Control of autoimmune arthritis by herbal extracts and their bioactive components
2017-01-19ShivprsdVenkteshBrinAstry
Shivprsd H.Venktesh,Brin Astry,
Siddaraju M.Nanjundaiaha,Hong R.Kima,Rajesh Rajaiaha, Yinghua Yanga,Li Tongb,Hua Yua,Brian M.Bermanc,
Kamal D.Moudgila,d,*
aDepartment of Microbiology and Immunology,University of Maryland School of Medicine,Baltimore,MD
21201,United States
bSouthern Medical University,Guangzhou,China
cCenter for Integrative Medicine,University of Maryland School of Medicine,Baltimore,MD 21201,United
States
dDivision of Rheumatology,Department of Medicine,University of Maryland School of Medicine,Baltimore,MD 21201,United States
Review
Control of autoimmune arthritis by herbal extracts and their bioactive components
Shivaprasad H.Venkateshaa,Brian Astrya,
Siddaraju M.Nanjundaiaha,Hong R.Kima,Rajesh Rajaiaha, Yinghua Yanga,Li Tongb,Hua Yua,Brian M.Bermanc,
Kamal D.Moudgila,d,*
aDepartment of Microbiology and Immunology,University of Maryland School of Medicine,Baltimore,MD
21201,United States
bSouthern Medical University,Guangzhou,China
cCenter for Integrative Medicine,University of Maryland School of Medicine,Baltimore,MD 21201,United
States
dDivision of Rheumatology,Department of Medicine,University of Maryland School of Medicine,Baltimore,MD 21201,United States
A R T I C L EI N F O
Article history:
Available online 15 February 2016
Drug discovery
Herbal medicine
Immune response
Traditional Chinese medicine
Natural products
Rheumatoid arthritis
Autoimmune diseases such as rheumatoid arthritis(RA)cause signifcant morbidity and loss of productivity.Many potent conventionally used drugs are available for these diseases,but their prolonged use is accompanied by severe adverse effects besides a high cost. Therefore,there is an unmet need for effective but less expensive medications for RA and other autoimmune diseases.Natural plant products belonging to the traditional systems of medicine,such as the traditional Chinese medicine and Indian Ayurvedic medicine,offer a vast and promising resource in this regard.However,herbal medicinal products are often poorly characterized for their composition as well as mechanisms of action.We review here the results of our systematically performed studies aimed at defning the anti-arthritic activity of three herbal extracts,namely,modifed Huo-luo-xiao-ling dan(HLXL),Celastrus aculeatusMerr.,and polyphenolic fraction of green tea(Camellia sinensis),as well as a purifedcompound Celastrol,a bioactive component of Celastrus.Specifcally,we examined the effects of these herbal products on the immunological,biochemical and molecular biological effector pathways in autoimmune arthritis.We have also reviewed here related studies on these herbal products by other investigators.Taken together,we suggest further testing of these herbal products in RA patients.
©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Autoimmune diseases result from deregulated immune responses that attack the body’s own tissues contrary to their traditional role in protecting the host against external infectious agents.Complex interplays among genetic and environmental factors are involved in the pathogenesis of autoimmunity[1,2].Cell-mediated and/or antibody-mediated effector responses contribute to autoimmune infammation and tissue damage[3,4].These processes can either affect multiple organs(systemic autoimmunity)or be limited primarily to one organ(organ-specifc autoimmunity)[3,5].Rheumatoid arthritis(RA),multiple sclerosis(MS),systemic lupus erythematosus(SLE),and type 1 diabetes(T1D)are examples of the major human autoimmune diseases[3,5].In general,the prevalence of these diseases is relatively higher in the developed countries compared to that in the developing countries. For example,the prevalence of RA is estimated to be approximately 1%in the United States compared to about 0.2–0.3%in China and a subset of population from rural South Africa,and the female to male ratio for RA is 2–3:1[6].Uncontrolled autoimmune pathology may result in severe disabilities and/or deformities,and loss of organ function.Due to their chronic nature,autoimmune diseases impose a heavy economical,psychological and social burden on the society.Therefore,effective safe therapeutic agents,and treatment regimen are critical to the management of patients with autoimmunity.The remaining section of this article will mostly cover RA and its experimental models,with some examples of other autoimmune diseases,where needed.
RA affects people all over the world,with geographical differences in prevalence[7–9].Major advances have been made in the treatment of RA over the past couple decades.Nonsteroidal anti-infammatory drugs(NSAIDs)(e.g.,aspirin, ibuprofen,and naproxen),corticosteroids,and disease-modifying anti-rheumaticdrugs(DMARDs)(e.g.,methotrexate, sulfasalazine,and lefunomide)represent conventionally used (allopathic)drugs for the management of RA[10–12].Recent additions to this arsenal against RA are the biologics composed of cytokine-/cytokine receptor-based drugs that belong to the DMARDs category[12].The biologics work by binding either to a particular cytokine(e.g.,TNF-α,IL-6,or IL-17)and neutralizing its function or to the cytokine receptor(e.g.,TNF-α receptor)and preventing the binding of the endogenous cytokine ligand to its cognate receptor.Consequently,biologics are quite potent and effective in controlling the progression of RA.However,their prolonged use is associated with severe adverse reactions,including severe infections.Furthermore, these mainstream drugs,particularly biologics,are very expensive,and it is very diffcult for many patients in the developing countries to afford them.Therefore,there is a continued search for relatively less expensive yet effective alternatives to conventional drugs for RA therapy.In this context,natural plant products constitute a vital and promising resource for identifying new therapeutic agents for RA that meet these criteria.
Plant products have been the source of a large number of bioactive compounds with therapeutic potential,of which many eventually have been developed into drugs that are consumed worldwide for diverse disorders,including infammatory and autoimmune diseases,infectious diseases,and cancer [13–19].Furthermore,a variety of herbal products belonging to the traditional systems of medicine are either already being used by patients with autoimmune diseases including RA,with or without the primary physician’s knowledge,or are under investigation for their therapeutic potential[13–15,18–20].Such medicinal herbs belong to the traditional Chinese medicine (TCM),Japanese traditional medicine(Kampo),Egyptian and other African traditional medicine,Indian Ayurvedic medicine,and other systems.
Adjuvant-induced arthritis(AA)is a well-established experimental model of human RA[21,22].The AA model has extensively been used for studies on the pathogenesis of autoimmune arthritis,for screening of potential anti-arthritic compounds,and for defning the mechanisms of action of such compounds.AA can be induced in Lewis rats(RT.1l)by subcutaneous immunization with heat-killedMycobacterium tuberculosisH37Ra(Mtb).The disease appears in about 10–12 days and it affects all paws.However,generally the disease is more severe in the hind paws than the fore paws.The severity of clinical arthritis can be assigned a semi-quantitative grade on a scale of 0(no disease)to 4(severe arthritis)on the basis of erythema and swelling of the paws as described in detail elsewhere[21,22].Such grading is helpful in quantifying the effect of natural products on the severity of arthritis. An alternative method used by some investigators is to measure the volume of the swollen paws by using an equipment called Plethysmograph.
In our laboratory,we have tested 3 herbal extracts(Huoluo-xiao-lingdan(HLXL),Celastrus,and Green tea)and one purifed compound(Celastrol)derived from one of them (Celastrus)in the rat AA model of RA.We also examined the infuence of these herbal products on various immunological,biochemical and molecular parameters associated with the disease process in RA(Fig.1).We have discussed below detailsof HLXL as a prototypic TCM herbal formula as an antiarthritic agent[23–25].This is followed by description of salient features of Celastrus extract[21,22,26,27],green tea polyphenolic extract[28],and Celastrol[22,26,27]for their suppressive effect on arthritis.
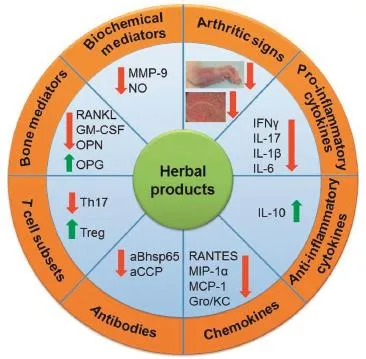
Fig.1–A schematic overview of the immunological, biochemical and molecular biological effector mechanisms that mediate the anti-arthritic activity of herbal products. Herbal extracts and their bioactive components can intervene at multiple steps in the pathogenesis of adjuvant arthritis.The processes/pathways affected by herbal products include cellular(T and B cells)and humoral (antibody)immune responses,cytokine response/balance, alterations in chemokines and chemokine receptors, balance between pathogenic(Th17)and protective(Treg) cells,balance among mediators of bone remodeling,and changes in gene expression.The net effect of these changes induced by herbal treatment is the suppression of the disease-related processes in autoimmune arthritis.
2.Anti-arthritic herbal extracts and their mechanisms of action
2.1.Huo-luo-xiao-ling dan(HLXL)
HLXL is a TCM and is a multi-herbal formulation.We tested a modifed version of original HLXL,and it consisted of 11 herbs (Chinese name,botanical names,and family names) [23–25,29–31]:Ruxiang(Boswellia carteriiBirdw.),Qianghuo (Notopterygium incisumTing ex H.T.Chang),Danggui(Angelica sinensis(Oliv.)Diels),Chishao(Paeonia lactiforaPall.),Gancao (Glycyrrhiza uralensisFisch.),Yanhusuo(Corydalis yanhusuoW.T. Wang.),Danshen(Salvia miltiorrhizaBge.),Chuanxiong(Ligusticum chuanxiongS.H.Qiu.),Qinjiao(Gentiana macrophyllaPall.),Guizhi (Cinnamomum cassiaPresl.),and Duhuo(Angelica pubescensMaxim).The precise composition including percent weight of individual herbs is described in our earlier studies[23–25,29–31]. This herbal mixture was thoroughly characterized by high performance liquid chromatography(HPLC)and mass spectrometry (MS)[24,25,29].Defned bioactive components belonging to several herbs in the mixture were used as biomarkers for quality control to ensure batch-to-batch variations.Furthermore,the mixture was prepared following the guidelines of good manufacturing practices,and the toxicity of the mixture was systematically assessed[24,25,29].This also formed the basis of the dose of HLXL tested in our study in arthritic rats.
Arthritic Lewis rats were treated with HLXL(2.3 g/kg/day) administered as a suspension in water and given by a gavage needle attached to a syringe[23,25,30,31].The treatment was started at the onset of AA and continued throughout the period of study.Arthritic severity was graded using a clinical scoring system.The effects on clinical aspects of the disease were validated by histological analysis of arthritic paws.The paw sections were also examined for histomorphometric parameters to assess bone and cartilage damage in the joints[30].The draining lymph nodes,splenic lymphoid cells,and sera of these rats were collected and tested for cytokines,chemokines,nitric oxide (NO),and other mediators of infammation and bone damage [25,30].Finally,the draining lymph node cells(LNC)were tested for HLXL-induced changes in gene expression using microarray analysis[31].
Our results[23,25,30,31]showed that HLXL was effective in suppressing both clinical and histological features of arthritis.This reduction in disease severity was associated with a decrease in several mediators of infammation,namely,proinfammatory cytokines interleukin-1β(IL-1β),IL-6,and IL-17; serum nitric oxide and matrix metalloproteinase-9(MMP-9); antibodies to mycobacterial heat-shock protein 65(Bhsp65); chemokines(regulated upon activation,normal T cell expressed,and secreted(RANTES),monocyte chemotactic protein-1(MCP-1),macrophage infammatory protein-1α (MIP-1α),and growth regulated oncogene/keratinocyte chemoattractant(GRO/KC))[30];and mediators of bone remodeling(e.g.,receptor activator of nuclear factor-κB ligand (RANKL),osteoprotegerin(OPG),and osteopontin(OPN))[23]. Microarray analysis of the draining LNC revealed distinct patterns of gene expression in HLXL-treated vs.vehicle-treated arthritic rats[31].The former showed 84 differentially expressed genes(DEG)(64 upregulated and 20 downregulated), whereas the latter displayed 120 such genes(94 upregulated and 26 downregulated).The two groups of rats shared 62 genes (45 upregulated and 17 downregulated).Several pathways associated with arthritis were represented in the genes that showed altered expression.These include metabolism,immune response,infammation,cellular proliferation and apoptosis. Taken together,these results validated the mechanistic aspects of the anti-arthritic activity of HLXL.
Comparative pharmacokinetic studies in normal and arthritic rats after oral administration of HLXL or another singleherb extract(e.g.,Angelica pubescens extractorNotopterygium incisumextract)have revealed interesting information about the pharmacokinetic profle of the anti-infammatory bioactive components of HLXL[32].Refned biochemical approaches such as ultra performance liquid chromatography-tandem mass spectrometry(UPLC-MS/MS)were employed to measure bioactiveingredients of herbal extracts in rat serum after oral feeding of the herbal extract.For example,seven coumarins that represent bioactive components of HLXL were tested in rat plasma.Normal(control)and arthritic rats displayed signifcant differences in the pharmacokinetic profles of the analytes tested.Similar differences in normal and arthritic rats were observed in a another study by the same investigators using a different herbal extract compared with HLXL[33].In that study, comparative pharmacokinetics of bioactive components shared between HLXL andBoswellia serrataextract was studied in normal and arthritic rats.The bioactive components included two boswellic acids.Additional insights were gained from this study in that the absorption of the two boswellic acids was much higher in the case of HLXL compared withBoswellia serrataextract alone.These results suggest synergism among component herbs in the multi-herbal HLXL,which consists of 11 different herbs.The scientifc evidence presented above validates an important theme ofTCM that the effcacy of an herbal formula(e.g.,HLXL)is superior to that of a single herb or its compound,when used for the treatment of a particular disease, in this case,infammatory arthritis.
2.2.Celastrus
Celastrus is aTCM,which has been used for many decades for the treatment of infammatory diseases including arthritis.We tested an ethanol fraction ofCelastrus aculeatusMerr.HPLC analysis revealed certain key components of Celastrus[34–39] such as triterpenes(e.g.,celastrol,celasdin C),favonoids(e.g., epiafzelechin),and sesquiterpenes(e.g.,orbiculin F)[22,26]. Celastrus was administered to arthritic rats at a dose of 1.5–3 g/kg/day orally by gavage.Treatment was started at arthritis onset and continued throughout the course of arthritis until rats were sacrifced.Immunological,biochemical and molecular testing of various arthritis-related pathways were performed as described above for HLXL.
Celastrus extract reduced the severity of clinical and histological parameters of arthritis[22,26].Major effect of Celastrus on cytokines was on anti-infammatory cytokine IL-10,which was increased compared to that in controls. Pro-infammatory cytokine interferon-γ(IFN-γ)was mostly unchanged,but indirectly this resulted in an altered ratio of anti-vs.pro-infammatory cytokines.The levels of nitric oxide(NO)in serum and supernate of lymph node cells (LNC)in Celastrus-treated rats were found to be reduced compared to that of control rats.Surprisingly,anti-Bhsp65 antibodies were increased in Celastrus-treated rats compared to controls[21].We proposed that these antibodies in Celastrus-treated rats include a subset that is protective against arthritis.In our follow-up studies[22,26],we tested additional pro-infammatory cytokines,namely,IL-1β,IL-6, IL-17,and IL-18.All these cytokines were inhibited by Celastrus. Surprisingly,tumor necrosis factor-α(TNF-α)level was increased and the reasons for that change were not fully clear. In our other study focused on bone remodeling in arthritis, we observed that Celastrus reduced the mediators of bone damage such as MMP-9,RANKL,GM-CSF(granulocyte macrophage colony-stimulating factor),and OPN[26].Furthermore, gene chip analysis revealed a distinct pattern of changes in several genes involved in immune response,cell proliferation,apoptosis,and cell signaling[40].Comparison of Celastrustreated and Water-treated rats at the onset of AA revealed 84 differentially expressed genes(DEG)(2 upregulated,82 downregulated)that were uniquely altered in Celastrustreated group.Water-treated group showed 8 DEG(6 upregulated, 2 downregulated)that were unique to that group.In addition,Celastrus-treated and Water-treated rats shared 20 genes (6 upregulated,14 downregulated).
Bai et al.tested an ethyl acetate extract fromC.aculeatusMerr in the rat AA model[41].This treatment suppressed both clinical arthritis and synovial infammation.Through systematic examination of the mechanisms,it was shown that Celastrus treatment increased the apoptosis of synoviocytes and peripheral lymphocytes,and facilitated the induction of CD4+CD25+Foxp3+regulatory T cells.In a study using a different type of celastrus,namely,Celastrus orbiculatus,methanol extract of that herb was tested in the chimeric SCID-HuRAg model of human RA[42].In this model,severe combined immunodefcient(SCID)mice were used,and the articular synovium from RA patient(test)or normal articular cartilage (control)was co-implanted subcutaneously into the back of mice.Treatment with celastrus extract caused signifcant reduction in synovial hyperplasia and cartilage erosion.Serum level of TNF-α was also reduced.
2.3.Green tea
Green tea is a popular beverage in several Asian countries,and its consumption is gradually increasing in other parts of the world.Besides several health maintenance benefts of green tea,there is great interest in exploring its therapeutic benefts in infammatory and autoimmune diseases and cancer [17].We tested an extract ofCamellia sinensiscontaining the polyphenolic fraction of green tea(PGT)for its anti-arthritic activity [28].This extract contained the major ingredients including epicatechin(EC),epigallocatechin(EGC),EC-3-O-gallate(ECG), and EGC-3-O-gallate(EGCG).Rats were immunized with heatkilled Mtb for induction of arthritis.Thereafter,rats were orally fed green tea polyphenolic extract in water(8–12 g/L)for 1–3 weeks before disease induction[28].The extract was fed daily in water until the day of Mtb injection for induction of arthritis.Thereafter,rats were followed regularly.Arthritic scores were recorded and tissues were harvested for immunological tests.After pilot testing as described above,we selected 8 g/L dose and a feeding period of 2 weeks for detailed mechanistic study.We observed that green tea extract inhibited the development of clinical arthritis,reduced pro-infammatory cytokine IL-17 but not IFN-γ production,increased antiinfammatory cytokine IL-10 but not IL-4 production,and suppressed serum levels of total immunoglobulins(Ig)as well as IgG2a subset of antibodies against Bhsp65[28].Thus,green tea given orally protected Lewis rats against development and progression of arthritis.
The benefcial effect of green tea in arthritis has also been demonstrated in another experimental model of RA,namely, the mouse collagen-induced arthritis(CIA)model[43].Feeding a polyphenolic fraction of green tea to arthritic mice suppressed clinical and histological features of arthritis.This reduction in clinical arthritis was associated with signifcant decrease in various mediators of infammation such as IFN-γ,TNF-α,and cyclooxygenase-2(COX-2)in the joints of arthritic mice.Also reduced were serum levels of antibodies to the disease-related antigen,type II collagen.Ahmed et al. reported in a study focused on mechanistic aspect of the anti-infammatory activity of green tea that EGCG induces alternative splicing of gp130 mRNA,which results in increased production of soluble gp130[44].This in turn inhibits IL-1-induced IL-6 production and trans-signaling,but reduces IL-6/IL-6 receptor-induced MMP-2 production in synovial fbroblasts.Another study but using the rat CIA model of RA was focused on the oxidant/anti-oxidant system[45].That study included testing of the levels of lipid peroxides,nitric oxide,ceruloplasmin,superoxide dismutase,uric acid,glutathione,prostaglandin E2,copper and zinc in the plasma of green tea extract-treated rats compared with control rats [45].The results revealed that green tea extract was able to reset the dysregulated oxidant/anti-oxidant system in arthritis,and thereby green tea might offer beneft against arthritis and its complications in RA patients.
Marotte et al.tested green tea extract in the rat AA model for its clinical effects and mechanisms of action against arthritis[46].The clinical disease was reduced,though the effect was not very marked.In addition,there was a decrease in the level of chemokines MCP-1 and GROα,but increased expression of chemokine receptors CCR-1,-2,-5 and CXCR1 in the joints of green tea extract-treated rats compared with control rats.The in vivo results were validated by in vitro culture system of human RA fbroblasts treated with IL-1.Another study comparing the relative effcacy of green tea extract and black tea extract in the rat AA model showed that green tea was more effective in suppressing arthritis than black tea[47].Furthermore,the effect of high dose of green tea extract was comparable to that of the allopathic drug indomethacin.Green tea treatment reduced systemic pro-infammatory cytokines, which play a vital role in arthritis pathogenesis.The bioactive component of green tea,EGCG,was also tested on the IL-1 receptor antagonist knockout(IL-1RaKO)model of RA[48].EGCG treatment showed protection against clinical arthritis and joint destruction.The latter was owing to the anti-osteoclastic activity of EGCG.Examination of the mechanisms of action of EGCG revealed multiple pathways that were infuenced.These included inhibition of expression of pro-infammatory cytokines and oxidative stress proteins.Also inhibited were biochemical pathways involving phosphor-signal transducer and activator of transcription 3(p-STAT3),mammalian target of rapamycin (mTOR)and hypoxia-inducible factor-1α(HIF-1α),which are involved in Th17/Treg differentiation.This is evident from the reduced Th17/Treg ratio in the spleen of EGCG-treated mice compared with control mice.
3.Anti-arthritic activity of purifed herbal compound,Celastrol
Following the experimental plan described above for Celastrus extract,we tested one of the purifed bioactive compounds of Celastrus,namely Celastrol,in arthritic Lewis rats[22].Celastrol is a triterpenoid,which along with other triterpenoids has been shown to possess anti-oxidant,anti-infammatory,and anticancer activities[16,49].In our study,rats were treated with Celastrol intraperitoneally(1 mg/kg/d)beginning at the onset of arthritis and continuing either for the entire duration of experiment or for about 10 days after initial injection in different experiments.Celastrol showed potent anti-arthritis activity[22]. In addition to inhibiting pro-infammatory cytokines(IFN-γ,IL-1β,IL-6,IL-17)and chemokines(RANTES and MIP-1α),Celastrol reduced anti-Bhsp65 and anti-cyclic ciltrullinated peptide(aCCP) antibody levels compared to control rats[22].Nanjundaiah et al. examined the effect of celastrol on immune system-bone interaction(osteoimmunology),and showed that celastrol treatment of arthritic rats inhibited RANKL,but increased OPG, thus altering the RANKL/OPG ratio[26].Also reduced were MMP-9,GM-CSF and OPN.A new set of parameters tested with Celastrol was the ratio between Th17 and Treg in the target organ,the joints[27].Interestingly,celastrol treatment reduced Th17 levels,but increasedTreg levels in the joints of Celastroltreated rats compared with control rats.Furthermore,in an in vitro system,Celastrol inhibited the differentiation ofTh17,but promoted the differentiation ofTreg[27].Thus,Celastrol altered the Th17/Treg ratio in vitro as well as in vivo.These changes were in part attributed to Celastrol-induced inhibition of p-STAT3.Gene chip analysis of the draining lymph node cells of celastrol-treated vs.vehicle-treated rats showed that 14 genes (12 upregulated,2 downregulated)were uniquely altered in their expression following celastrol treatment,whereas 57 genes(38 upregulated,19 downregulated)showed differential expression in vehicle-treated rats[50].Furthermore,another 19 genes (7 upregulated,12 downregulated)were shared between celastrol-treated and vehicle-treated rats.Altered genes were related to the immune cells,cellular proliferation and infammatory responses.
Celastrol is also known as tripterine.In a study in the rat AA model,it was shown that tripterine had anti-arthritic activity[51].Intragastric administration of tripterine suppressed paw swelling and bone damage in ongoing AA.This was associated with reduced levels of mRNA expression of IL-1 and TNF-α in the joints as tested using paw homogenates.Cascao et al.showed that Celastrol inhibits the production of IL-1 and TNF-α in vitro,as well as inhibits clinical arthritis in the rat AA model[52].Authors concluded that Celastrol possesses antiinfammatory and anti-proliferative properties,and further suggested that Celastrol might constitute a potential therapeutic for RA.
Another study was focused on the effect of celastrol on IL-17-inducedmigrationandinvasionoffbroblast-like synoviocytes(FLSs),which play an important role in the pathogenesis of RA[53].Celastrol treatment suppressed IL-17-induced migration and invasion of RA-FLSs as well as IL-17-induced MMP-9 and its proteolytic activity.Furthermore, celastrol reduced NF-kB-mediated MMP-9 expression.Gan et al. tested celastrol in the mouse CIA model,with particular emphasis on bone erosion[54].Celastrol treatment suppressed both clinical arthritis and bone damage.In addition,celastrol inhibited the formation and function of osteoclasts.For example,there was a reduction of osteoclasts in the joints,of serum tartrate-resistant acid phosphatase,and of the expression of specifc osteoclastic genes and transcriptional factors. It was suggested that celastrol could directly inhibit osteoclast formation and function.
4.Conclusions
Plant-derived natural products offer a vital and promising resource for new therapeutic agents for RA and other autoimmune diseases.Practitioners of the traditional systems of medicine prefer to use herbal extracts,either singly or in a formulation using multiple herbs.However,as part of its drug discovery process,the pharmaceutical industry frequently solicits purifed herbal compounds which possess bioactivity that replicates,albeit exceeds,the bioactivity of the parental herbal extract.An unforeseen but not unexpected scenario in that case is that the purifed compounds might be more potent,but at the same time they also might be more toxic,than the whole natural extract.Carefully planned dosing studies with suitable modifcations in the product following an active collaboration between the academia and the industry would help further expand the applications of natural products in the treatment of autoimmune and other disorders.Similarly,there is a need for practitioners of the mainstream(allopathic)medicine and those of CAM to work together on the use of these products for the treatment of various diseases.This is important to anticipate and manage unwanted interactions between conventional(allopathic)and CAM products being used concurrently by patients with autoimmunity and other diseases.
Acknowledgement
This work was supported by NIH grants R01 AT004321(KDM), R21AT001608-01A2(KDM),P01AT002605,and 5F31AT007278-02(BMB).
R E F E R E N C E S
[1]Viatte S,Plant D,Raychaudhuri S.Genetics and epigenetics of rheumatoid arthritis.Nat Rev Rheumatol 2013;9:141–153.
[2]Pollard KM.Environment,autoantibodies,and autoimmunity.Front Immunol 2015;6:60.
[3]Comerford I,Kara EE,McKenzie DR,et al.Advances in understanding the pathogenesis of autoimmune disorders: focus on chemokines and lymphocyte traffcking.Br J Haematol 2014;164:329–341.
[4]Mihai S,Nimmerjahn F.The role of Fc receptors and complement in autoimmunity.Autoimmun Rev 2013;12:657–660.
[5]Crow MK,Olferiev M,Kirou KA.Targeting of type I interferon in systemic autoimmune diseases.Transl Res 2015;165:296–305.
[6]Mackie S,Quinn M,Emery P.Rheumatoid arthritis.In:Rose N,Mackay I,editors.The autoimmune diseases.St.Louis, MO:Elsevier;2006.p.417–436.
[7]Rudan I,Sidhu S,Papana A,et al.Prevalence of rheumatoid arthritis in low-and middle-income countries:a systematic review and analysis.J Glob Health 2015;5:010409.
[8]Abdel-Nasser AM,Rasker JJ,Valkenburg HA.Epidemiological and clinical aspects relating to the variability of rheumatoid arthritis.Semin Arthritis Rheum 1997;27:123–140.
[9]Alamanos Y,Drosos AA.Epidemiology of adult rheumatoid arthritis.Autoimmun Rev 2005;4:130–136.
[10]Finckh A,Bansback N,Marra CA,et al.Treatment of very early rheumatoid arthritis with symptomatic therapy, disease-modifying antirheumatic drugs,or biologic agents:a cost-effectiveness analysis.Ann Intern Med 2009;151:612–621.
[11]Muller RB,Kempis JV,Haile SR,et al.Effectiveness, tolerability,and safety of subcutaneous methotrexate in early rheumatoid arthritis:a retrospective analysis of realworld data from the St.Gallen cohort.Semin Arthritis Rheum 2015;45:28–34.
[12]Her M,Kavanaugh A.Advances in use of immunomodulatory agents-a rheumatology perspective. Nat Rev Gastroenterol Hepatol 2015;12:363–368.
[13]Akerele O.Nature’s medicinal bounty:don’t throw it away. World Health Forum 1993;14:390–395.
[14]Amirghofran Z.Herbal medicines for immunosuppression. Iran J Allergy Asthma Immunol 2012;11:111–119.
[15]Kolasinski SL.Herbal medicine for rheumatic diseases: promises kept?Curr Rheumatol Rep 2012;14:617–623.
[16]Aggarwal B,Prasad S,Sung B,et al.Prevention and treatment of colorectal cancer by natural agents from mother nature.Curr Colorectal Cancer Rep 2013;9:37–56.
[17]Khan N,Mukhtar H.Tea and health:studies in humans.Curr Pharm Des 2013;19:6141–6147.
[18]Basnyat S,Kolasinski SL.Ayurvedic medicine for rheumatoid arthritis.Curr Rheumatol Rep 2014;16:435.
[19]Furst R,Zundorf I.Evidence-based phytotherapy in Europe: where do we stand?Planta Med 2015;81:962–967.
[20]Chang CL,Chen YC,Chen HM,et al.Natural cures for type 1 diabetes:a review of phytochemicals,biological actions,and clinical potential.Curr Med Chem 2013;20:899–907.
[21]Tong L,Moudgil KD.Celastrus aculeatus Merr.suppresses the induction and progression of autoimmune arthritis by modulating immune response to heat-shock protein 65. Arthritis Res Ther 2007;9:R70.
[22]Venkatesha SH,Yu H,Rajaiah R,et al.Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses.J Biol Chem 2011;286:15138–15146.
[23]Nanjundaiah SM,Lee DY,Berman BM,et al.Chinese herbal formula Huo-Luo-Xiao-Ling Dan protects against bone damage in adjuvant arthritis by modulating the mediators of bone remodeling.Evid Based Complement Alternat Med 2013;2013:429606.
[24]Rajaiah R,Lee DY,Ma Z,et al.Huo-Luo-Xiao-Ling Dan modulates antigen-directed immune response in adjuvant-induced infammation.J Ethnopharmacol 2009;123:40–44.
[25]Yang YH,Rajaiah R,Lee DY,et al.Suppression of ongoing experimental arthritis by a Chinese herbal formula(Huo-Luo-Xiao-Ling Dan)involves changes in antigen-induced immunological and biochemical mediators of infammation. Evid Based Complement Alternat Med 2011;2011:642027.
[26]Nanjundaiah SM,Venkatesha SH,Yu H,et al.Celastrus and its bioactive celastrol protect against bone damage in autoimmune arthritis by modulating osteoimmune crosstalk.J Biol Chem 2012;287:22216–22226.
[27]Astry B,Venkatesha SH,Laurence SH,et al.Celastrol,a Chinese herbal compound,controls autoimmune infammation by altering the balance of pathogenic and regulatory T cells in the target organ.Clin Immunol 2015;157:228–238.
[28]Kim HR,Rajaiah R,Wu QL,et al.Green tea protects rats against autoimmune arthritis by modulating disease-related immune events.J Nutr 2008;138:2111–2116.
[29]Zhang RX,Fan AY,Zhou AN,et al.Extract of the Chinese herbal formula Huo Luo Xiao Ling Dan inhibited adjuvant arthritis in rats.J Ethnopharmacol 2009;121:366–371.
[30]Nanjundaiah SM,Lee DY,Ma Z,et al.Modifed huo-luo-xiaoling dan suppresses adjuvant arthritis by inhibiting chemokines and matrix-degrading enzymes.Evid Based Complement Alternat Med 2012;2012:589256.
[31]Yu H,Lee DY,Nanjundaiah SM,et al.Microarray analysis reveals the molecular basis of antiarthritic activity of huoluo-xiao-ling dan.Evid Based Complement Alternat Med 2013;2013:524746.
[32]Wu Y,Wang F,Ai Y,et al.Simultaneous determination of seven coumarins by UPLC-MS/MS:application to a comparative pharmacokinetic study in normal and arthritic rats after oral administration of Huo Luo Xiao Ling Dan or single-herb extract.J Chromatogr B Analyt Technol Biomed Life Sci 2015;991:108–117.
[33]Wang H,Zhang C,Wu Y,et al.Comparative pharmacokinetic study of two boswellic acids in normal and arthritic rat plasma after oral administration of Boswellia serrata extract or Huo Luo Xiao Ling Dan by LC-MS.Biomed Chromatogr 2014;28:1402–1408.
[34]Spivey AC,Weston M,Woodhead S.Celastraceae sesquiterpenoids:biological activity and synthesis.Chem Soc Rev 2002;31:43–59.
[35]Guo YQ,Li X,Xu J,et al.Sesquiterpene esters from the fruits of Celastrus orbiculatus.Chem Pharm Bull 2004;52:1134–1136.
[36]Kim SE,Kim YH,Lee JJ,et al.A new sesquiterpene ester from Celastrus orbiculatus reversing multidrug resistance in cancer cells.J Nat Prod 1998;61:108–111.
[37]Jin HZ,Hwang BY,Kim HS,et al.Antiinfammatory constituents of Celastrus orbiculatus inhibit the NF-kappaB activation and NO production.J Nat Prod 2002;65:89–91.
[38]Min KR,Hwang BY,Lim HS,et al.(-)-Epiafzelechin: cyclooxygenase-1 inhibitor and anti-infammatory agent from aerial parts of Celastrus orbiculatus.Planta Med 1999;65:460–462.
[39]Westerheide SD,Bosman JD,Mbadugha BN,et al.Celastrols as inducers of the heat shock response and cytoprotection.J Biol Chem 2004;279:56053–56060.
[40]Yu H,Venkatesha SH,Nanjundaiah S,et al.Celastrus treatment modulates antigen-induced gene expression in lymphoid cells of arthritic rats.Int J Immunopathol Pharmacol 2012;25:455–466.
[41]Bai ST,Chen PH,Chen YY,et al.Ethyl acetate extract from Celastrus aculeatus Merr.suppresses synovial infammation in adjuvant arthritis rats through apoptosis induction of CD4(+)CD25(+)FOXP3(+)T cells.Evid Based Complement Alternat Med 2014;2014:460136.
[42]Xiao CH,Gu WW,Zhang JN,et al.Methanol extract of Celastrus orbiculatus suppresses synovial hyperplasia and cartilage erosion and degradation in rheumatoid arthritis. Nan Fang Yi Ke Da Xue Xue Bao 2007;27:945–950.
[43]Haqqi TM,Anthony DD,Gupta S,et al.Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea.Proc Natl Acad Sci U S A 1999; 96:4524–4529.
[44]Ahmed S,Marotte H,Kwan K,et al.Epigallocatechin-3-gallate inhibits IL-6 synthesis and suppresses transsignaling by enhancing soluble gp130 production.Proc Natl Acad Sci U S A 2008;105:14692–14697.
[45]Meki AR,Hamed EA,Ezam KA.Effect of green tea extract and vitamin C on oxidant or antioxidant status of rheumatoid arthritis rat model.Indian J Clin Biochem 2009;24:280–287.
[46]Marotte H,Ruth JH,Campbell PL,et al.Green tea extract inhibits chemokine production,but up-regulates chemokine receptor expression,in rheumatoid arthritis synovial fbroblasts and rat adjuvant-induced arthritis. Rheumatology(Oxford)2010;49:467–479.
[47]Ramadan G,El-Beih NM,Talaat RM,et al.Anti-infammatory activity of green versus black tea aqueous extract in a rat model of human rheumatoid arthritis.Int J Rheum Dis 2015;doi:10.1111/1756-185X.12666.[Epub ahead of print].
[48]Yang EJ,Lee L,Lee SY,et al.EGCG attenuates autoimmune arthritis by inhibition of STAT3 and HIF-1alpha with Th17/ Treg control.PLoS ONE 2014;9:e86062.
[49]Yadav VR,Prasad S,Sung B,et al.Targeting infammatory pathways by triterpenoids for prevention and treatment of cancer.Toxins(Basel)2010;2:2428–2466.
[50]Yu H,Venkatesha SH,Moudgil KD.Microarray-based gene expression profling reveals the mediators and pathways involved in the anti-arthritic activity of Celastrus-derived Celastrol.Int Immunopharmacol 2012;13:499–506.
[51]Li H,Zhang YY,Tan HW,et al.Therapeutic effect of tripterine on adjuvant arthritis in rats.J Ethnopharmacol 2008;118:479–484.
[52]Cascao R,Vidal B,Raquel H,et al.Effective treatment of rat adjuvant-induced arthritis by celastrol.Autoimmun Rev 2012;11:856–862.
[53]Li GQ,Zhang Y,Liu D,et al.Celastrol inhibits interleukin-17A-stimulated rheumatoid fbroblast-like synoviocyte migration and invasion through suppression of NF-kappaB-mediated matrix metalloproteinase-9 expression.Int Immunopharmacol 2012;14:422–431.
[54]Gan K,Xu L,Feng X,et al.Celastrol attenuates bone erosion in collagen-induced arthritis mice and inhibits osteoclast differentiation and function in RANKL-induced RAW264.7. Int Immunopharmacol 2015;24:239–246.
Abbreviations:AA,adjuvant arthritis;aBhsp65,antibodies to Bhsp65;aCCP,antibodies to cyclic citrullinated peptides;Bhsp65,mycobacterial heat-shock protein 65;CAM,complementary and alternative medicine;DEG,differentially expressed genes;FLS,fbroblast-like synoviocyte;GM-CSF,granulocyte macrophage colony-stimulating factor;GRO/KC,growth regulated oncogene/keratinocyte chemoattractant (GRO/KC);HLXL,Huo-luo-xiao-ling dan;IFN-γ,interferon gamma;IL-1β,interleukin 1 beta;MCP-1,monocyte chemotactic protein-1(MCP-1);MIP-1a,macrophage infammatory protein-1α(MIP-1α);MMP-9,matrix metalloproteinase-9;OPG,osteoprotegerin;OPN,osteopontin; PGT,polyphenolic fraction of green tea;NO,nitric oxide;RA-FLS,Rheumatoid arthritis-fbroblast-like synoviocyte;RANKL,receptor activator of nuclear factor-κB ligand(RANKL);RANTES,regulated upon activation,normal T cell expressed,and secreted;TCM,Traditional Chinese medicine;Th17,T helper 17 cell;Treg,T regulatory cell.
*< class="emphasis_italic">Corresponding author.
.Department of Microbiology and Immunology,University of Maryland School of Medicine,685 W.Baltimore Street, HSF-1,Suite 380,Baltimore,MD 21201,United States.Tel.:(410)-706-7804;fax:+1 410 706 2129.
E-mail address:kmoudgil@som.umaryland.edu(K.D.Moudgil).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2016.02.003
1818-0876/©2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Ameliorated effects of Lactobacillus delbrueckii subsp. lactis DSM 20076 and Pediococcus acidilactici NNRL B-5627 on Fumonisin B1-induced Hepatotoxicity and Nephrotoxicity in rats
- Validation and application of Vierordt’s spectrophotometric method for simultaneous estimation of tamoxifen/coenzyme Q10 in their binary mixture and pharmaceutical dosage forms
- Introduction of antineoplastic drug NSC631570 in an inpatient and outpatient setting: Comparative evaluation of biological effects
- The 1st Euro-Mediterranean Workshop:Natural Products in Health and Diseases:Cairo,Egypt, March 2,2015
- Effect of process parameters on the recrystallization and size control of puerarin using the supercritical fuid antisolvent process
- Rapid and sensitive analysis of melatonin by LC-MS/MS and its application to pharmacokinetic study in dogs
