Surgical treatment of intrahepatic cholangiocarcinoma: a retrospective study of 104 cases
2017-01-13XiaoDongXunQiangLi
Xiao-Dong Xun, Qiang Li
Department of Hepatobiliary Cancer, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin Key Laboratory of Cancer Prevention and Therapy, Tianjin Clinical Research Center for Cancer, Tianjin 300060, China
Surgical treatment of intrahepatic cholangiocarcinoma: a retrospective study of 104 cases
Xiao-Dong Xun, Qiang Li
Department of Hepatobiliary Cancer, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin Key Laboratory of Cancer Prevention and Therapy, Tianjin Clinical Research Center for Cancer, Tianjin 300060, China
Objective: To explore the clinicopathological features, surgical treatment techniques, and prognostic risk factors of intrahepatic cholangiocarcinoma (ICC).
Methods: A total of 104 ICC cases were collected from January 2008 to December 2013 at Tianjin Medical University Cancer Institute and Hospital and divided into the hepatic hilum lymphadenectomy (HLL, 21 cases), extended hepatic hilum lymphadenectomy (EHLL, 12 cases), and non-lymphadenectomy (NL, 71 cases) groups. The clinical data of the patients were retrospectively analyzed, and the prognostic differences were compared among different groups.
Results: The 1-, 2-, and 3-year overall survival (OS) rates of all cases were 72.1%, 56.1%, and 43.7%, respectively. The median survival duration was 34 months. The 1-, 2-, and 3-year OS rates of the HLL group (42.9%, 28.6%, and 28.6%, respectively) were significantly lower than those of the NL group (78.9%, 62.5%, and 47.8%, respectively). Meanwhile, the 1-, 2-, and 3-year OS rates of the EHLL group (75.0%, 56.1%, and 33.3%, respectively) were not significantly different from those of the other two groups. Univariate analysis showed that age, gender, American Joint Committee on Cancer (AJCC) stage, differentiation, ferritin (Fer), carbohydrate antigen19-9 (CA19-9) and carcinoembryonicantigen (CEA) levels, lymph node metastasis (LNM), and lymph node dissection (LND) were prognostic factors for the long-term survival of ICC. Meanwhile, multivariate analysis revealed that age, AJCC stage, differentiation, Fer levels, and LNM were independent risk factors for survival.
Conclusions: ICC patients will not benefit from lymphadenectomy in the absence of LNM. However, systematic lymphadenectomy may improve ICC outcomes if the location of lymphatic metastasis is known. Age, AJCC stage, differentiation, Fer level, and LNM are independent risk factors for survival in ICC.
Intrahepatic cholangiocarcinoma; surgical treatment; lymph node dissection; prognosis
Introduction
Intrahepatic cholangiocarcinoma (ICC), a primary liver cancer, initially presents in the intrahepatic biliary tree and is the second most common liver malignancy after hepatocellular carcinoma, accounting for 10% of all primary liver cancers1. Surgical resection is currently the most effective treatment for ICC, but has several intrinsic limitations and disadvantages, such as poor curative effect, high recurrence rate, and low survival rate. The liver is the most common target organ for ICC metastasis, whereas lymph node metastasis (LNM) has been reported in 30%-70% of cases. Therefore, controlling LNM is the most important consideration for ICC treatment. For lymph node dissection (LND), there are various questions and controversies regarding the following aspects: when to perform LND, the extent of LND, and whether prophylactic LND should be performed. This study retrospectively analyzed the clinical data of 104 ICC cases collected from January 2008 to December 2013 at Tianjin Medical University Cancer Institute and Hospital. The clinical treatment strategies and prognostic factors for ICC were then explored.
Materials and methods
General clinical data
A total of 148 ICC cases were admitted to our hospital from January 2008 to December 2013. We ruled out 44 cases,including 4 cases diagnosed as hepatocellular carcinoma (HCC), 13 cases without surgical and interventional therapy, and 27 cases that were not followed up. The final 104 cases were investigated and divided into the hepatic hilum lymphadenectomy (HLL; 21 cases, 20.19%), extended hepatic hilum lymphadenectomy (EHLL; 12 cases, 11.54%), and the non-lymphadenectomy (NL; 71 cases, 68.27%) groups. Table 1 shows the clinical features of the case groups.
Therapeutic modalities
The tumor diameters of the 104 cases ranged from 1 cm to 15 cm. All the cases that underwent liver resection were pathologically confirmed as R0 resections. The excision areas of the HLL group included the gallbladder neck and any single or several lymph node areas in zones 12 (12A, 12B, and 12P), 5, 7, 8, and 9. The EHLL group further underwent extraction of any single or several areas of zone 13, peritoneal lymph nodes, and intestinal region, in addition to the excision areas of the HLL group. No case in the NL group underwent lymph node removal. Additionally, 43 cases underwent regular hepatic resection (monosegmentectomy), 22 cases underwent left hemihepatectomy, 24 cases underwent right hemihepatectomy, 5 cases underwent extended left hemihepatectomy, 6 cases underwent extended right hemihepatectomy, and 4 cases underwent liver wedge resection.
Follow up
The follow-up was performed via telephone conversations. The median follow-up duration was 41 months (1-92 months). The overall survival (OS) time was defined as the time from surgery to death or end of follow-up. The followup data of the 104 cases were completed.
Statistical analysis
Statistical analysis was conducted by SPSS 19.0 statistical software. Survival analysis was performed by the Kaplan-Meier method, and the prognostic factors were compared by the log-rank test. Independent ICC prognostic factors were analyzed by the Cox regression model. Clinical parameters included gender, age, hepatitis B status, cirrhosis, tumor number, American Joint Committee on Cancer (AJCC) stage, differentiation, maximum tumor diameter, tumor marker levels [ferritin (Fer), alpha fetoprotein, CEA, and CA19-9], vascular invasion, LNM, and lymph node excision. P < 0.05 was considered as statistically significant.

Table 1 Summary of clinicopathological features of ICC, n (%)
Results
Survival rate
The 1-, 2-, and 3-year OS rates of the 104 cases were 72.1%, 56.1%, and 43.7%, respectively. The median survival time was 34 months. The 1-, 2-, and 3-year OS rates of HLL were 42.9%, 28.6%, and 28.6%, respectively. The 1-, 2-, and 3-year OS rates of EHLL were 75.0%, 66.7%, and 33.3%, respectively. The 1-, 2-, and 3-year OS rates of NL were 78.9%, 62.5%, and 47.8%, respectively. The overall differences among the three groups were statistically significant. The 1-, 2-, and 3-year OS were significantly higher in the NL group than in the HLL group (P < 0.05, Table 2).
Prognostic factors
Univariate analysis (Table 3) revealed that age, gender, AJCC stage, differentiation, tumor marker levels, LNM, and LND significantly affected the prognosis of ICC patients. Meanwhile, multivariate analysis (Table 4) showed that age, AJCC stage, differentiation, Fer, and LNM were independent risk factors for survival. Furthermore, 62 of the 104 cases once received adjuvant therapy before or after surgery, including 37, 10, and 15 cases that received transcatheter arterial chemoembolization, radiofrequency ablation, and chemotherapy, respectively. After further analysis, we found that the 1-, 2-, and 3-year OS did not significantly differ between the adjuvant therapy (71.7%, 51.0%, and 38.6%, respectively) and non-adjuvant therapy groups (72.7%, 63.5%, and 51.2%, respectively). Therefore, the effects of adjuvant therapy can be excluded.
Discussion
Currently, there are many controversies on the application of LND in ICC patients. According to the AJCC guidelines on ICC, ICC and combined hepatocellular intrahepatic cholangiocarcinoma (cHCC-ICC) are not partitioned. Tumor diameter has been reported to be closely related to LNM and vascular invasion4. This study set the boundary value of the maximum tumor diameter at 5 cm. According tosome ICC guidelines, surgical excision is suitable for patients in AJCC stages I and II, whereas conservative treatment is suitable for patients in stages III and IV (IVa and IVb)5. Therefore, the stage I and II cases were classified into one group, and stage III and IV cases were classified into another group. The effect of surgical resection on the prognosis of ICC was then investigated. Univariate analysis showed that age, gender, AJCC stage, differentiation, Fer, CA19-9, and CEA levels, LNM, and LND significantly affected the prognosis of ICC patients. Multivariate analysis revealed that age, AJCC stage, differentiation, Fer, and LNM were independent risk factors of survival. This study found that hepatitis B was not an independent risk factor for the prognosis of ICC patients, which is consistent with the view of some researchers5. No study had reported that Fer is an independent risk factor for the survival of ICC patients. However, plasma Fer concentrations were reported to be closely related to the occurrence and development of HCC, breast cancer, and gastrointestinal tumors7-9. Additionally, some researchers posited that in male patients, excessively high or low preoperative Fer concentrations are an independent risk factor for hepatitis C in HCC7. In this study, only Fer had statistically significant effects on survival rate whereas Fer, gender, and hepatitis B were covariates, which varied from the results reported by some studies performed in China10. In this study, we found that the risk of death was 1.7 times higher for patients with high preoperative Fer concentrations than for patients with normal Fer levels.

Table 2 Comparison of the OS according to the LND

Table 3 Results of univariate analysis
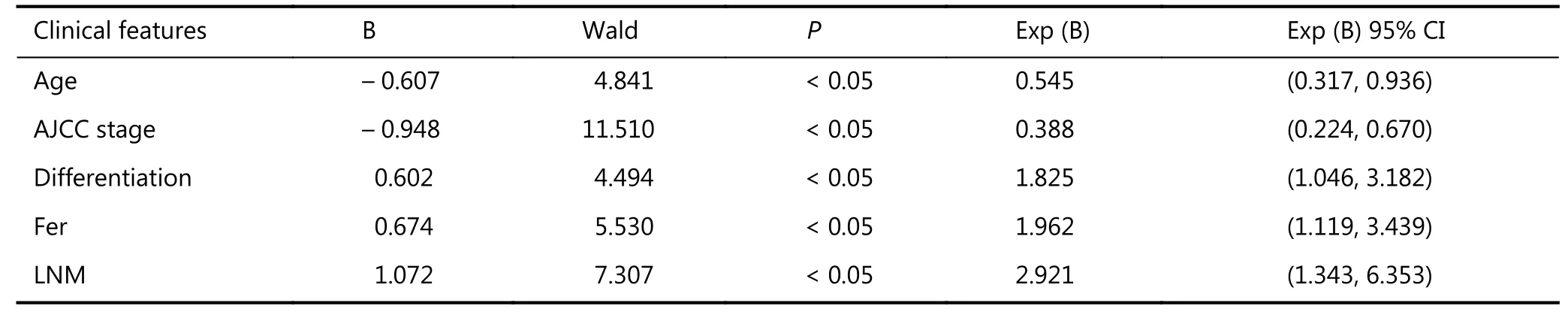
Table 4 Results of multivariate analysis
This study analyzed the application of LND in ICC, although the results of multivariate analysis showed that LND was not an independent risk factor for ICC survival. A total of 104 cases were divided into the HLL, EHLL, and NL groups. Univariate analysis showed that LND significantly affected the prognosis of ICC patients (P < 0.05). This finding coincides with a previous perspective that ICC patients without LNM do not benefit from routine LND11. Furthermore, this study observed some differences among groups. The EHLL group had considerably improved prognosis compared with the HLL group, as noted from the OS, although the difference between groups was not statistically significant. Moreover, 5 cases in the EHLL group (5/12) and 6 cases in the HLL group (6/21) exhibited lymph metastases. LNM had been confirmed to be rarely confined to the first lymph node, and has an apparent tendency for skip metastasis11. Therefore, we suspected that ICC patients will not benefit from lymphadenectomy when LNM is absent. However, systematic lymphadenectomy may improve the outcomes of ICC if we identify the location of lymphatic metastasis13. Some researchers recommend that ICC patients without LNM should not undergo routine LND, and extended systemic LND is not recommended for ICC patients with LNM14. Therefore, this study analyzed LND and LNM as covariates. The results showed that only the effects of LNM on the prognosis were significantly different. LND did not significantly improve the prognosis of some ICC patients. Therefore, establishing a comprehensive, clear, and standardized LND system for ICC is critical. Multivariate analysis revealed that LNM was an independent factor for ICC prognosis, which is consistent with most research perspectives15,16. Li et al.11found that the ligamentum hepatoduodenale is the most common LNM site in ICC. In this study, 11 cases exhibited ICC in LNM, including 5 cases in zone 12, 5 cases in zone 8, 2 cases in zone 13, and 1 case in the retroperitoneum. Hence, the results suggested that zones 12 and 8 are the most common LNM sites.
Some shortcomings of this study must be acknowledged. First, the study performed retrospective analyses, and some clinical data may not be reliable. Several reports showed that vascular invasion and tumor number are independent prognostic factors16, which contradict our results. This discrepancy possibly resulted from the inaccuracy of some clinical information, selective bias of the treatment, or insufficient sample size. Some previous studies identified CEA and CA19-9 as prognostic factors for ICC, a conclusion not verified by this study15,16. This incongruity might have resulted from the insufficient sample size, such that individual differences may have affected the result.

Figure 1 Survival curves of patients in the HLL group and NL group.
Moreover, this study collected cases that were in relatively early stages of ICC progression, and the majority of the AJCC stage I and II cases have increased in recent years. Therefore, the median survival time (34 months) in our study was higher than in some reports17,18. In conclusion, we recommend that ICC patients without or with uncertain LNM should not undergo routine LND, but that ICC patients with LNM should undergo LND. A comprehensive, clear, and standardized LND system for early-stage ICC must be established.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Brown KM, Parmar AD, Geller DA. Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. 2014; 23: 231-46.
2.Bagante F, Gani F, Spolverato G, Xu L, Alexandrescu S, Marques HP, et al. Intrahepatic cholangiocarcinoma: prognosis of patients who did not undergo lymphadenectomy. J Am Coll Surg. 2015; 221: 1031-40.e4.
3.Sakamoto Y, Kokudo N, Matsuyama Y, Sakamoto M, Izumi N, Kadoya M, et al. Proposal of a new staging system for intrahepatic cholangiocarcinoma: analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer. 2016; 122: 61-70.
4.Spolverato G, Ejaz A, Kim Y, Sotiropoulos GC, Pau A, Alexandrescu S, et al. Tumor size predicts vascular invasion and histologic grade among patients undergoing resection of intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2014; 18: 1284-91.
5.Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014; 60: 1268-89.
6.Horino K, Beppu T, Komori H, Masuda T, Hayashi H, Okabe H, et al. Evaluation of mass-forming intrahepatic cholangiocarcinoma with viral hepatitis. Hepatogastroenterology. 2012; 59: 1217-9.
7.Uchino K, Tateishi R, Fujiwara N, Minami T, Sato M, Enooku K, et al. Impact of serum ferritin level on hepatocarcinogenesis in chronic hepatitis C patients. Hepatol Res. 2016; 46: 259-68.
8.Chekhun SV, Lukyanova NY, Shvets YV, Burlaka AP, Buchinska LG. Significance of ferritin expression in formation of malignant phenotype of human breast cancer cells. Exp Oncol. 2014; 36: 179-83.
9.Usnarska-Zubkiewicz L, Strutyńska-Karpińska M, Zubkiewicz-Kucharska A, Zarebski P, Grabowski K. Soluble urokinase-type plasminogen activator receptor and ferritin concentration in patients with advanced alimentary tract carcinoma. Relationship to localization, surgical treatment and the stage of the disease--preliminary report. Adv Clin Exp Med. 2014; 23: 959-67.
10.Lin PY, Zhou XP, Chen ZS, Lyu LS, Tang ZH. Research advances on pathogenic mechanisms of HBV-related intrahepatic cholangiocarcinoma. Chin J Hepatobiliary Surg. 2014; 20: 617-20.
11.Li DY, Zhang HB, Yang N, Quan Y, Yang GS. Routine lymph node dissection may be not suitable for all intrahepatic cholangiocarcinoma patients: results of a monocentric series. World J Gastroenterol. 2013; 19: 9084-91.
12.Zhang L, Bi XY, Zhao P. Advancement in surgical treatment of intrahepatic cholangiocarcinoma. Chin J Hepatobiliary Surg. 2010; 16: 718-20.
13.Choi SB, Kim KS, Choi JY, Park SW, Choi JS, Lee WJ, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009; 16: 3048-56.
14.Morine Y, Shimada M. The value of systematic lymph node dissection for intrahepatic cholangiocarcinoma from the viewpoint of liver lymphatics. J Gastroenterol. 2015; 50: 913-27.
15.Saxena A, Chua TC, Sarkar A, Chu F, Morris DL. Clinicopathologic and treatment-related factors influencing recurrence and survival after hepatic resection of intrahepatic cholangiocarcinoma: a 19-year experience from an established Australian hepatobiliary unit. J Gastrointest Surg. 2010; 14: 1128-38.
16.Luo XW, Yuan L, Wang Y, Ge RL, Sun YF, Wei GT. Survival outcomes and prognostic factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: a large single-center cohort study. J Gastrointest Surg. 2014; 18: 562-72.
17.Dhanasekaran R, Hemming AW, Zendejas I, George T, Nelson DR, Soldevila-Pico C, et al. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol Rep. 2013; 29: 1259-67.
18.Zhou QB, Lai DM, Lin Q, Chen YJ, Wang J, Chen JS, et al. Surgical management and prognosis of 80 cases with intrahepatic cholangiocarcinoma. Chin Arch Gen Surg (Electronic Edition). 2012; 6: 12-6.
Cite this article as:Xun X, Li Q. Surgical treatment of intrahepatic cholangiocarcinoma: a retrospective study of 104 cases. Cancer Biol Med. 2016; 13: 469-73. doi: 10.20892/j.issn.2095-3941.2016.0063
Qiang Li
E-mail: liqiang4016@yahoo.com
Received July 25, 2016; accepted October 21, 2016.
Available at www.cancerbiomed.org
Copyright © 2016 by Cancer Biology & Medicine
杂志排行
Cancer Biology & Medicine的其它文章
- Liver cancer: challenge and prospect
- Role of microRNAs in inflammation-associated liver cancer
- Portal vein embolization for induction of selective hepatic hypertrophy prior to major hepatectomy: rationale, techniques, outcomes and future directions
- Three-dimensional printing: review of application in medicine and hepatic surgery
- Portal vein tumor thrombus is a bottleneck in the treatment of hepatocellular carcinoma
- Bcl-2 expression is a poor predictor for hepatocellular carcinoma prognosis of andropause-age patients
