Synthesis of 6-naphthoxymethyl-[1,3]dioxolo[4,5-g]quinoline-7-carboxylic acid derivatives
2017-01-03FUXinboCAIWeiZHANGHongLIYangCHANGMingqin
FU Xinbo, CAI Wei, ZHANG Hong, LI Yang, CHANG Mingqin*
(1.Institute of Superfine Chemicals, Bohai University, Jinzhou 121000, Liaoning, China;2.Sebest Pharmaceutical Technology Co., Ltd, Shenyang 110000, Liaoning, China)
Synthesis of 6-naphthoxymethyl-[1,3]dioxolo[4,5-g]quinoline-7-carboxylic acid derivatives
FU Xinbo1, CAI Wei2, ZHANG Hong1, LI Yang1, CHANG Mingqin1*
(1.InstituteofSuperfineChemicals,BohaiUniversity,Jinzhou121000,Liaoning,China;2.SebestPharmaceuticalTechnologyCo.,Ltd,Shenyang110000,Liaoning,China)
A novel synthesis of naphthalene-containing quinolines, namely, 2-(naphthoxymethyl)quinoline-3-carboxylic acids has been achieved in one-pot procedure in good yields through Williamson ether synthesis reaction of ethyl 6-(chloromethyl)-[1,3]dioxolo[4,5-g]quinoline-7-carboxylate with 1- and 2-naphthols followed by ethyl ester hydrolysis reaction.The new synthesized compounds would be good candidates for the development of compounds for use in medicinal chemistry.
naphthalene; quinoline; Williamson ether synthesis; ester hydrolysis; one-pot
Received date:2016-01-03.
Foundation item:National Natural Science Foundation of China (21476028 and 21402011).
Biography:FU Xinbo(1991-), male, post-graduate, research field: organic synthesis.*Corresponding author, E-mail: bhuzh@163.com.
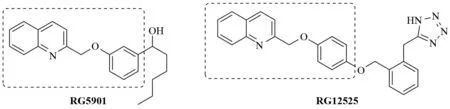
It has been proven that the 2-(aroxymethyl)quinoline structural motif is useful in the design of several leukotriene biosynthesis inhibitors or leukotriene receptor antagonists[1-3].For example, compound RG5901 as shown in Fig.1 exhibited potent inhibition of 5-lipoxygenase (5-LO) activity and slow reacting substance of anaphylaxis (SRSA)-mediated bronchospasm[4].(2-Quinolinylmethoxy)phenoxy-containing compound RG12525 was used in clinical studies as an antiasthmatic agent[5].
On the other hand, the naphthalene nucleus is commonly found in compounds of commercial importance, and a number of pharmaceutical and agricultural agents have a naphthalene framework[6-8].For example, MAYA et al reported that the 2-naphthyl system is a good surrogate for the 3-hydroxy-4-methoxyphenyl (ring B) of combretastatins (Fig.2)[9-10].Considering the wide variety of practical applications of the naphthalene nucleus as a building block, it is of paramount importance to develop synthetic strategies for this nucleus to gain easy access to a variety of naphthalene-containing derivatives.
The construction of structurally novel 2-(naphthoxymethyl)quinoline hybrid molecules as possible drug candidates for the development of new medicinal products with interesting properties, would be important in medicinal chemistry.In recent years, our group has synthesized a number of 2-aroxymethylquinoline compounds[11-15].Building upon this evolving expertise and diversifying our work on the synthesis of new quinoline compounds, we would like to report, herein, the synthesis of naphthalene-containing compounds, namely, 2-(naphthoxymethyl)quinoline-3-carboxylic acids.To the best of our know-ledge, this simple quinoline-naphthalene system has never been previously described in the literature.

1 Experimental section
1.1 Apparatus and chemicals
The melting points were determined by using a WRS-1B melting point apparatus and were unco-rrected.The IR spectra of the compounds in KBr pellets were obtained in the range of 400-4 000 cm-1on a Shimadzu FTIR-8400S spectrophotometer.1H (600 MHz) and13C (150 MHz) NMR spectra were recorded on a Bruker AVANCE NMR spectrometer using DMSO-d6as the solvent.The reported chemical shifts (δvalues) are given in parts per million downfield from tetramethylsilane (TMS) as the internal standard.Elemental analyses were performed for C and H using an Elementar Vario EL-III element analyzer.The progress of reactions was monitored by thin layer chromato-graphy (TLC) on silica gel GF254 using ethyl acetate/petroleum ether (1∶2) as eluent.
1.2 Procedure for the preparation 6-naphthoxymethyl-[1,3]dioxolo[4,5-g]quinoline-7-carboxylic acids
A mixture of ethyl 6-(chloromethyl)-[1,3]dioxolo[4,5-g]quinoline-7-carboxylate (1) (1.0 mmol, 0.294 g), 1- or 2-naphthol 2a, 2b (1.1 mmol, 0.158 g) and anhydrous K2CO3(3.0 mmol, 0.414 g) was stirred in refluxing MeCN (10 mL) for 3 h.After the reaction was complete, MeCN was evaporated.Then a solution of KOH (20.0 mmol, 1.120 g) in 80% ethanol (25 mL) was added directly to the residue and the mixture was heated under reflux for an additional 2 h.After completion, the reaction mixture was cooled, acidified to pH 4-5 with 1 mol/L HCl.The resulting crude product was crystallized from ethanol to afford compounds 3a, 3b.
6-((Naphthalen-1-yloxy)methyl)-[1,3]dioxolo[4,5-g]quinoline-7-carboxylic acid (3a): Brown solid, yield 90%, m.p.200-202 ℃.IR (KBr)ν: 3 442 (COOH), 1 719 (C=O), 1 618, 1 579, 1 510, 1 453, 1 402, 1 389 cm-1;1H NMR (600 MHz, DMSO-d6)δ: 5.69 (s, 2H, ArCH2O), 6.28 (s, 2H, OCH2O), 7.10 (d,J= 7.8 Hz, 1H, ArH), 7.41 (d,J= 8.4 Hz, 1H, ArH), 7.43 (s, 1H, ArH), 7.45 (t,J= 7.8 Hz, 1H, ArH), 7.48 (t,J= 8.4 Hz, 1H, ArH), 7.51 (t,J= 8.4 Hz, 1H, ArH), 7.55 (s, 1H, ArH), 7.87 (d,J= 8.4 Hz, 1H, ArH), 8.08 (d,J= 8.4 Hz, 1H, ArH), 8.73 (s, 1H, ArH), 13.24 (s, 1H, COOH);13C NMR (150 MHz, DMSO-d6)δ: 71.00, 102.63, 103.44, 104.90, 105.62, 120.12, 121.74, 123.78, 125.06, 125.28, 126.26, 126.45, 127.43, 134.11, 138.24, 146.23, 148.54, 152.54, 152.96, 154.26, 167.69; Anal.Calcd for C22H15NO5: C, 70.77; H, 4.05; N, 3.75.Found: C, 77.54; H, 3.87; N, 3.91%.
6-((Naphthalen-2-yloxy)methyl)-[1,3]dioxolo[4,5-g]quinoline-7-carboxylic acid (3b): Brown solid, yield 92%, m.p.214-216 ℃.IR (KBr)ν: 3 427 (COOH), 1 714 (C=O), 1 614, 1 585, 1 510, 1 465, 1 400, 1 389 cm-1;1H NMR (600 MHz, DMSO-d6)δ: 5.64 (s, 2H, ArCH2O), 6.27 (s, 2H, OCH2O), 7.80 - 7.84 (m, 3H, ArH), 7.79 (s, 1H, ArH), 7.55 (s, 1H, ArH), 7.44 - 7.47 (m, 2H, ArH), 7.42 (s, 1H, ArH), 7.35 (t,J= 7.8 Hz, 1H, ArH), 7.18 (dd,J= 9.0, 2.4 Hz, 1H, ArH), 13.18 (s, 1H, COOH);13C NMR (150 MHz, DMSO-d6)δ: 70.55, 102.64, 103.43, 104.85, 107.23, 118.80, 122.93, 123.66, 123.73, 126.45, 126.78, 127.58, 128.63, 129.29, 134.31, 138.37, 146.35, 148.53, 152.59, 152.86, 156.75, 167.51; Anal.Calcd for C22H15NO5: C, 70.77; H, 4.05; N, 3.75.Found: C, 77.95; H, 4.23; N, 3.61%.
2 Results and discussion
We recently reported on the reaction of ethyl 2-chloromethylquinoline-3-carboxylate with 8-hydroxylquinolines and dihydroxy arenes phenols for the synthesis of novel quinoline-based hybrid molecules[14-15].The inspiring result obtained prompted us to further study its synthetic application for synthesis of naphthalene-containing quinoline-3-carboxylic acid.As shown in Fig.3, ethyl 6-(chloromethyl)-[1,3]dioxolo[4,5-g]quinoline-7-carboxylate (1) was underwent the Williamson reaction with 1.1 equiv.of 1-naphthol (2a) or 2-naphthol (2b) to give the corresponding naphthoxymethyl quinoline ether, which was further hydrolyzed to give the title compound 2-(naphthoxymethyl)quinoline-3-carboxylic acid 3a or 3b.
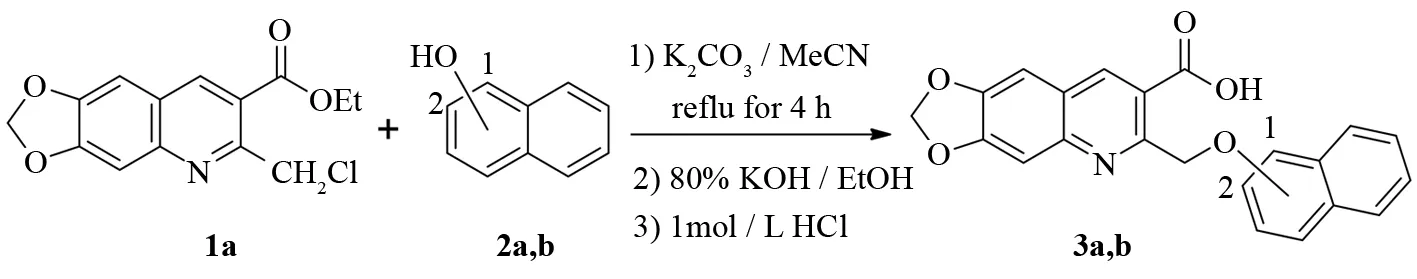
In Willamson reaction step, acetonitrile was employed due to its low boiling point leading to a more convenient workup procedure.As the formed naphthoxymethyl quinoline ether from the reaction did not interfere with further ester hydrolysis reaction, purification at this step was unnecessary.After the Williamson reaction was complete as observed on TLC, we evaporated MeCN to dryness, and added 80% ethanolic potassium hydroxide solution (15 mL) to the residue and the mixture was heated to reflux.The alkaline hydrolysis reaction proceeded smoothly and became completed in 2 hours.After acidification with 1 mol/L HCl solution followed by purification of the crude products by recrystallization from ethanol, the products 3a and 3b were obtained in good overall yields of 90% and 92%, respectively.In addition, Williamson reaction can also be run in other solvents such as N,N-dimethylformamide (DMF), which, might bring much convenience in the workup procedure.
To the best of our knowledge, none of the synthesized compounds have yet been reported, and their structures were easily established based on spectral data and elemental analyses.For instance, the IR spectrum of 3a exhibited the presence of hydroxyl and carbonyl groups of carboxyl moiety at 3 442 and 1 719 cm-1, respectively.Its1H NMR spectrum showed no signals attributable to chloromethyl and ester groups but contained a distinct singlet atδ5.69 attributable to the nascent oxymethylene protons and a broad singlet atδ13.24 for carboxylic proton.The signals due to 10 aromatic ring protons in the range ofδ7.10 - 8.73 were consistent with the molecular structure suggested.The structure of 3a was further confirmed by its13C NMR spectrum, which revealed the presence of carboxyl carbon and naphthalenyloxymethyl carbon atδ167.69 and 102.63, respectively, along with the signals due to the aromatic carbons.Another synthesized compound exhibited similar spectral characteristics.
3 Conclusions
In view of the potential synergism of the naphthalene unit and the quinoline ring in a mole-cular framework, these newly synthesized compounds could be good candidates for the development of lead compounds.Additionally, these compounds belong to a new class of quinoline-carboxylic acids systems, and thus, a further investigation of the synthetic potential of these scaffolds should provide access to significant chemical diversity via further derivatizations.
[1] YOUSSEFYEH R D, MAGNIEN E, LEE T D Y, et al.Development of a novel series of (2-quinolinymethoxy)phenyl-containing compounds as high-affinity leukotriene receptor antagonists.1.Initial structure-activity relationships [J].J Med Chem, 1990, 33(4): 1186-1194.
[2] HUANG F C, GALEMMO J R A, POLI G B, et al.Development of a novel series of (2-quinolinylmethoxy)phenyl-containing compounds as high-affinity leukotriene D4receptor antagonists.4.Addition of chromone moiety enhances leukotriene D4receptor binding affinity [J].J Med Chem, 1991, 34(5): 1704-1707.
[3] ZWAAGSTRA M E, TIMMERMAN H, STOLPE A C V D, et al.Synthesis and structure-activity relationships of carboxyflavones as structurally rigidCysLT1(LTD4) receptor antagonists [J].J Med Chem, 1998, 41(9): 1428-1438.
[4] GALEMMO R A, JOHNSON W H, LEARN K S, et al.The development of a novel series of (quinolin-2-ylmethoxy)phenyl-containing compounds as high-affinity leukotriene receptor antagonists.3.Structural variation of the acidic side chain to give antagonists of enhanced potency [J].J Med Chem, 1990, 33(10): 2828-2841.
[5] HUANG F C, GALEMMO R A, JOHNSON W H, et al.Development of a novel series of (2-quinolinylmethoxy)phenyl-containing compounds as high-affinity leukotriene D4receptor antagonists.2.Effects of an additional phenyl ring on receptor affinity [J].J Med Chem, 1990, 33 (4): 1194-1200.
[6] MATTHIEU S, RUBEN A, MAXIM T.Nuclear hormone receptor targeted virtual screening [J].J Med Chem, 2003, 46(14): 3045-3059.
[7] BLACK J W, DUNCAN W A M, SHANKS R G.Comparison of some properties of pronethalol and propranolol [J].Br J Pharmacol, 1997, 25(S1): 577-591.
[8] KYLE A A, DAHL M V.Topical therapy for fungal infections [J].Am J Clin Dermatol, 2004, 5(6): 443-451.
[9] MAYA A B S, REY B D, CLAIRAC R P L D, et al.Design, synthesis and cytotoxic activities of naphthyl analogues of combretastatin A-4 [J].Bioorg Med Chem Lett, 2000, 10(22): 2549-2551.
[10] MAYA A B S, CONCEPCION P M, CAEMEN M, et al.Further naphthylcombretastatins.An investigation on the role of the naphthalene moiety [J].J Med Chem, 2005, 48(2): 556-68.
[11] 张红, 李阳.“一锅法”合成2-[(2,4-二叔丁基苯氧基)甲基]-6,7-二氧戊环并[1,3-g]喹啉-3-羧酸[J].化学研究与应用, 2009, 21(5): 738-740.
[12] GAO W T, LIN G H, LI Y, et al.An efficient access to the synthesis of novel 12-phenylbenzo[6,7]oxepino[3,4-b]quinolin-13(6H)-one derivatives [J].Beilstein J Org Chem, 2012, 8: 1849-1857.
[13] LI Y, GAO W T.Synthesis of 2-[(quinolin-8-yloxy)methyl]quinoline-3-carboxylic acid derivatives [J].He-terocycl Commun, 2013, 19(6): 405-409.
[14] 常明琴, 张红, 李阳.含4-苯基喹啉结构的双喹啉类衍生物的合成 [J].化学研究与应用, 2015, 27(3): 358-363.
[15] LI Y.A facile synthesis and application of ethyl 6-(bromomethyl)-[1,3]dioxolo[4,5-g]quinoline-7-carboxylate [J].Res Chem Intermed, 2015, 41(7): 4977-4985.
[责任编辑:张普玉]
6-萘氧甲基-[1,3]二氧戊环并[4,5-g]喹啉-7-甲酸衍生物的合成
符鑫博1,蔡 巍2,张 红1,李 阳1,常明琴1*
(1.渤海大学 超精细化学品研究所,辽宁 锦州 121000; 2.辽宁思百得医药科技有限公司,辽宁 沈阳 110000)
介绍了通过6-氯甲基-[1,3]二氧戊环并[4,5-g]喹啉-7-甲酸乙酯与1-和2-萘酚的Williamson醚合成反应及其随后的酯水解反应,“一锅法”高收率的合成了结构新颖的含有萘环结构的喹啉化合物,即2-(萘氧甲基)喹啉-3-羧酸.这种新合成的化合物可以为开发有用的药物活性先导化合物提供很好的底物.
萘;喹啉;Williamson醚合成;酯水解;一锅法
O625
A
1008-1011(2016)06-0725-04
