Antibiotic Resistance Evaluation and Resistance Gene Profile of Epibiotic Lactic Acid Bacteria on Red Bell Peppers Used for Sichuan Pickle Fermentation
2017-02-08CAITingLUQianwenXIANGWenliangZHANGQingZHANGQishengCHENGongCAIYimin
CAI Ting, LU Qianwen, XIANG Wenliang,*, ZHANG Qing, ZHANG Qisheng, CHEN Gong, CAI Yimin
(1. Provincial Key Laboratory of Food Biotechnology of Sichuan, Institute of Ancient Brewing Technology, College of Food and Bioengineering, Xihua University, Chengdu 610039, China; 2. Sichuan Academy of Food and Fermentation Industries, Chengdu 611130, China; 3. International Research Center for Agricultural Sciences of Japan, Tsukuba 30528686, Japan)
Antibiotic Resistance Evaluation and Resistance Gene Profile of Epibiotic Lactic Acid Bacteria on Red Bell Peppers Used for Sichuan Pickle Fermentation
CAI Ting1, LU Qianwen1, XIANG Wenliang1,*, ZHANG Qing1, ZHANG Qisheng2, CHEN Gong2, CAI Yimin3
(1. Provincial Key Laboratory of Food Biotechnology of Sichuan, Institute of Ancient Brewing Technology, College of Food and Bioengineering, Xihua University, Chengdu 610039, China; 2. Sichuan Academy of Food and Fermentation Industries, Chengdu 611130, China; 3. International Research Center for Agricultural Sciences of Japan, Tsukuba 30528686, Japan)
For formulating reasonable measures for the prevention and control of bacterial antibiotic resistance to ensure food safety, penicillin (PEN), erythromycin (ERY), tetracycline (TET), streptomycin (STR) and chloramphenicol (CHL) resistance of lactic acid bacteria (LABs) and the corresponding resistance genes were evaluated, including Enterococcus mundtii (n = 5), Enterococcus faecalis (n = 2), Enterococcus hirae (n = 2), Lactococcus lactis (n = 7), Leuconostoc mesenteroides (n = 2), Leuconostoc holzapfelii (n = 3) and Weissella cibaria (n = 79) from fresh red bell peppers used for Sichuan pickle fermentation. All of the isolated strains were susceptible to PEN or ERY, but they had solo, double or triplicate resistance to TET, STR and CHL. All the isolates of L. mesenteroides as well as some strains of E. hirae, E. faecalis and L. holzapfelii showed solo STR resistance. Some strains of E. faecalis, E. hirae, L. lactis and W. cibaria had double resistance to STR and TET, as well as STR and CHL. However, isolates with triplicate resistances to STR, TET and CHL were only found in W. cibaria. It was found that except norA, sepA, tet(A), tet(O) and aac(6’)-aph(2’) genes, all antibiotic resistance genes were harbored by the resistant isolates partly or completely. The multiple-drug resistance efflux pump genes efrA, tolC, norC, sugE and mdfA showed higher positive rates (which were 49%, 41%, 48%, 41% and 47%, respectively) than the ribosomal protection protein genes and the enzymatic modification genes in the corresponding polymerase chain reaction (PCR). Even though the dissemination of these antibiotic resistances needs to be further studied, such results demonstrated that food safety concerns will be partly focused on antibiotic resistance of LABs on fresh red bell peppers according to Qualified Presumption of Safety criteria.
red bell peppers; lactic acid bacteria; food safety; antibiotic resistance; antibiotic resistance genes
Sichuan pickle is the typical representative of Chinese traditional vegetable fermentation. It normally serves as a key flavor for Sichuan cuisine or is used as an appetizer because of its unique flavor in many regions of China. Like the kimchi, Sichuan pickle also has various beneficial properties on general health for the consumers, including anti-oxidative activity, antiaging effects, antimutagenic, antigenotoxic and antitumor activities, antimicrobial activity, immune stimulation, weight-controlling, lipidlowering, and anti-atherogenic activities[1]. The material basis of beneficial properties is closely linked to fermentation process dominated by strains of Lactobacillus, Leuconostoc, Weissella and Pediococcus genera from the old salt brine and the fresh vegetable materials. In the last years, the lactic acid bacteria (LAB) genera which are involved in traditional lactic fermentation, were generally considered to be safe for human according to the “Generally Recognized as Safe (GRAS)”principles and the “Qualitative Presumption of Safety (QPS)”risk assessment approach based on a long history of safe use[2]. However, in the recent years, along with antibiotic resistance genes (ARGs) polluting intensively, several antibiotic resistance genes have already been found in the Sichuan pickle[3]. This gives a new challenge to the traditional GRAS and QPS state of Sichuan pickle, more specifically to those without heat-treated before consumption.
The antibiotic resistance genes (ARGs), as emerging contaminants, were first proposed in 2006[4]. They fleetly became a new research topic in the food safety and environmental science because their health risks resulted from spreading among different hosts were often greater than the harm caused by antibiotics themselves. In 2000, the World Health Organization (WHO) report focused on antibiotic resistance as one of the most critical human health challenges of the next century and heralded the need for “a global strategy to contain resistance”. The food chain was considered as the main route of transmission of antibiotic resistance[5]. The development of antibiotic resistance among bacteria introduced in the food chain is of great novel concern in the food safety[6]. Recently, several spontaneously fermented foods have been considered as important potential transmission vehicles of ARGs from environment to human gastrointestinal tract[7-9], moreover the transfer of ARGs in the commensal or bacteria may be also induced by low pH, high salt concentration, antimicrobial compounds and the high number of living bacteria. And thus the European Food Safety Authority (EFSA) requires that bacteria which are to be introduced into the food chain should lack acquired or transferable ARGs to prevent their spread among different bacteria[10]. Unfortunately, with the aggravation of pollution of ARGs in the environment, the bacteria with transferable ARGs would be inevitably introduced into food produce chains[11-12].
Bell pepper, a vegetable of nightshade (Solanaceae) family, is one of the best vegetable to serve in a cruditéplatter because of its bright color, thick flesh, great favor, crunchy high texture capsorubin and high vitamin content[13]. In the southwest of China, it is also usually used to make the Sichuan pickle for the flavor refreshments to stimulate the appetite before the meal, or to relieve oleaginous taste after the meal in the summer. However, in the recent years, LABs with ARGs were often found in the Sichuan pickle fermentation system after the bell peppers were introduced to the old salt brine[3], which would make the GRAS and QPS state of Sichuan pickle worse if the transfer of ARGs took place between different LABs. Therefore, it is necessary to evaluate antibiotic resistance and ARGs of epibiotic LABs from the fresh red bell peppers. In current study, we have investigated their resistance to 5 important antibiotics including penicillin (PEN), erythromycin (ERY), tetracycline (TET), streptomycin (STR) and chloramphenicol (CHL), and their ARGs were also detected by polymerase chain reaction (PCR). This study would be very significant to food safety of epibiotic LABs on the fresh red bell peppers used for the Sichuan pickle.
1 Materials and Methoddss
1.1 LABs and growth condition
In the present study, 100 LAB strains were previously obtained from the fresh red bell peppers used for the Sichuan pickle fermentation. They were identified according to the methods described by Pan Lu et al[14]and then stored as frozen stocks at -20 ℃ in de Man Rogosa and Sharpe (MRS) broth containing 20% (V/V) glycerol for long term storage. They were routinely propagated at 30 ℃ in MRS broth (Fluka, Madrid, Spain) or agar slants under aerobic conditions for 24-48 h.
1.2 Antimicrobial susceptibility testing
Antimicrobial susceptibility tests were performed by broth micro-dilution method[15]. Brief y, a 96-well plate was inoculated with 2 μL of fresh LAB cultures and 198 μL of MRS broth with serial two-fold dilutions of antibiotics (0.125-64.000 μg/mL PEN, 0.25-128.00 μg/mL ERY, 1-512 μg/mL TET, 2-1 024 μg/mL STR, 0.5-256.0 μg/mL CHL). LABs were f rst cultured in 2 mL of MRS for 24 h at 30 ℃and subsequently diluted in 0.85 g/100 mL physiological saline to the concentration of approximately 1×105CFU/mL. LABs inoculated in MRS were used as positive control, and a LAB-free well as negative. Plates were incubated under anaerobic conditions at 30 ℃ for 48 h.
The minimal inhibitory concentration (MIC) of each antibiotic was visually evaluated as the lowest concentration at which no growth was observed. All the tests were repeated at least thrice. In duplicate experiments, the differences of MIC for independent sample never exceeded 1 order of dilution. Interpretation for susceptibility status was based on the threshold X defined also as Extended Common Object File Forma (ECOFF) according to the EFSA (2012)[16-17]and the European Committee on Antimicrobial Susceptibility Testing (EUCAST, http://www.eucast.org). When MIC ≤ECOFF value, the strain was sensitive to the antibiotic; on the contrary, it was resistant.
1.3 Detection of ARGs
The temple DNA for PCR was prepared as methods by Xiang et al[18]. The genes associated with resistance to chloramphenicol acetyltransferase gene (cat)[14,19], TET (tet(A), tet(B), tet(C), tet(D), tet(G), tet(H), tet(K), tet(M), tet(O), tet(S), tet(W) and tet(X))[19-20], and STR (strA, strB, aadA, aad6, aph(3’)-Ⅲa and aac(6’)-aph(2’)) were detected by PCR amplification[19]. The efflux pump genes mediating antibiotic resistance eff ux were also investigated according to the PCR methods described by Swick et al[21]for acrA, acrB, tolC, mdfA and norE; He et al[22]for sugE; Noguchi et al[23]for smr; Lee et al[24]for efrA and efrB; Patel et al[25]for mdeA, mepA, norA, norB, norC, sdrM and sepA. The PCR primers were listed in Table 1.
All the amplified ARGs were respectively cloned into the pGEM-T plasmid vector (Promega, Madison, WI, USA) and transformed into the chemically competent E. coli DH5α cells for sequencing. Then the antibiotic resistance genes were further verified by sequence BLASTx program in the National Center for Biotechnology Information (NCBI).
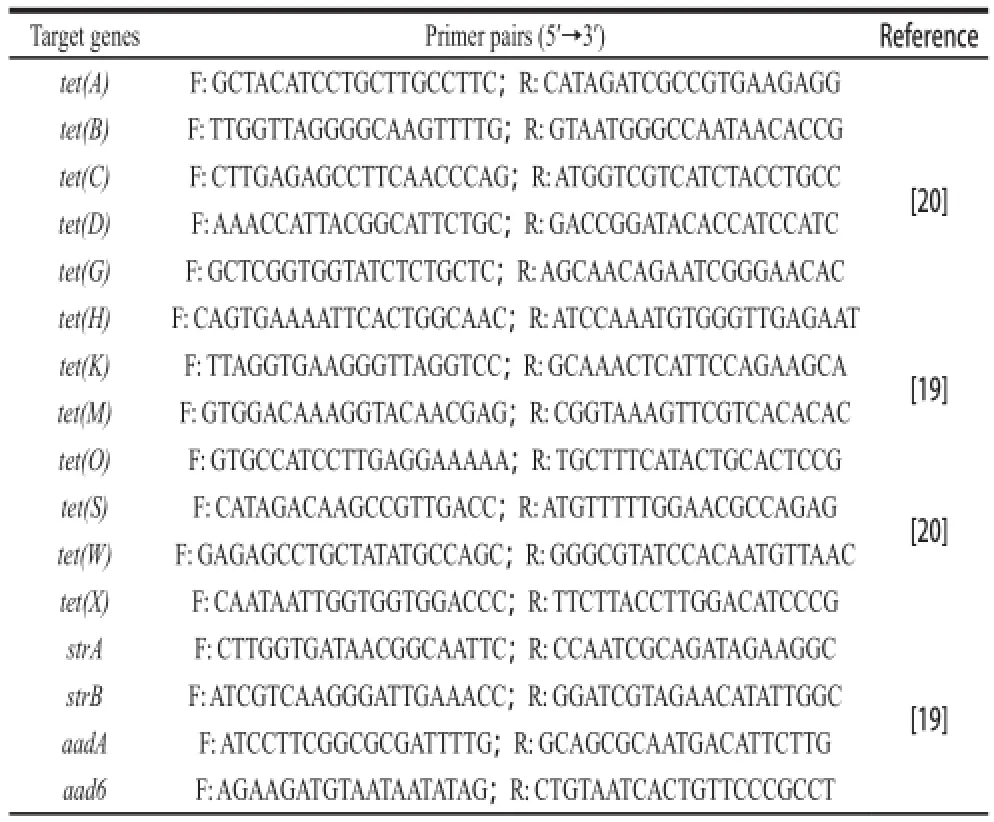
Table1 PCR primers for antibiotic resistance genes

Table1 continued
1.4 Statistical analysis
The MIC distribution of 5 antibiotics was statistically analyzed using Excel 2010 (Microsoft, Redmond, Washington, USA). Distribution of antibiotic susceptibility and antibiotic resistance genes were performed using SPSS version 17.0 (IBM, Armonk, New York, USA).
2 Results and Analysis
2.1 Antibiotic susceptibility
Antibiotics had been spread in the environment when used as growth promoters in livestock years ago, leading to the selection of antibiotic resistant bacteria[26]. These resistant bacteria may inhabitat in or on fruits, vegetables and animal feeds, and may further disseminate during the food fermentation[27-28]. Therefore, it is important to evaluate the antibiotic resistance incidences of bacteria in fermented vegetables[29]. A total of one hundred isolates were initially identified as LAB by 16S rRNA sequence analysis, and these LABs were further verified by physiological and biochemical methods. They were turned out to be seventy-nine strains of Weissella cibaria, five strains of Enterococcus mundtii, two strains of Enterococcus faecalis, two strains of Enterococcus hirae, two strains of Leuconostoc mesenteroides, three strains of Leuconostoc holzapfelii and seven strains of Lactococcus lactis (Table 2).
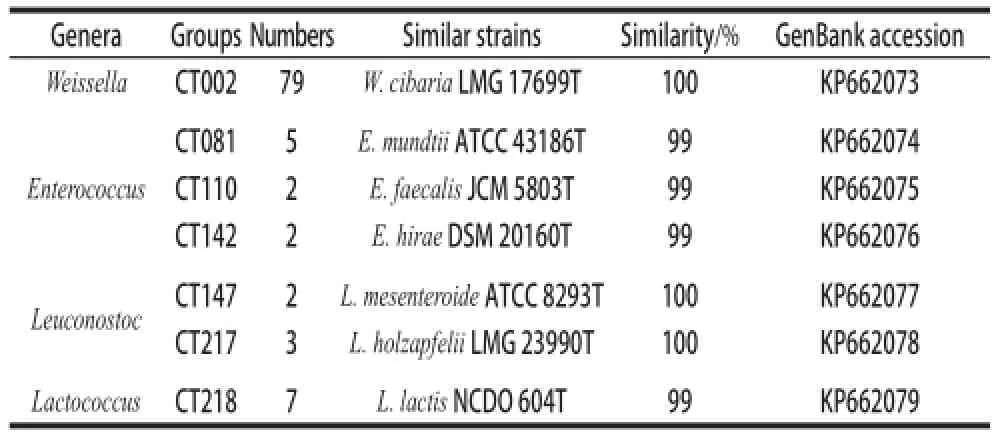
Table2 Microbial classification of LABs isolated from red bell peppers using 16S rRNA gene sequence analysis

Table3 MIC distribution of 5 antibiotics for LABs isolated from red peppers used for Sichuan pickle production
The susceptibility determination was performed with epibiotic LABs to PEN, ERY, TET, STR and CHL. The results indicated that the MICs to PEN and ERY did not exceed the ECOFF values posed by the EFSA 2012 for E. mundtii, E. faecalis, E. hirae, L. mesenteroides, L. holzapfelii, L. lactis and W. cibaria (Table 3). It suggested that all epibiotic LABs on the fresh red bell peppers are sensitive to PEN and ERY. Conversely, except L. holzapfelii, most of them displayed resistance to the STR (84 strains LABs), with 100% of E. hirae and L. mesenteroides, 60% of E. mundtii, 50% of E. faecalis, 85.7% L. lactis and 88.6% W. cibaria (Table 3), and these resistant LABs showed high MIC values as previously reported by Elkins et al[30]. For TET, all strains of E. mundtii, L. mesenteroides and L. holzapfelii showed susceptibility, while 50% of E. faecalis, 100% of E. hirae and 28.6% of L. lactis strains had higher MIC than their corresponding ECOFF values, suggesting resistance to TET (Table 3). In the CHL, only 3.8% of W. cibaria strains had obtained resistance, the other species and 96.2% of W. cibaria strains were sensitive to CHL (Table 3).
2.2 Antibiotic resistance phenotype and distribution
Statistical analysis showed that none of strains were resistant to PEN and ERY, but there were some strains with solo, or double or triplicate resistance to TET, STR and CHL. As one of the most widespread agricultural antibiotics, the use of STR has lead STR resistance bacteria to grow in the environment, and thus unavoidably gathered at the surface of the vegetable. Therefore, STR resistant bacteria were often found on the surface of the vegetables[5]. It was also verified by our results that 84% of LAB isolates were resistant to STR, and the strains with solo resistance to STR almost existed in all species except E. hirae and E. faecalis (Fig. 1). All the strains of L. mesenteroides only showed solo STR resistance. In E. faecalis, E. hirae, L. lactis and W. cibaria, some isolates have double resistance to STR and TET or STR and CHL. However, the triplicate resistant strain was found only in W. cibaria, and it was against STR, TET and CHL (Fig. 1). Two E. hirae strains both displayed STR and TET double resistance. In two E. faecalis strains, one was sensitive to antibiotics tested, the other with TET and STR double resistance. In five E. mundtii strains, which have two strains with susceptibility to all testing antibiotics, three solo STR resistant. Among seven L. lactis strains, four strains were found to be with solo STR resistance, two strains with TET and STR double resistance, and one strain with susceptibility to all testing antibiotics. Among seventy nine W. cibaria strains, only one W. cibaria strain with triplicate resistance to TET, STR and CHL was observed. Furthermore, there were nine susceptibility isolates, fifty-eight solo STR resistant isolates, nine TET and STR double resistant isolates and two STR and CHL double resistant isolates.
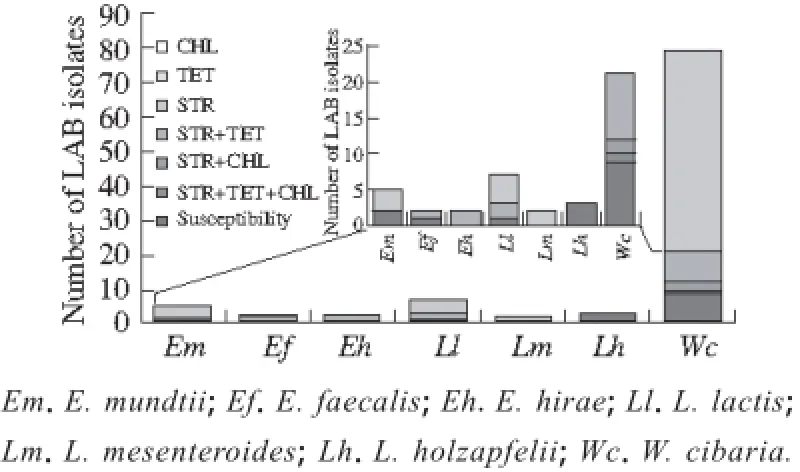
Fig. 1 Distribution of antibiotic susceptibility and resistant LAB isolates
2.3 Antibiotic resistance genes
The overuse and misuse of antibiotics have created a tremendous selective pressure toward antibiotic resistant bacteria[30]. Different mechanisms for the resistance to various antibiotics have been found in bacteria, including antibiotic degrading, pump efflux, altering and metabolism in cell[31]. The emergence of antibiotic resistance is a global threat because it reduces the efficiency of the antibiotic therapy, which is getting worse by the horizontal transfer of ARGs between bacteria[32-33]. Fermentative materials have been considered as potential vehicles of resistant genes from environment to products[5].
To identify resistant determinants responsible for the resistance phenotypes observed, all the strains were screened by PCR for the presence of resistant genes as described above. In current investigation, these genes served the antibiotic resistance were detected and displayed in Fig. 2. Except the efflux genes norA and sepA, TET resistance genes tet(A) and tet(O), STR resistance gene aac(6’)-aph(2’), the other resistant genes were harbored by resistance isolates partly or completely. The multiple-drug resistant efflux pump genes, including efrA, tolC, norC, sugE and mdfA, showed higher positive ratios than the others in the corresponding PCR reactions, in which their detected ratios were 49, 41, 48, 41 and 47%, respectively. The results were similar to the eff ux pump genes in the LAB described by del Carmen et al[19]. The STR and TET double resistant W. cibaria CT023 carried most resistant genes, including seven drug eff ux pump genes efrA, efrB, acrB, sugE, norC, mdfA and mepA, three TET efflux genes tet(B), tet(C) and tet(K), two ribosomal protection protein genes tet(S) and tet(W), one enzymatic modif cationgene tet(X), and three STR resistance genes strB, aad6 and aph(3’)-Ⅲa. While, E. mundtii CT080, CT081, W. cibaria CT012, CT014, CT098 and CT206 have only possessed one resistance gene, norE for CT080, mdfA for CT081, strB for CT012 and CT206, acrA for CT014 and CT098. And all of them were positive for solo STR resistance. The detection of ARGs in fermentative vegetable materials implies that there is a potential food safety risk when ARGs spread to other microorganisms during the fermentation by horizontal gene transfer.

Fig. 2 Distribution of ARGs in different antibiotic resistance LAB isolates
3 Conclusions
Traditionally fermented vegetables play an important role in the food systems in China. However, no investigation has been conducted to assess the antibiotic resistance incidences and ARGs of LAB. In current investigation, all the LAB isolates from the fresh red bell peppers were susceptible to PEN and ERY. Concerning TET, STR and CHL, all the strains of L. mesenteroides showed solo STR resistance. In E. faecalis, E. hirae, L. lactis and W. cibaria, some isolates had double resistance to STR and TET or STR and CHL. However, the triplicate resistance was found only in W. cibaria. Except for norA, sepA, tet(A), tet(O) and aac(6’)-aph(2’), the other resistance genes were harbored by resistant isolates partly or completely. The genes efrA, tolC, norC,
sugE and mdfA showed higher positive ratios, which were 49%, 41%, 48%, 41% and 47% respectively. Even though the dissemination of these ARGs during vegetable fermentation need to be further studied, such studies will be conducive to safety assessment of fresh red bell peppers when being used as the material for fermentation.
[1] JI Y, KIM H, PARK H, et al. Functionality and safety of lactic bacterial strains from Korean kimchi[J]. Food Control, 2013, 31(2): 467-473. DOI:10.1016/j.foodcont.2012.10.034.
[2] ANADÓN A, MART☒NEZ-LARRAŇAGA M R, MART☒NEZ M A. Probiotics for animal nutrition in the European Union. regulation and safety assessment[J]. Regulatory Toxicology and Pharmacology, 2006, 45(1): 91-95. DOI:10.1016/j.yrtph.2006.02.004.
[3] SONG Feifei, XU Gurong, CAI Ting, et al. Detection of streptomycin resistance and resistance genes in lactic acid bacteria from Sichuan Pickle of China[J]. Journal of Food Safety and Quality, 2014, 5(12): 4032-4039.
[4] PRUDEN A, PEI R, STORTEBOOM H, et al. Antibiotic resistance genes as emerging contaminants: studies in northern Colorado[J]. Environmental Science and Technology, 2006, 40(23): 7445-7450. DOI:10.1021/es060413l.
[5] VERRAES C, van BOXSTAEL S, van MEERVENNE E, et al. Antimicrobial resistance in the food chain: a review[J]. International Journal of Environmental Research and Public Health, 2013, 10(7): 2643-2669. DOI:10.3390/ijerph10072643.
[6] WANG H, McENTIRE J C, ZHANG L, et al. The transfer of antibiotic resistance from food to humans: facts, implications and future directions[J]. International Office of Epizootics, 2012, 31(1): 249-260. DOI:10.20506/rst.31.1.2117.
[7] BAUTISTA-GALLEGO J, ARROYO-L☒PEA F N, RANTSIOU K, et al. Screening of lactic acid bacteria isolated from fermented table olives with probiotic potential[J]. Food Research International, 2013, 50(1): 135-142. DOI:10.1016/j.foodres.2012.10.004.
[8] AHAOTU I, ANYOGU A, NLOKU O H, et al. Molecular identification and safety of Bacillus species involved in the fermentation of African oil beans (Pentaclethra macrophylla Benth) for production of Ugba[J]. International Journal of Food Microbiology, 2013, 162(1): 95-104. DOI:10.1016/j.ijfoodmicro.2013.01.001.
[9] HUDDLESTON J R. Horizontal gene transfer in the human gastrointestinal tract: potential spread of antibiotic resistance genes[J]. Infecition and Drug Resistance, 2014(7): 167-176. DOI:10.2147/IDR.S48820.
[10] van REENEN C A, DICKS L M T. Horizontal gene transfer amongst probiotic lactic acid bacteria and other intestinal microbiota: what are the possibilities? a review[J]. Archives of Microbiology, 2011, 193(3): 157-168. DOI:10.1007/s00203-010-0668-3.
[11] ZHANG Xiangxu, ZHANG Tong, FANG H H P. Antibiotic resistance genes in water environment[J]. Applied Microbiology and Biotechnology, 2009, 82(3): 397-414. DOI:10.1007/s00253-008-1829-z.
[12] FUENTES M A F, MORENTE E O, ABRIOUEL H, et al. Antimicrobial resistance determinants in antibiotic and biocide resistant gram-negative bacteria from organic foods[J]. Food Control, 2014, 37: 9-14. DOI:10.1016/j.foodcont.2013.08.041.
[13] OUYANG Jing, TAO Xianglin, LI Ziming, at el. Analysis of changes in the main components and volatile components in fermented pepper with high salt content[J]. Food Science, 2014, 35(4): 174-179. DOI:10.7506/spkx1002-6630-201416038.
[14] PAN Lu, HU Xiaoqing, WANG Xiaoyuan. Assessment of antibiotic resistance of lactic acid bacteria in Chinese fermented foods[J]. Food Control, 2011, 22(8): 1316-1321. DOI:10.1016/ j.foodcont.2011.02.006.
[15] KLARE I, KONSTABEL C, M☒LLER-BERTLING S, et al. Evaluation of new broth media for microdilution antibiotic susceptibility testing of Lactobacilli, Pediococci, Lactococci, and Bif dobacteria[J]. Applied and Environmental Microbiology, 2005, 71(12): 8982-8986. DOI:10.1128/aem.71.12.8982-8986.2005.
[16] DANIELSEN M, WIND A. Susceptibility of Lactobacillus spp. to antimicrobial agents[J]. International Journal of Food Microbiology, 2003, 82(1): 1-11. DOI:10.1016/S0168-1605(02)00254-4.
[17] FLOREZ H, SLIVA E, FERN☒NDEZ V, et al. Prevalence and risk factors associated with the metabolic syndrome and dyslipidemia in White, Black, Amerindian and Mixed Hispanics in Zulia State, Venezuela[J]. Diabetes Research and Clinical Practice, 2005, 69(1): 63-77. DOI:10.1016/j.diabres.2004.11.018.
[18] XIANG Wenliang, LI Ke, LIU Sen, et al. Microbial succession in the traditional Chinese Luzhou-flavor liquor fermentation process as evaluated by SSU rRNA profiles[J]. World Journal of Microbiology and Biotechnology, 2012, 29(3): 559-567. DOI:10.1007/s11274-012-1210-3.
[19] del CARMEN CASADO MU☒OZ M, BENMAR N, LERMA L L, et al. Antibiotic resistance of Lactobacillus pentosus and Leuconostoc pseudomesenteroides isolated from naturally-fermented Alore☒a table olives throughout fermentation process[J]. International Journal of Food Microbiology, 2014, 172(17): 110-118. DOI:10.1016/ j.ijfoodmicro.2013.11.025.
[20] JIA Shuyu, HE Xiwei, BU Yuanqing, et al. Environmental fate of tetracycline resistance genes originating from swine feedlots in river water[J]. Journal of Environmental Science and Health, 2014, 49(8): 624-631. DOI:10.1080/03601234.2014.911594.
[21] SWICK M C, MORGAN-LINNELL S K, CARLSON K M, et al. Expression of multidrug efflux pump genes acrAB-tolC, mdfA, and norE in Escherichia coli clinical isolates as a function of fluoroquinolone and multidrug resistance[J]. Antimicrobial Agents and Chemotherapy, 2011, 55(2): 921-924. DOI:10.1128/AAC.00996-10.
[22] HE Guixin, ZHANG Chu, CROW R R, et al. SugE, a new member of the SMR family of transporters, contributes to antimicrobial resistance in Enterobacter cloacae[J]. Antimicrobial Agents and Chemotherapy, 2011, 55(8): 3954-3957. DOI:10.1128/aac.00094-11.
[23] NOGUCHI N, HASE M, KITTA M, et al. Antiseptic susceptibility and distribution of antiseptic-resistance genes in methicillin-resistant Staphylococcus aureus[J]. FEMS Microbiology Letters, 1999, 172(2): 247-253. DOI:10.1111/j.1574-6968.1999.tb13475.x.
[24] LEE E W, HUDA M N, KURODA T, et al. EfrAB, an ABC multidrug efflux pump in Enterococcus faecalis[J]. Antimicrobial Agents and Chemotherapy, 2003, 47(12): 3733-3738. DOI:10.1128/ aac.47.12.3733-3738.2003.
[25] PATEL D, KOSMIDIS C, SEO S M, et al. Ethidium bromide MIC screening for enhanced efflux pump gene expression or efflux activity in Staphylococcus aureus[J]. Antimicrobial Agents and Chemotherapy, 2010, 54(12): 5070-5073. DOI:10.1128/aac.01058-10.
[26] DEVIRGILIIS C, CARAVELLI A, COPPOIA D, et al. Antibiotic resistance and microbial composition along the manufacturing process of Mozzarella di Bufala Campana[J]. International Journal of Food Microbiology, 2008, 128(2): 378-384. DOI:10.1016/ j.ijfoodmicro.2008.09.021.
[27] ZHANG Hongmei, HUANG Shaosong, ZHOU Hanji, et al. Two kinds of antibiotics resistance of lactic acid bacteria isolated from yogurt[J]. Chinese Journal of Public Health, 2010, 26(4): 511-512. DOI:10.11847/zgggws2010-26-04-73.
[28] LIN Kai, CAI Ting, XU Gurong, et al. Antibiotic resistance of epibiotic lactic acid bacteria on the surface of organic white radish[J]. Food Science, 2015, 36(11): 145-149. DOI:10.7506/spkx1002-6630-201511028.
[29] FU Mingchun, XI Huiping, LIU Yanzhao. Current antibiotic residues and control countermeasures of milk and meat[J]. Chinese Journal of Animal Quarantine, 2008, 25(6): 20-22. DOI:10.3969/j.issn.1005-944X.2008.06.010.
[30] ELKINS C A, MULLIS L B. Bile-mediated aminoglycoside sensibility in Lactobacillus species likely results from increased membrane permeability attributable to cholic acid[J]. Applied and Environmental Microbiology, 2004, 70(12): 7200-7209. DOI:10.1128/ aem.70.12.7200-7209.2004.
[31] SHARMA P, TOMAR S K, GOSWAMI P, et al. Antibiotic resistance among commercially available probiotics[J]. Food Research International, 2014, 57(1): 176-195. DOI:10.1016/ j.foodres.2014.01.025.
[32] NAWAZ M, WANG Juan, ZHOU Aiping, et al. Characterization and transfer of antibiotic resistance in lactic acid bacteria from fermented food products[J]. Current Microbiology, 2011, 62(3): 1081-1089. DOI:10.1007/s00284-010-9856-2.
[33] TOOMEY N, BOLTON D, FANNING S. Characterisation and transferability of antibiotic resistance genes from lactic acid bacteria isolated from Irish pork and beef abattoirs[J]. Research in Microbiology, 2010, 161(2): 127-135. DOI:10.1016/ j.resmic.2009.12.010.
四川泡菜发酵原料-灯笼辣椒附生乳酸菌的抗生素耐药性评估与耐药基因分析
蔡 婷1,卢倩文1,向文良1,*,张 庆1,张其圣2,陈 功2,蔡义民3
(1.西华大学食品与生物工程学院,四川省食品生物技术重点实验室,古法发酵(酿造)生物技术研究所,四川 成都 610039;2. 四川省食品发酵工业研究设计院,四川 成都 611130;3.日本国际农业科学研究中心,日本 筑波 30528686)
以四川泡菜蔬菜原料——新鲜灯笼辣椒为对象,分析其表面附生乳酸菌Enterococcus mundtii(5 株)、Enterococcus faecalis(2 株)、Enterococcus hirae(5 株)、Lactococcus lactis(7 株)、Leuconostoc mesenteroides(2 株)、Leuconostoc holzapfelii(3 株)和Weissella cibaria(79 株)对青霉素(penicillin,PEN)、红霉素(erythromycin,ERY)、四环素(tetracycline,TET)、链霉素(streptomycin,STR)和氯霉素(chloramphenicol,CHL)的抗生素耐药性和耐药基因分布,为制定合理的食品安全防控措施提供科学依据。研究表明:所有分离菌株均无PEN和ERY耐药性,其他种属部分菌株对TET、STR和CHL表现出单一、二重或三重耐药性。除E. hirae、E. faecalis和L. holzapfelii部分菌株对STR表现出单一耐药性外,所有L. mesenteroide菌株只表现出了STR单一耐药性;STR和TET、STR和CHL二重耐药菌株在E. faecalis、E. hirae、L. lactis和W. cibaria分离菌株中都有发现,但是STR、TET、CHL三重耐药菌株仅在W. cibaria中发现。聚合酶链式反应检测发现:除基因norA、sepA、tet(A)、tet(O)和aac(6’)-aph(2’)未被检出外,其他耐药菌株都有相应1 个或多个耐药基因被检出。多重耐药外排泵基因efrA、tolC、norC、sugE和mdfA较核糖体蛋白质保护和酶修饰基因检出率高,分别达到了49%、41%、48%、41%和47%。虽然辣椒表面附生乳酸菌的抗生素耐药基因在四川泡菜发酵过程中的扩散行为需要进一步研究,但根据食品加工过程安全规范标准,也应关注其表面附生的乳酸菌抗生素耐药性存在的潜在食品安全问题。
灯笼辣椒;乳酸菌;食品安全;抗生素耐药性;抗生素耐药性基因
TS201.3
A
1002-6630(2017)02-0027-07
nces
2016-03-11
国家自然科学基金面上项目(31571935);教育部春晖计划项目(Z2014061);四川省应用基础项目(2014JY0045);四川省教育厅重点项目(14ZA0110)
蔡婷(1991—),女,硕士研究生,主要从事食品微生物分子生态研究。E-mail:caiting1124@sina.com
10.7506/spkx1002-6630-201702005
*通信作者:向文良(1973—),男,教授,博士,主要从事中国西南地区特色发酵食品微生物分子生态与生物过程学研究。
E-mail:biounicom@mail.xhu.edu.cn
CAI Ting, LU Qianwen, XIANG Wenliang, et al. Antibiotic resistance evaluation and resistance gene profile of epibiotic lactic acid bacteria on red bell peppers used for Sichuan pickle fermentation[J]. 食品科学, 2017, 38(2): 27-33.
10.7506/ spkx1002-6630-201702005. http://www.spkx.net.cn
CAI Ting, LU Qianwen, XIANG Wenliang, et al. Antibiotic resistance evaluation and resistance gene profile of epibiotic lactic acid bacteria on red bell peppers used for Sichuan pickle fermentation[J]. Food Science, 2017, 38(2): 27-33. DOI:10.7506/spkx1002-6630-201702005. http://www.spkx.net.cn
