Ameliorated effects of Lactobacillus delbrueckii subsp. lactis DSM 20076 and Pediococcus acidilactici NNRL B-5627 on Fumonisin B1-induced Hepatotoxicity and Nephrotoxicity in rats
2017-01-19
Department of Protein Technology,Institute of Genetic Engineering and Biotechnology,City of Scientifc Research and Technological Applications,Borg El-Arab,Alexandria,Egypt
Ameliorated effects of Lactobacillus delbrueckii subsp. lactis DSM 20076 and Pediococcus acidilactici NNRL B-5627 on Fumonisin B1-induced Hepatotoxicity and Nephrotoxicity in rats
Amira A.Abdellatef,Ashraf A.Khalil*
Department of Protein Technology,Institute of Genetic Engineering and Biotechnology,City of Scientifc Research and Technological Applications,Borg El-Arab,Alexandria,Egypt
A R T I C L EI N F O
Article history:
Available online 11 February 2016
Fumonisin B1
Lactic acid bacteria
Oxidative stress
Antioxidant activity
Lipid peroxidation
Apoptosis
Oxidative stress has been implicated in a number of human regeneration and disease processes including atherosclerosis,pulmonary fbrosis,cancer,and different neurodegenerative diseases.The aim of this study was to evaluate the protective effects ofLactobacillus delbrueckiisubsp.lactisDSM 20076(LL-DSM)andPediococcus acidilacticiNNRL B-5627(PA-NNRL)against the hepatic-and nephro-toxicity of fumonisin B1(FB1)in FB1-treated rats for an experimental period of 4-weeks.Eighty mature male Sprague-Dawley rats were divided to 12 groups:1 untreated group;3 groups fed by a FB1-contaminated diet(50,100 and 200 mg FB1/kg diet, respectively);1 group fed orally by LL-DSM(1 ml/d);1 group fed orally by PA-NNRL(1 ml/d);3 groups co-administered by FB1-contaminated diet and LL-DSM(1 ml/d),and 3 groups coadministered by FB1-contaminated diet and PA-NNRL(1 ml/d).Malonaldehyde(MDA)nitric oxide,glutathione content,SOD activity,total antioxidant capacity(TAC),total oxidant status (TOS)and oxidative stress index(OSI)were determined.DPA assay was used to assess apoptosisinliverandkidneytissues.TheanimalsfedwithFB1-contaminateddietshowedasignifcant increase in oxidative stress markers and DNA fragmentation accompanied with signifcant decrease in GSH content,SOD activity,andTAC in liver and kidney tissues,especially at highdosageofFB1(T200).Probioticsantioxidantstrains(LL-DSMandPA-NNRL)relativelysucceeded to restore almost all parameters investigated as well as to reduce DNA fragmentation in liver and kidney tissues.As a conclusion,probiotics may induce its protective roleviaincreasing the antioxidant capacity,inhibition of lipid peroxidation,scavenging of free radicals and decreasing DNA lesions in liver and kidney of experimental animals tested.
©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Oxidative stress is classically defned as an imbalance between pro-oxidants and anti-oxidants in favor of the former,resulting in an overall increase in cellular levels of reactive oxygen species(ROS)[1].ROS can damage or cause complete degradation(i.e.,peroxidation)of essential complex molecules in the cells,including fat molecules(i.e.,lipids),proteins,and DNA[2].Although lipid peroxidation evidently may not contribute directly to killing in all instances of oxidative stress, products of oxidized lipids may themselves initiate further oxidative damage that could prove fatal.Thus reactive products such as malondialdehyde and 4-hydroxynonenal may attack amino acid side chains in proteins and cause fragmentation of DNA[3].
Apoptosis is considered to be a common result of oxidative stress caused by ROS production,disturbance of GSH generation and lipid peroxidation.In addition,activation of caspase-3 may be one of the events causing an increase in ROS production,and subsequent lipid peroxidation and reduction of intracellular GSH levels[4].Another mechanism of apoptosis induction probably involves the inhibition of protein kinase C,a key hallmark of apoptosis signaling and destructuring of the endothelial barrier[5].Endogenous cell metabolism and different chemicals,drugs,mycotoxins,ionizing radiation,solar light,cigarette smoking,and air pollution can induce oxidative damage to DNA.It has been considered that oxidative DNA damage is involved in the development of different diseases, aging,and cancer[6].
It is well known that some mycotoxins may induce the production of free radicals and/or the reduction of antioxidant defenses[7].In addition detrimental effects of mycotoxins on DNA,RNA and protein synthesis together with pro-apoptotic action further compromise important metabolic pathways,and consequently,changes in physiological functions including growth,development and reproduction occur[4].The impact of the mycotoxins on the immune system of exposed animals is a matter of concern because,by this way,these naturaloccurring toxins may predispose farm animals to the infectious diseases,which could result in economic losses for the livestock industry[8].
Carcinogenic and neurotoxic fumonisins,another class of fungal mycotoxins produced byFusariumspecies and other fungal species that ubiquitous in Nature,contaminate food, mainly corn(Zea mays L)and other grains throughout theWorld, represent a signifcant hazard to the food chain[9].Major fumonisin fungi species–mycotoxin associations are derived fromF.verticilliodes(formerly known asF.moniliforme)andF.proliferatum.Minor Fumonisin sources includeF.nygamai,F.napiforme,F.thapsinum,F.anthophilumandF.dlamini[10]. Fumonisin induced porcine pulmonary edema(PPE)is a wellestablished toxin specifc adverse effect[11],and fumonisin also has the potential to negatively impact the food and feed market due to contaminated grain[12].One of the initial events that occur in the target organs exposed to fumonisin is apoptosis, which might be a consequence of the inhibition of ceramide synthetase and alterations in sphingolipid metabolism. Fumonisin B(FB)evokes oxidative stress,which may contribute at least in part to FB toxicity and carcinogenicity[13].El-Nekeety et al.[14]demonstrated that FB administration enhanced lipid peroxidation as indicated by the signifcant increase in MDA level,which directly results to free radicalmediated toxicity.
Fumonisin B1(FB1),the most common and highest toxic of fumonisins species,is the focus of governments and scientists throughout the world due to the strong toxicity and potent carcinogenicity shown in animal studies[15].FB1 is a nephrotoxin in all species tested,a carcinogen in rodents and a reproductive toxicant in rodents and likely in humans[16]. It can induce apoptosisin vitroin different cultured cell lines andin vivoin liver and kidney of rodents,where production of proinfammatory cytokines such asTNFα and IFNγ is an important mediator of apoptosis[17].The Food and Drug Administration(FDA)has issued maximum residue limits in maize,maize byproducts in food and animal feeds,which are 2000–4000and5000–100,000 μg/kgtotalfumonisins (FB1+FB2+FB3)for human foods and animal feeds,respectively[18,19].
Probiotics have been proven to exert health promoting infuences in human and animals[20,21].Probiotics are effective compounds in the treatment and protection of some alimentary track infections as they alter the intestinal fora in favor of benefciary microorganisms.They are used to increase feed conversion as well as to decrease malnutrition and stress conditions[22].MostLactobacillalesare rather tolerant to H2O2. For exampleLactobacillus lactisIL1403,generates H2O2by NADH dehydrogenation but does not possess catalase for H2O2removal [23–25].Some of the benefcial properties of probiotics are related to their capacity to adhere to or bind different targets.L.rhamnosusGG strain(ATCC 53103)andL.rhamnosusLC-705 strain(DSM 7061)can bind toxic compounds,such as afatoxin B1[26],mutagens from food[27],or microcystin-LR[28]. Recently,manyin vitroandin vivoinvestigations indicated that probiotics can play an important role in the body’s natural processes of detoxifcation and elimination of FB1[29–31].The present study describes the ameliorating infuences ofLactobacillus delbrueckiisubsp.lactisDSM 20076 andPediococcus acidilacticiNNRL B-5627 on FB1-induced hepatotoxicity and nephrotoxicity in male rats fed with incremented doses of FB1-contaminated diets along an experimental period of 4-weeks.
2.Materials and methods
2.1.Chemicals
Ortho-dianisidine dihydrochloride,sodium tungestate,Tris-HCl,diphenylamine(DPA),and H2O2were purchased from Sigma Chemical Company(St.Louis,Mo,USA).5,5 dithiobis-25-nitrobenzoic acid(DNTB)and NADH were bought from MP Biomedicals,LLC,France.Napthylethyline diamine hydrochloride(NEDD)was purchased from Park Scientifc Limited, Northampton,UK.Phenazine methosulphate(PMS)was purchased from Lobachemie,USA.Nitro blue tetrazolium(NBT) was purchased fromAlliance Bio,USA.Thiobarbituric acid(TBA) was purchased from Acros,New Jersey,USA.Xylenol orange and sodium pyrophosphate were purchased from Alpha Chemika,Mumbai,India.Trichloroacetic acid was purchasedfrom SD Fine-Chem Limited,Mumbai,India.Fumonisin B1 was purchased from Cayman Chemicals Company,USA,dissolved in acetonitrile-water in the ratio 1:1(v/v)and kept at−20oC until used.All other chemicals and reagents were of analytical grade and highest quality available commercially.Corn samples were purchased from Egyptian local market.
2.2.Preparation of Fusarium-contaminated corn and probiotic cultures
Culture material ofFusarium moniliforme(EMF1)as FB1-producing isolate was prepared as previously described[32]and used in the experiment in order to study the protective infuence ofLactobacillus delbrueckiisubsp.lactisDSM 20076 andPediococcus acidilacticiNNRL B-5627 against fumonsin B1 produced in corn culture contaminated byF.moniliforme(EMF1)strain.This strain was previously isolated and assessed for its ability to produce FB1 toxin(GB accession number:KJ546424)[30].
Pediococcus acidilacticiNNRL B-5627(PA-NNRL)strain was obtained from the Northern Regional Research Laboratory(NRRL, Peoria,IL,USA)andLactobacillus delbrueckiisubsp.lactisDSM 20076(LL-DSM)strain was obtained from German Collection of Microorganisms and Cell Cultures(DSM),Braunschweig, Germany.Stock cultures of probiotics were maintained at−80°C on MRS medium with 25%(v/v)glycerol.To produce fresh cultures,the probiotic strains were propagated at 30°C for 14–16 h,then at 37°C for 14–16 h,before experimental use. Whenever needed,probiotics cultures were given orally by gavages at a daily dose of 1010CFU/ml for 4-weeks.
2.3.Animals and experimental design
All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals,National Institutes of Health(Institute of Laboratory Animal Resources,1996). Male Sprague-Dawley rats(weight 100–120 g)were obtained from the animal house of the Faculty ofVeterinary,Cairo University,Egypt.The rats were maintained at approximately 23–25°C with a 12 h light/dark cycle and received basal diet and tap waterad-libitumfor one week acclimation period.Eighty rats were housed in metal cages in which they chosen randomly then were divided into 12 experimental groups(6 or 7 rats each).The experimental design was conducted as shown in Table 1.
After 4-weeks,rats were anesthetized by sodium pentobarbital(4%,40 mg/kg),then kidney as well as liver were quickly removed and placed in chilled phosphate buffer,pH 7.4.The tissues were freed from adhering blood by repeated washing with the same buffer and then divided into two portions:one kept as a stock and the other used for preparation of tissue homogenates.
2.4.Preparation of liver and kidney homogenates
Liver and kidney homogenate were prepared using the method determined by Guidet and Shah[33].One gram of tissue was washed in ice cold isotonic saline containing 1 mM EDTA.The tissues were then homogenized separately in 8 ml of cold buffer (50 mM potassium phosphate buffer,pH 7.4,containing 1 mM EDTA and 1 mM 2-mercaptoethanol)using a Potter-Elvejham homogenizer at 4°C.The crude tissue homogenate was then centrifuged at 8000×gfor 15 min at 4°C and the supernatant was removed and kept at−80°C for estimation of malondialdehyde(MDA),reduced glutathione(GSH),superoxide dismutase(SOD)and nitric oxide(NO),total oxidative statues (TOS),total antioxidant capacity(TAC)and oxidative stress index (OSI).The protein concentration of the clarifed homogenate was estimated according to the method of Lowry et al.[34].
2.5.Markers of oxidative stress and antioxidant capacity
2.5.1.Determination of lipid peroxidation in terms of malonaldehyde(MDA)
The lipid peroxidation was measured byTBARS formation[35]. The reaction mixture contained 0.1 ml tissue homogenate, 0.2 ml 8.1%sodium dodecyl sulphate(SDS),1.5 ml 20%acetic acid(pH 3.5 adjusted with 1 Ν NaOH)and 1.5 ml 0.8%aqueous solution of thiobarbituric acid(TBA).The mixture was fnally made up to 4.0 ml with distilled water,then heated at 95°C for 30 min on a water bath.After cooling under tap water,1.0 ml of distilled water and 5.0 ml of a mixture of n-butanol and pyridine(15:1 v/v)were added and then the mixture was shakenvigorously on a vortex mixer.After centrifugation at 2200×gfor 5 min the absorbance of the organic layer was measured immediately at 535 nm.MDA concentration was calculated:

Table 1–The experimental design of rat treatments along 4-weeks exposure to Fumonisin B1 and/or probiotic lactic acid bacteria.
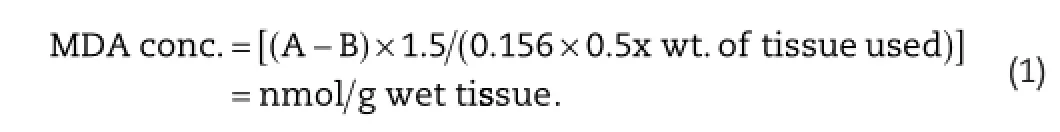
where:A,the absorbance of sample;B,the absorbance of blank; 1.5,the total volume used for measurement;0.156,the absorbance for 1 nM solution of MDA measured in 1 cm thick cell at 535 nm;0.5,the used volume of tissue(liver or kidney) homogenate.
2.5.2.Determination of superoxide dismutase(SOD)activityMeasurement superoxide radical scavenging activity was carried out following a standard method[36].The reaction mixture contained 1 ml of nitro blue tetrazolium(NBT)solution(312 μM prepared in phosphate buffer,pH 7.4),1 ml NADH solution (936 μM prepared in phosphate buffer,pH 7.4).Standardized 50 times methanol diluted different extracts of the samples were then added.Finally,reaction was accelerated by adding 100 μl phenazine methosulphate(PMS)solution(120 μM prepared in phosphate buffer,pH 7.4)to the mixture.The reaction mixture was incubated at 25°C for 5 min and absorbance at 560 nm was measured against methanol as control.Percentage of inhibition was calculated:
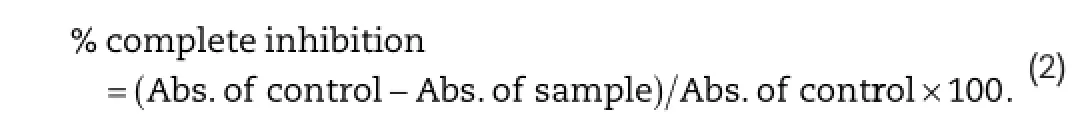
2.5.3.Determination of reduced glutathione(GSH)content
Reduced glutathione(GSH)was assayed by the method of Prins and Loose[37].The method utilized metaphosphoric acid for protein precipitation and 5,5-dithiobis(2-nitrobenzoic acid) (DTNB)for color development.The absorbance was measured at 412 nm.
2.5.4.Nitric oxide(NO)scavenging activity
Nitric oxide generated from sodium nitroprusside in aqueous solution at physiological pH interacts with oxygen to produce nitrite ions,which were measured by Griess reaction[38,39]. The reaction mixture(3 ml)containing sodium nitroprusside (10 mM)in phosphate buffer saline and the tested extracts(10, 25,50 or 100 μg/ml)was incubated at 25°C for 150 min.After incubation,1.5 ml of the reaction mixture was removed and 1.5 ml of the Griss reagent(1%sulphanilamide,2%orthophosphoric acid and 0.1%napthylethyline diamine hydrochloride) was added.The absorbance of the chromophore formed was read at 546 nm.A decrease in absorbance indicated a high scavenging activity[40].
2.5.5.Measurement of total antioxidant capacity(TAC)
Total antioxidant capacity(TAC)of liver and kidney homogenates was determined using an automated measurement method as previously described[41].In brief,200 μl of Reagent 1[(o-dianisidine(10 mM),ferrous ion(45 mM)in the Clark and Lubs solution(75 mM,pH 1.8)]was mixed with 10μl Reagent 2[H2O2(7.5 mM)in the Clark and Lubs solution].Then 5 μl of the sample was added.Where,Clark and Lubs solution(75 mM,pH 1.8)was prepared by dissolving 5.591 g of KCl in 1000 ml of deionized water(fnal concentration,75 mM)and diluting 6.41 ml hydrochloric acid(36.5%)to 1000 ml with deionized water(fnal concentration,75 mM).The prepared KCl solution(800 ml)was mixed with 200 ml of HCl solution under pH meter(fnal pH 1.8).The absorbance was measured at 412 nm.The results are expressed as mM Trolox equivalent/l. The assay has excellent precision with coeffcients of variation less than 3%.
2.5.6.Measurement of total oxidant status(TOS)
Total oxidant status(TOS)of liver and kidney homogenates was determined using an automated measurement method as previously described[42].In brief,225 μl solution of reagent 1 (xylenol orange 150 μM,NaCl 140 mM and glycerol 1.35 M in 25 mM H2SO4solution,pH 1.75)was mixed with 11 μl reagent 2(ferrous ion 5 mM and o-dianisidine 10 mM in 25 mM H2SO4solution).Then 35 μl of the sample was added.The absorbance was measured at 560 nm.The color intensity,which could be measured spectrophotometrically,was related to the total amount of oxidant molecules present in the sample.The assay was calibrated with hydrogen peroxide and the results were expressed in terms of μM H2O2equivalent/l.
2.5.7.Determination of oxidative stress index(OSI)
The ratio of TOS to TAC was accepted as the oxidative stress index(OSI).For calculation,the resulting unit of TAC was changed to mM,and the OSI value(Arbitrary Unit)was calculated according to a formula stated by Horoz et al.[43]:

2.6.Apoptosis assay:quantifcation of fragmented DNA using diphenylamine(DPA)
DPA assay was conducted following the method of Gibb et al. [44].DPA solution was prepared by dissolving 150 mg DPA in 10 ml glacial acetic acid,150 ml of sulfuric acid and 50 ml acetaldehyde(16 mg/ml).The tissues of livers and kidneys were prepared in 0.5 ml of lyses buffer containing 10 mM tris-HCl (pH 8),1 mM EDTA,0.2%triton X-100,centrifuged at 10,000×gfor 20 min at 4°C.The pellets were re-suspended in 0.5 ml of lyses buffer.Half ml of 25%trichloroacetic acid(TCA)was added to the pellets(P)and the supernatants(S)then incubated at 4°C for 24 h.The samples were centrifuged for 20 min at 10,000×gat 4°C and the pellets were suspended in 80 ml of 5%TCA,followed by incubation at 83°C for 20 min.Subsequently,160 ml DPA solution was added to each sample and incubated at room temperature for 24 h.The proportion of fragmented DNA was calculated from absorbance reading at 600 nm using the formula:
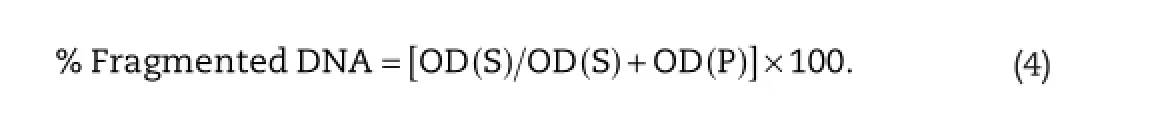
2.7.Statistical analysis
Data from these studies were obtained from a minimum of six independent experiments(n=6,for each treatment),and assessed by a one-way ANOVA followed by either Tukey Kramermultiple comparisons test or Bonferroni’s post hoc test using SPSS computer program software,version 20.All data were analyzed through a completely randomized design(CRD). Differences were considered signifcant at theP≤0.05 level.

Table 2–Values of MDA and GSH levels in liver and kidney of rats treated with FB1 and/or probiotic LAB.
3.Results
3.1.Markers of oxidative stress and antioxidant capacity
The oxidative stress played a role in FB1 hepatotoxicity and nephrotoxicity.The data show that FB1 treatment signifcantly increased the production of MDA,NO and TOS starting from the concentration of 50 mg/kg diet accompanied with a signifcant decrease in SOD,GSH,and TAC.FB1-induced lipid peroxidation was measured as an increase in the concentration of MDA in both liver and kidney tissues(Table 2).In FB1-treated rats,the level of MDA in liver and kidney tissues were signifcantly increased compared to untreated and probiotics supplemented groups atP<0.05.Increased MDA concentrations were observed in T50,T100,T200 groups already after a 4-weeks treatment with FB1 by 1.97,3.81 and 4.67,respectively in liver tissues and by 1.90,2.95 and 4.09,respectively in kidney tissues.On the other hand,rats received either LLDSM or PA-NNRL,showed a notable reduction in MDA levels when compared to controlP>0.05.Furthermore,coadministration of LL-DSM or PA-NNRL along with FB1 resulted in a signifcant reduction in MDA levels in both liver and kidney tissues compared to FB1-treated groups atP<0.05.In this groups,the levels of MDA in liver and kidney tissues were signifcantly higher than that of control group(P<0.05),exception in T50-LL and T50-PA which were normalized to control level.
Regarding GSH content,rats given either LL-DSM or PANNRL exhibited an increase in GSH content as compared to control,which was signifcant atP<0.05 in case of liver and kidney tissues(Table 2).GSH was markedly low in liver tissues of rats groups T50,T100 and T200,by 3.33,26.7 and 65.6%respectively,and by 0.96,32 and 68%in kidney tissues in comparison with control;this reduction was statistically signifcant atP<0.05.A signifcant increase in GSH level was shown in rats received FB1 and co-administrated with LLDSM or PA-NNRL when compared with FB1 groups atP<0.05. This enhancement was signifcantly higher than that of control with an exception inT200-LL andT200-PA groups,which were just normalized to control level.
As shown in Table 3,FB1 toxicity(T50,T100 and T200)signifcantly decreased activities of SOD enzyme in liver and kidney tissues,as compared to control group atP<0.05.However,the activity of SOD in FB1 groups’co-administrated with LL-DSM or PA-NNRL was signifcantly increased as compared to the FB1 groups.This increase in the protective groups T50-LL,T50-PA, T100-LL and T100-PA was still signifcantly higher or equal to that of control atP<0.05.In addition,we observed a higher elevation in SOD activity of the liver tissues response to probiotics in protective groups(T200-LL:0.29+0.070;T200-PA:0.35+0.037)in relation to infected groupT200(0.14+0.049). Besides,high elevation in SOD activity of the kidney tissues response to probiotics in protective groups(T200-LL:0.25+0.09; T200-PA:0.33+0.02)in comparison to infected group T200 (0.17+0.01)was also observed.Meanwhile,liver and kidney tissues of rat groups LL-DSM and PA-NNRL showed a signifcant enhancement in SOD activity atP<0.05 compared to those of the control group.
A positive correlation was observed between increasing concentrations of FB1 groups and nitric oxide levels in both liver and kidney tissues.As shown in Table 2,liver tissues of FB1-treated groups(T50,T100 andT200)have recorded a signifcant elevation of NO levels,by 25%,69.2%and 151.2%respectively; however,NO recorded lesser values in kidney tissues by 19.5%, 63.4%and 134%respectively.The administration of either LLDSM or PA-NNRL along with FB1 succeeded to decrease NO levels in both liver and kidney tissues but still their levels were higher than that of the untreated group.Furthermore,no differences were detected in NO levels in LL-DSM and PA-NNRL groups compared to control group.
TAC,TOS levels and OSI in FB1-treated groups,protective groups and controls are shown in Table 4.TOS and OSI were signifcantly higher in FB1-treated rats than control atP<0.05, while TAC was signifcantly lower(P<0.05).A signifcant and positive correlation was observed between TAC and adminis-tration of either LL-DSM or PA-NNRL(P<0.05).On the contrary, TOS level and OSI were negatively correlated with probiotic treatments(P<0.05).In addition,liver tissues ofT100 andT200 groups have recorded signifcant elevations inTOS by 60.82 and 130.72%,respectively.However,in kidney tissues the elevation reached 90.13 and 145.50%,respectively,compared to those of control group.Such oxidative modifcation is an index of oxidative stress and these abnormalities were prevented by supplementation of probiotics.It was,also found that rats administrated either LL-NNRL or PA-DSM along with FB1 doses showed an increase in the antioxidant capacity.Moreover,this capacity decreased when the oxidative stress increased,as revealed by the higher OSI values in rats treated with FB1.The overall data indicated that LL-NNRL and PA-DSM have a broad range of bio-modulatory properties,which alleviated the FB1-oxidative stress and improved their antioxidant capacity.

Table 3–Values of SOD and NO activity in liver and kidney of rats treated with FB1 and/or probiotic LAB.
3.2.DPA assay(DNA fragmentation)
FB1-induced apoptosis in liver and kidney tissues was evaluated by determination of genomic DNA fragmentation using the DPA assay.As shown in Figs 1 and 2,treatment with FB1 triggered a marked increase in the percentage of DNA fragmentation in liver and kidney tissues compared to that in the untreated rats.It was found that FB1 induced a dose-dependent genomic DNA fragmentation.The percentage of DNA fragmentation was more pronounced in rats treated with the higher dose of FB1(200 mg/kg diet)compared to the other treated groups:T50 and T100.The percentage of genomic DNA fragmentation in FB1-treated groups(T50,T100 andT200)recorded 18,22.5 and 37.40%,respectively,in liver tissues(Fig.1),while in the kidney tissues(Fig.2),recorded 15.25,20.5 and 28.8%, respectively.Moreover,the data revealed that liver was more sensitive to FB1 toxicity than kidney.DNA fragmentation in LLDSM and PA-NNRL groups was comparable to that of the control group in both liver and kidney tissues.However,those treated with FB1 and co-administrated with LL-DSM or PA-NNRL showed a decrease in the percentage of DNA fragmentation in liver and kidney tissues compared to FB1-treated groups. This reduction was comparable to that of control group,except of T200-LL and T200-PA groups,where it remains signifcantly high atP≤0.05.Furthermore,treatment with LL-DSM or PA-NNRL signifcantly brought down the percentage of the DNA damage in liver tissues of theT200-LL andT200-PA groups to 25.05 and 23.4%respectively,while the levels of reduction in the kidney tissues were 22.3 and 21.5%,respectively.As overall,rat groups treated with FB1 and co-administrated orally by PA-NNRL presented much reduction in DNA fragmentation compared to LL-DSM.
4.Discussion
Authorization of probiotics for use in pharmaceutical products and food supplements,as there is an increase demand for natural products as alternative medicines,focuses the attention to verify the ability of these bacteria to reduce pathogens and toxins adhesion to surfaces.Thus,our study focused on the protective role ofP.acidilacticiNNRL B-5627 andLb.delbrueckiisubsp.lactisDSM 20076 against the oxidative stress and apoptosis of FB1 toxicity in male rats.Fumonisins is well known to disrupt sphingolipid metabolism,altering the cell membrane and causing cytotoxicity[45],while FB1 is reported to induce lipid peroxidation,which may affect DNA integrity, leading to DNA oxidized bases[46,47].
In this study,treatment of male rats with FB1 resulted in a signifcant increase in lipid peroxidation marker(MDA)and decrease in GSH content and SOD activity in both liver and kidney tissues.Similarly,several reports indicated that FB1 increased lipid peroxidation[48,49]and the production of reactive oxygen species(ROS)in animal models or exposed cells[14,50]. Recently,Hassan et al.[51]showed that the levels of lipid peroxidation as an oxidative stress marker induced in liver and kidney were signifcantly increased while the levels of antioxidant GSH were signifcantly decreased in FB1-treated groups compared to their levels in the normal controls.
FBs inducing hepatotoxicity by their oxidative damage effects have been observed in few studiesin vitroandin vivoat high doses,such as in rats fed high dose of 250 mg FB1/kg diet for21 d[52],rats fed 0.08–0.16 mg FB1/100g b.w/d for 2 years[53], rats injected IP with 0.5 mg FB1/kg b.w/d for 7 d[54],and also in cell culture[55].In contrary to our results,FBs failure at low dose to increase oxidative damages parameters was reported in ducks,which received 45 mg FB1/kg b.w.by daily oral administration over 12 d[56],and other animal species,such as rats,which consumed contaminated diet at level of 10 mg FB1/ kg diet for 21 d[52].Although our results were in concordance with the studies mentioned above with respect to the increase in oxidative stress,this would be the frst study on oxidation capacity of FB1 subjects as determined using measurement of TOS along with measurement of TAC level and calculation of OSI.
On the other hand,nitric oxide(NO)was defned as an important chemical mediator generated by endothelial cells, macrophages,neurons,etc.and was involved in the regulation of various physiological processes.Excess concentration of NO is associated with several diseases.Oxygen reacts with the excess NO to generate nitrite and peroxynitrite anions, which act as free radicals[57].In our study,levels of NO in liver and kidney tissues were signifcantly increased in FB1-treated rats compared to both untreated and probiotic supplemented groups atP<0.05.In another study,it was demonstrated that pretreatment with FB signifcantly enhanced NO release by both resident untreated and IFN-g-activated macrophages,suggesting the potential involvement of FB in promoting an infammatory response[58].
Numerousin vitro,in vivo,human,and epidemiological studies have provided evidence of the chemopreventive effects of LAB.These effects actviadiverse mechanisms,including alteration of the gastrointestinal microfora,enhancement of the host’s immune response,and antioxidative and antiproliferative activities[59].Kaizu et al.[60]found thatLactobacillussp.SBT 2028 exerted the strongest antioxidant effects out of 570 strains of LAB.Lin and Yen[61]demonstrated that 19 strains of LAB exhibited antioxidant activities of 7–12%in intracellular cellfree extracts,which was due to their metal-ion-chelating and ROS-scavenging abilities.Lactobacillus acidophilusandBifdobacterium longuminhibit lipid peroxidation,as demonstrated by two methods in which linoleic acid and the cell membrane of osteoblasts were used for lipid peroxidation.Both intact-cell and intracellular-cell-free extracts exerted antioxidant activities[62].Heat-killed cells ofLactobacillus acidophilus606 also exert antiproliferative activity[63],which is due to the soluble polysaccharide fraction;this fraction also exhibits potent antioxidant activity.Although,this study observed that inLactococcus lactiseven in a wild-type strain and mutant KatE could contribute to limiting the DNA degradation[64].In view of the benefcial effects of probiotics mentioned above,we designed the experimental procedure.
In response to probiotics treatment,our results showed that the administration of LL-DSM or PA-NNRL to FB1-intoxicated rats ameliorated the induced oxidative stress comparable to control,where MDA,NO,TOS and OSI decreased,along with increased SOD activity GSH andTAC contents.Zoghi et al.[65] reported that probiotic LAB have a signifcant potential for inactivation of toxins by surface binding due to great adhesive properties of S-layer proteins in their cell wall.Deabes et al. [66]also reported that mice receivedLactobacillus rhamnosusstrain GG(ATCC 53013)before afatoxins gavage,and showeda signifcant amelioration in oxidative status in both liver and kidney,by increasing the contents of GSH and SOD activity.

?
Apoptosis is a specialized process of cell death that is part of the normal development of organs and tissue maintenance,and may also occur as a response to various environmental stimuli,indicating toxicity.Since apoptosis can play a critical role in the development of cancer,the ability of toxins to induce apoptosis appears to be related to their toxicological effects[67].Regarding genotoxicity our results clearly showed that FB1 has cytotoxic effect to liver and kidney tissues along a genotoxic effect,causing a DNA fragmentation in both tissues.A previous study in our lab also reported that administration of FB1 resulted in excessive malfunctions in liver and kidney with concomitant increase in DNA damage of rat blood plasma tested[31].Omar[68]describedin vivoFB1 increases oxidative damage of the DNA,as measured by increased breaks of its strands and MDA adducts in rat liver and kidney.Another action mechanism of FB1 involves the disruption of thede novosphingolipid biosynthesis pathway by inhibition of the enzyme ceramide synthase.The inhibition of sphingolipid biosynthesis disrupts numerous cell functions and signaling pathways, including apoptosis and mitosis,thus potentially contributing to carcinogenesis through an altered balance of cell deathand replication[68].Furthermore,Domijan[69]suggested that FB1 has different effects on cell death/cell growth from proapoptotic and growth inhibitory to anti-apoptotic and growthstimulatory.A previous study hypothesized that the TNF/Fas pathway involved with FB1-induced apoptosis and the induction of TNF-like pathway in FB1-treated cells has relevance to its toxic and carcinogenic properties[70].FB1 also causes DNA damage and induces caspase-3 activity,suggesting a role in a cascade of events leading to apoptosis,in rat astrocytes[50]. In agreement with the previous studies,our fndings indicate thein vivoFB1-induced apoptotic DNA fragmentation in liver and kidney tissues,which was restored by probiotics supplementation.
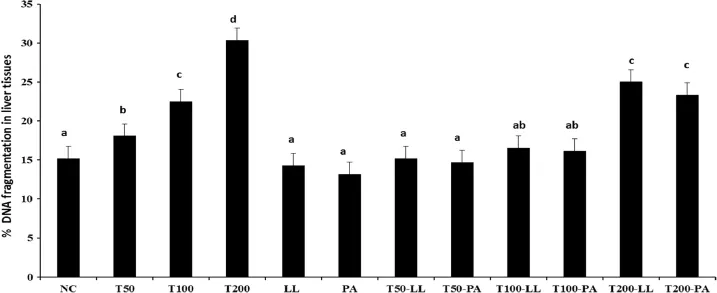
Fig.1–The effect of probiotic lactic acid bacteria on FB1-induced DNA damage in rat liver exposedin vivo.The level of DNA strand breaks is expressed as the percentage of DNA in the supernatant.Values are expressed as means±SD of six replicates.Statistical analysis is carried out using one way analysis of variance(ANOVA)by SPSS program,V 20.Means with the same superscript letters are not signifcantly different(P≤0.05).

Fig.2–The effect of probiotic lactic acid bacteria on FB1-induced DNA damage in rat kidney exposedin vivo.The level of DNA strand breaks is expressed as the percentage of DNA in the supernatant.Values are expressed as means±SD of six replicates.Statistical analysis is carried out using one way analysis of variance(ANOVA)by SPSS program,V 20.Means with the same superscript letters are not signifcantly different(P≤0.05).
5.Conclusion
Our results confrmed that the liver and kidney as the target organs in FB1-induced rat toxicosis and indicated that exposure to FB1 would generate free radicals,which resulted in the elevation of hepatic and renal lipid peroxidation along with a reduction in the antioxidant enzymes such as SOD and GSH content.In addition,FB1 caused severe apoptotic effects assets by increasing the genomic DNA fragmentation in both liver and kidney tissues.In addition,the current results indicate that oral administration of LL-DSM or PA-NNRL cultures to rats signifcantly attenuates FB1-induced toxicity by means of preventing oxidative stress,recovering nitric oxide content to the control level,and maintaining GSH content,as well as a stable activity of SOD,and a protection against FB1-induced DNA fragmentation.A probiotic antioxidant strain itself was safe at the tested doses and it may be a candidate for the prevention as well as treatment of liver and kidney diseases.It restores the different parameters tested.The protective effects ofP. acidilacticiNNRL B-5627 andLb.delbrueckiisubsp.lactisDSM 20076, which may be due to the ability to eliminate ROS in the digestive tract of animals and humans,could have applications for treatment of infammatory diseases or post-cancer drug treatments.Consequently,probiotic lactic acid bacteria are relatively useful and reasonable for treatment of FB1 toxicity.Thus, further studies would be necessary to assess the protective role of these probiotic bacteria after FB1 intoxication of rats.
Acknowledgments
This work was supported byThe City of Scientifc Research and Technological Applications(SRTA-City)at Bourg Elarab,Egypt. Special thanks to the Scientists New Generation(SNG)Program (Grant No.CG017)funded by The Egyptian Academy of Scientifc Research and Technology(ASRT)(CG 017).
R E F E R E N C E S
[1]Valko M,Leibfritz D,Moncol J,et al.Free radicals and antioxidants in normal physiological functions and human disease.Int J Biochem Cell Biol 2007;39:44–84.
[2]Wu D,Cederbaum AI.Alcohol,oxidative stress,and free radical damage.Alcohol Res Health 2003;27:277–284.
[3]Avery S.Molecular targets of oxidative stress.Biochem J 2011;434:201–210.
[4]Doi K,Uetsuka K.Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int J Mol Sci 2011;12:5213–5237.
[5]Ribeiro DH,Ferreira FL,Da Silva VN,et al.Effects of afatoxin B1 and fumonisin B1 on the viability and induction of apoptosis in rat primary hepatocytes.Int J Mol Sci 2010;11:1944–1955.
[6]Domijan AM,Peraica M.Determination of 8-hydroxy-2’deoxyguanosine in urine using HPLC with electrochemical detection.Arch Indust Hygiene Toxicol 2008;59:277–282.
[7]Bernabucci U,Colavecchia L,Danieli PP,et al.Afatoxin B1 and fumonisin B1 affect the oxidative status of bovine peripheral blood mononuclear cells.Toxicol in Vitro 2011;25:684–691.
[8]Fink-Gremmels J.The role of mycotoxins in the health and performance of dairy cows.Vet J 2008;176:84–92.
[9]Friedman M,Rasooly R.Review of the inhibition of biological activities of food-related selected toxins by natural compounds.Toxins 2013;5:743–775.
[10]Frisvad JC,Thrane U,Samson RA,et al.Important mycotoxins and the fungi which produce them.In:Hocking AD,Pitt JI,Samson RA,Thrane U,editors.Advances in food mycology.New York:Springer;2006.p.3–31.
[11]Haschek WM,Gumprecht LA,Smith G,et al.Fumonisin toxicosis in swine:an overview of porcine pulmonary edema and current perspectives.Environ Health Persp 2001;109(Suppl.2):251.
[12]Wu F,Munkvold GP.Mycotoxins in ethanol co-products: modeling economic impacts on the livestock industry and management strategies.J Agi Food Chem 2008;56:3900–3911.
[13]Stockmann-Juvala H,Mikkola J,Naarala J,et al.Oxidative stress induced by fumonisin B1 in continuous human and rodent neural cell cultures.Free Rad Res 2004;38:933–942.
[14]El-Nekeety AA,El-Kholy W,Abbas NF,et al.Effcacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon 2007;50:256–269.
[15]US Food and Drug Agency(FDA).Background Paper in Support of Fumonisin Levels in Animal Feeds.(Draft) Guidance for Industry:Fumonisin Levels in Human Foods and Animal Feeds;availability.In:Fed.Regist.Washington, DC:2002;65:p.35945.Available from:http://www.fda.gov/ food/guidanceregulation/guidancedocumentsregulatory information/ucm109231.htm.
[16]Bolger M,Coker R,DiNovi M,et al.Fumonisins.WHO/IPCS safety evaluation of certain mycotoxins in food.WHO Food Addit Ser 2001;47:557–680.
[17]Sharma N,Suzuki H,He Q,et al.Tumor necrosis factor α-mediated activation of c-Jun NH2-terminal kinase as a mechanism for fumonisin B1 induced apoptosis in murine primary hepatocytes.J Biochem Mol Toxicol 2006;19:359–367.
[18]Hussein HS,Brasel JM.Toxicity,metabolism,and impact of mycotoxins on humans and animals.Toxicol 2001;167:101–134.
[19]Zain ME.Impact of mycotoxins on humans and animals.J Saudi Chem Soc 2011;15:129–144.
[20]Ouwehand AC,Salminen S,Isolauri E.Probiotics:an overview of benefcial effects.Antonie Van Leeuwenhoek 2002;82:279–289.
[21]Saxelin M,Tynkkynen S,Mattila-Sandholm T,et al. Probiotic and other functional microbes:from markets to mechanisms.Current Opinion Biotech 2005;16:204–211.
[22]Cenesiz S,Yaman H,Ozcan A,et al.Effects of kefr as a probiotic on serum cholesterol,total lipid,aspartate aminotransferase and alanine amino transferase activities in broiler chicks.Med Weter 2008;64:168.
[23]Marty-Teysset C,De La Torre F,Garel JR.Increased production of hydrogen peroxide byLactobacillus delbrueckiisubsp.bulgaricusupon aeration:involvement of an NADH oxidase in oxidative stress.Appl Environ Microbiol 2000;66:262–267.
[24]Bolotin A,Wincker P,Mauger S,et al.The complete genome sequence of the lactic acid bacteriumLactococcus lactisssp.lactisIL1403.Gen Res 2001;11:731–753.
[25]Rochat T,Gratadoux JJ,Gruss A,et al.Production of a heterologous nonheme catalase byLactobacillus casei:an effcient tool for removal of H2O2and protection ofLactobacillus bulgaricusfrom oxidative stress in milk.Appl Environ Microbiol 2006;72:5143–5149.
[26]Haskard CA,El-Nezami HS,Kankaanpää PE,et al.Surface binding of afatoxin B1 by lactic acid bacteria.Appl Environ Microbiol 2001;67:3086–3091.
[27]Turbic A,Ahokas J,Haskard C.Selectivein vitrobinding of dietary mutagens,individually or in combination,by lactic acid bacteria.Food Addit Contam 2002;19:144–152.
[28]Halttunen T,Salminen S,Tahvonen R.Rapid removal of lead and cadmium from water by specifc lactic acid bacteria.Int J Food Microbiol 2007;114:30–35.
[29]Niderkorn V,Morgavi D,Aboab B,et al.Cell wall component and mycotoxin moieties involved in the binding of fumonisin B1 and B2 by lactic acid bacteria.J Appl Microbiol 2009;106:977–985.
[30]Khalil AA,Abou-Gabal AE,Elfaramawy AM,et al.Lactic acid bacteria as antimycotic and antimycotoxins agents against toxigenic fusarium species associated to maize grains stored in Egyptian markets.J Pure Appl Microbiol 2013;7(Spl. Edn.):93–105.
[31]Khalil AA,Abou-Gabal AE,Abdellatef AA,et al.Protective role of probiotic lactic acid bacteria against dietary fumonisin B1-induced toxicity and DNA-fragmentation in Sprague-Dawley Rats.Prep Biochem Biotechnol 2015;45:530–550.
[32]Ross P,Nelson P,Richard J,et al.Production of fumonisins by Fusarium moniliforme andFusarium proliferatumisolates associated with equine leukoencephalomalacia and a pulmonary edema syndrome in swine.Appl Environ Microbiol 1990;56:3225–3226.
[33]Guidet BR,Shah SV.In vivogeneration of hydrogen peroxide by rat kidney cortex and glomeruli.Am J Physiol 1989;256:F158–F164.
[34]Lowry OH,Rosebrough NJ,Farr AL,et al.Protein measurement with the Folin phenol reagent.J Biol Chem 1951;193:265–275.
[35]Ohkawa H,Ohishi N,Yagi K.Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction.Anal Biochem 1979;95:351–358.
[36]Nishikimi M,Appaji Rao N,Yagi K.The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen.Biochem Biophysic Res Commun 1972;46:849–854.
[37]Prins HK,Loose JA.Glutathione“chapter 4”Biochemical methods in red cell genetics.edited by J.Yanis,NYD London: Academic Press;1969.p.126–129.
[38]Green LC,Wagner DA,Glogowski J,et al.Analysis of nitrate, nitrite,and[15 N]nitrate in biological fuids.Anal Biochem 1982;126:131–138.
[39]Marcocci C,Golia F,Bruno-Bossio G,et al.Carefully monitored levothyroxine suppressive therapy is not associated with bone loss in premenopausal women.J Clin Endocrinol Met 1994;78:818–823.
[40]Green DT,Bolanos H,Alesi DE,et al.,Apparatus and method for placing staples in laparoscopic or endoscopic procedures.1994,Google Patents.
[41]Erel O.A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 2004;37:112–119.
[42]Erel O.A new automated colorimetric method for measuring total oxidant status.Clin Biochem 2005;38:1103–1111.
[43]Horoz M,Bolukbas C,Bolukbas FF,et al.Oxidative stress in hepatitis C infected end-stage renal disease subjects.BMC Infec Dis 2006;6:114.
[44]Gibb RK,Taylor DD,Wan T,et al.Apoptosis as a measure of chemosensitivity to cisplatin and taxol therapy in ovarian cancer cell lines.Gynecol Oncol 1997;65:13–22.
[45]Riley R,Voss K,Norred W,et al.Fumonisins:mechanism of mycotoxicity.Rev Med Vet-Toulouse 1998;6:617–626.
[46]Mobio TA,Anane R,Baudrimont I,et al.Epigenetic properties of fumonisin B 1:cell cycle arrest and DNA base modifcation in C6 glioma cells.Toxicol Appl Pharmacol 2000;164:91–96.
[47]Mobio TA,Tavan E,Baudrimont I,et al.Comparative study of the toxic effects of fumonisin B 1 in rat C6 glioma cells and p53-null mouse embryo fbroblasts.Toxicol 2003;183:65–75.
[48]Stockmann-Juvala H,Mikkola J,Naarala J,et al.Fumonisin B 1-induced toxicity and oxidative damage in U-118MG glioblastoma cells.Toxicol 2004;202:173–183.
[49]Klaric´MŠ,Pepeljnjak S,Domijan AM,et al.Lipid peroxidation and glutathione levels in porcine kidney PK15 cells after individual and combined treatment with fumonisin B1,beauvericin and ochratoxin A.Basic Clin Pharmacol Toxicol 2007;100:157–164.
[50]Galvano F,Campisi A,Russo A,et al.DNA damage in astrocytes exposed to fumonisin B1.Neurochem Res 2002;27:345–351.
[51]Hassan AM,Abdel-Aziem SH,El-Nekeety AA,et al.Panax ginseng extract modulates oxidative stress,DNA fragmentation and up-regulate gene expression in rats sub chronically treated with afatoxin B1 and fumonisin B1. Cytotechnol 2014;67:1–11.
[52]Abel S,Gelderblom W.Oxidative damage and fumonisin B 1-induced toxicity in primary rat hepatocytes and rat liverin vivo.Toxicol 1998;131:121–131.
[53]Gelderblom W,Abel S,Smuts CM,et al.Fumonisin-induced hepatocarcinogenesis:mechanisms related to cancer initiation and promotion.Environ Health Persp 2001;109(Suppl.2):291.
[54]Domijan A-M,Želježic´D,Milic´M,et al.Fumonisin B 1: oxidative status and DNA damage in rats.Toxicol 2007;232:163–169.
[55]Abado-Becognee K,Mobio TA,Ennamany R,et al. Cytotoxicity of fumonisin B1:implication of lipid peroxidation and inhibition of protein and DNA syntheses. Arch Toxicol 1998;72:233–236.
[56]Bailly J,Benard G,Jouglar J-Y,et al.Toxicity of Fusarium moniliforme culture material containing known levels of fumonisin B1 in ducks.Toxicol 2001;163:11–22.
[57]Hemnani T,Parihar M.Reactive oxygen species and oxidative DNA damage.Indian J Physiol Pharm 1998;42:440–452.
[58]Dombrink-Kurtzman MA,Gomez-Flores R,Weber RJ. Activation of rat splenic macrophage and lymphocyte functions by fumonisin B 1.Immunopharmacol 2000;49:401–409.
[59]Kim J-E,Kim JY,Lee KW,et al.Cancer chemopreventive effects of lactic acid bacteria.J Microbiol Biotechnol 2007;17:1227–1235.
[60]Kaizu H,Sasaki M,Nakajima H,et al.Effect of antioxidative lactic acid bacteria on rats fed a diet defcient in vitamin E.J Dairy Sci 1993;76:2493–2499.
[61]Lin M-Y,Yen C-L.Antioxidative ability of lactic acid bacteria. J Agri Food Chem 1999;47:1460–1466.
[62]Lin M-Y,Chang F-J.Antioxidative effect of intestinal bacteria Bifdobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356.Dig Dis Sci 2000;45:1617–1622.
[63]Choi S,Kim Y,Han K,et al.Effects ofLactobacillusstrains on cancer cell proliferation and oxidative stressin vitro.Letters Appl Microbiol 2006;42:452–458.
[64]Rochat T,Miyoshi A,Gratadoux J,et al.High-level resistance to oxidative stress inLactococcus lactisconferred byBacillus subtiliscatalase KatE.Microbiol 2005;151:3011–3018.
[65]Zoghi A,Khosravi-Darani K,Sohrabvandi S.Surface binding of toxins and heavy metals by probiotics.Mini Reviews Medicinal Chem 2014;14:84–98.
[66]Deabes M,Darwish HR,Abdel-Aziz KB,et al.Protective effects ofLactobacillus rhamnosusGG on afatoxins-induced toxicities in male albino mice.J Environ Anal Toxicol 2012;2:132.
[67]Dragan YP,Bidlack WR,Cohen SM,et al.Implications of apoptosis for toxicity,carcinogenicity,and risk assessment: fumonisin B1 as an example.Toxicol Sci 2001;61:6–17.
[68]Omar HE.Mycotoxins-Induced Oxidative Stress and Disease In Mycotoxin and Food Safety in Developing Countries.In: Makun HA,editor.Mycotoxin and Food Safety in Developing Countries.Rijeka,Croatia:InTech;2013.p.63–92.
[69]Domijan AM.Fumonisin B1:a Neurotoxic Mycotoxin/ Fumonizin B1:Neurotoksicˇni Mikotoksin.Arch Indust Hygiene Toxicol 2012;63:531–544.
[70]Jones C,Ciacci-Zanella JR,Zhang Y,et al.Analysis of fumonisin B1-induced apoptosis.Environ Health Perspect 2001;109(Suppl.2):315.
*< class="emphasis_italic">Corresponding author.
.Department of Protein Technology,Institute of Genetic Engineering and Biotechnology,City of Scientifc Research and Technological Applications,Borg El-Arab,Alexandria,Egypt.Tel.:+203-459-9384;fax:+203-459-9384.
E-mail address:ashraf_khalil@msn.com(A.A.Khalil).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2016.02.006
1818-0876/©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Validation and application of Vierordt’s spectrophotometric method for simultaneous estimation of tamoxifen/coenzyme Q10 in their binary mixture and pharmaceutical dosage forms
- Introduction of antineoplastic drug NSC631570 in an inpatient and outpatient setting: Comparative evaluation of biological effects
- Control of autoimmune arthritis by herbal extracts and their bioactive components
- The 1st Euro-Mediterranean Workshop:Natural Products in Health and Diseases:Cairo,Egypt, March 2,2015
- Effect of process parameters on the recrystallization and size control of puerarin using the supercritical fuid antisolvent process
- Rapid and sensitive analysis of melatonin by LC-MS/MS and its application to pharmacokinetic study in dogs
