
[16] GOLMAKANI A,FATEMI S,TAMNANLOO J.CO2capture from the tail gas of hydrogen purification unit by vacuum swing adsorption process,using SAPO-34[J].Ind Eng Chem Res,2016,55(1):334-350.
[17] MONAZAM E R,SHADLE L J,MILLER D C,etal.Equilibrium and kinetics analysis of carbon dioxide capture using immobilized amine on a mesoporous silica[J].AIChE J,2013,59(3):923-935.
[18] KUWAHARA Y,KANG D Y,COPELAND J R,etal.Dramatic enhancement of CO2uptake by poly(ethyleneimine) using zirconosilicate supports[J].J Am Chem Soc,2012,134(26):10757-10760.
[19] YANG F M,CHEN L,AU C T,etal.Preparation of triethylenetetramine-modified zirconosilicate molecular sieve for carbon dioxide adsorption[J].Environ Prog Sustain Energy,2015,34(6):1814-1821.
[20] WANG X,GUO Q J,ZHAO J,etal.Mixed amine-modified MCM-41 sorbents for CO2capture[J].Int J Greenh Gas Control,2015,37(1):90-98.
[21] REZAEI F,SAKWA-NOVAK M A,BALI S,etal.Shaping amine-based solid CO2adsorbents:effects of pelletization pressure on the physical and chemical properties[J].Micropor Mesopor Mater,2015,204:34-42.
[22] VILARRASA-GARCIA E,ORTIGOSA Moya E M,CECILIA J A,etal.CO2adsorption on amine modified mesoporous silicas:effect of the progressive disorder of the honeycomb arrangement[J].Micropor Mesopor Mater,2015,209:172-183.
[23] OLEA A,SANZ-PEREZ E S,ARENCIBIA A,etal.Amino-functionalized pore-expanded SBA-15 for CO2adsorption[J].Adsorpt,2013,19(2/3/4):589-600.
[24] BELMABKHOUT Y,SERNA-GUERRERO R,SAYARI A.Adsorption of CO2-containing gas mixtures over amine-bearing pore-expanded MCM-41 silica:application for gas purification[J].Ind Eng Chem Res,2010,49(1):359-365.
[25] HARLICK P J E,SAYARI A.Applications of pore-expanded mesoporous Silica.5.triamine grafted material with exceptional CO2dynamic and equilibrium adsorption performance[J].Ind Eng Chem Res,2007,46(2):446-458.
[26] ZHAO Y,SHEN Y,BAI L,etal.Carbon dioxide adsorption on polyacrylamide-impregnated silica gel and breakthrough modeling[J].Appl Surf Sci,2012,261:708-716.
[27] ZHANG Z H,YIN L,WANG Y M,An expeditious synthesis of benzimidazole derivatives catalyzed by Lewis acids[J].Catal Commun,2007,8(7):1126-1131.
[28] FIROUZABADA H,IRANPOOR N,JAFARPOUR M,etal.ZrOCl2·8H2O as a highly efficient and the moisture tolerant Lewis acid catalyst for Michael addition of amines and indoles toα,β-unsaturated ketones under solvent-free conditions[J].J Mol Catal A:Chem,2006,252(1/2):150-155.
[29] CHEN W H,KO H H,SAKTHIEVL A,etal.A solid-state NMR,FT-IR and TPD study on acid properties of sulfated and metal-promoted zirconia:Influence of promoter and sulfation treatment[J].Catal Today,2006,116(2):111-120.
[30] MARTIN P J,BENDAVID A,CAIRNEY J M,etal.Nanocomposite Ti-Si-N,Zr-Si-N,Ti-Al-Si-N,Ti-Al-V-Si-N thin film coating deposited by vacuum arc deposition[J].Surf Coat Technol,2005,200(7):2228-2235.
[31] XUE A,ZHOU S,ZHAO Y,etal.Effective NH2-grafting on attapulgite surfaces for adsorption of reactive dyes[J].J Hazard Mater,2011,194:7-14.
[32] RODRIGUEZ-MOSQUEDA R,PFEIFFER H.Thermokinetic analysis of the CO2chemisorption on Li4SiO4by using different gas flow rates and particle sizes[J].J Phys Chem A,2010,114(13):4535-4541.
[33] LIAO Y,CAO S W,YUAN Y,etal.Efficient CO2capture and photoreduction by amine-functionalized TiO2[J].Chem Euro J,2014,20(33):10220-10222.
[34] TSENG C L,CHEN Y K,WANG S H,etal.2-ethanolamine on TiO2investigated by in situ infrared spectroscopy,adsorption,photochemistry,and its interaction with CO2[J].J Phys Chem C,2010,114(27):11835-11843.
(编辑 WJ)
2016-10-12
国家自然科学基金资助项目(21401054);湖南省自然科学基金资助项目(2015JJ3033);国家科技支撑计划资助项目(2013BAC11B03)
O614.81+2
A
1000-2537(2017)01-0051-09
三乙烯四胺修饰介孔ZrO2的合成及其CO2吸附性能研究
陈 盛2,杨泛明1,陈 浪1*
(1.湖南大学化学化工学院,化石能源清洁利用湖南省重点实验室,中国 长沙 410082;2.雅礼中学,中国 长沙 410007)
合成介孔ZrO2并采用三乙烯四胺修饰制备CO2吸附剂.采用X-射线粉末衍射、低温N2吸附-脱附、傅里叶红外、X-射线光电子能谱、色散光谱、热重及CO2程序升温脱附等表征手段对合成材料的理化性质进行表征.吸附剂的吸附性能在CO2浓度为5%的流动气流中进行测定.研究结果表明通过三乙烯四胺改性的介孔ZrO2对CO2有较好的吸附性能,适当增加CO2气体流速能促进吸附而提高吸附温度对吸附不利.当三乙烯四胺的负载量为200 mg,CO2气体流速为20 cm3·min-1,吸附温度为75 ℃时,吸附剂表现出最好的吸附性能,最大吸附容量达到4.16 mmol·g-1,该条件下吸附剂还具有很好的重复使用性能.因此其突出的吸附能力和良好的重复利用性表明三乙烯四胺改性的介孔ZrO2在CO2吸附方面具有潜力.
三乙烯四胺; 氧化锆; 吸附剂; 二氧化碳
10.7612/j.issn.1000-2537.2017.01.008
* 通讯作者,E-mail:huagong042cl@163.com
