广东鹤山南亚热带植被重建对土壤动物群落的影响
2016-12-27徐国良方碧真周丽霞刘占锋
徐国良, 方碧真, 周丽霞, 刘占锋
(1. 广州大学 地理科学学院, 广东 广州 510006;2. 中国科学院华南植物园, 广东 广州 510650)
广东鹤山南亚热带植被重建对土壤动物群落的影响
徐国良1, 方碧真1, 周丽霞2, 刘占锋2
(1. 广州大学 地理科学学院, 广东 广州 510006;2. 中国科学院华南植物园, 广东 广州 510650)
恢复生态学常常聚焦于地上变化过程,却较少关注地下过程,尤其是土壤动物的响应.文章对中国南亚热带鹤山一个持续了6 a,占地50 hm2的生态恢复实验中的土壤动物群落进行了研究.结果发现,在此生态重建阶段,由于人工纯林郁闭度过大及外来树种的负面效应,多数测试指标都显示了对照灌丛草坡处理效应的优势.草坡样地土壤动物整体群落密度和土壤螨密度显著高于厚荚相思林和尾叶桉林.不同的恢复措施对土壤动物类群丰富度也有显著影响.尾叶桉林中的土壤动物类群数显著低于对照草坡和乡土红椎林;厚荚相思林中的土壤跳虫类群丰富度和多样性也显著低于草坡和乡土红椎林.混交林对土壤动物群落没有明显的影响.对环境因子的测试分析表明,灌丛草坡样地蕨类盖度和土壤pH值显著高于其他各处理样地;而在厚荚相思林中,发现土壤水分偏低.本研究表明,在中国南亚热带地区自然恢复具有较好的生态效应;然而,相对来说,外来速生树种对土壤生态系统恢复表现了一些负效应.
生态恢复; 土壤动物; 南亚热带; 鹤山
The serious degraded environment began in the 50~60 s’ last century due to the much requirement on natural resources, which proceeded the development of restoration ecology[1]. The degradation of environment also developed very fast in China, and many related studies have been conducted[2-3]. It is necessary to develop strategies of forests restoration for biodiversity protection or productive land uses. Given the sparse knowledge of tropical forests, particularly highly disturbed tropical forests, it is even more significant to conduct researches to promote our understanding of tropical ecosystems and informs selection of restoration strategies in this area[4].
Different trees with different ecological and physiological properties can yield different recoverage results, because of the varied responses of biota and abiota factors of the ecosystem. The selection of plantation species will depend on local management objectives and the ecological characteristics of the candidate species. Because of recolonizing easily after disturbances and fast-growing to reforest, exotic species have been widely used to ameliorate harsh site conditions[5]. For example, legumes are widely used to help restore degraded soils because of the nitrogen-rich leaf litter and its biological nitrogen fixation belowground[6]. However, the use of exotic species, is in great dispute at the same time[5]. Non-native exotic species colonization and invasion were recognized as one of the most serious ongoing ecological problems[7]. For example, the plantation ofEucalyptusinduces significant changes of the vegetation development and soil characteristics, and microbial communities by the influence of allelochemicals[8]. In recent years, due to increasing concerns on biodiversity, native species plantation was encouraged in establishing “Ecological forests” in southern China.
Present restoration studies focused mostly only on the responses aboveground, and little attention on the belowground[9]. The relationships between vegetation community aboveground and decomposer biodiversity belowground have received much attention over the past years[10-11]. The bottom-up control of plantation on soil organisms have been studied in many researches, In fact, there are vast biodiversity belowground and soil biota fulfill important functions on the ecosystem in the soil[12-13], and the vegetation pattern aboveground will affect the fauna community belowground. In a microcosm experiment in Germany, the resource inputs by manipulations in plant species and functuional compositions affected differently the decomposer groups(earthworms, springtails and microorganisms)[14]. CHAUVAT, et al. reported that Collembola were relatively to changes in environmental conditions in four different successional phases of spruce forest stands[15], and suggested that substantial ecosystem-level implications of changes in the soil food web during forest rotation. At the same time, soil fauna can act good indicators for the environmental changes[16].
This study was conducted in southern subtropical China. In this area, there are vast natural biodiversity, and also serious land degrading with the economic development. Many restoration researches on the plants, microbials, physical and chemical soil characteristics have been reported, however, little is still known about the responses of soil fauna community to different reforestation models. In the present study, we selected five reforestation models, including two exotic and fast-growing tree monoculture plantationsEucalyptusurophylla(Myrtaceae) andAcaciacrassicarpa(Leguminosae), a native tree species monoculture plantationCastanopsishystrix(Fagaceae), a mixture native tree species plantation, and a natural restoration shrubland as control, to evaluate the ecological effects on soil fauna. We hypothesize that fast-growing exotic tree plantations are beneficial for the build-up of soil organic matter, and subsequently, in favor of soil biota.
1 Methods and materials
1.1 Site description
This study was conducted at Heshan station (112 °54′ E, 22 °41′ N, 0~90 m a.s.l.), which is located in Heshan County, Guangdong Province, China. Heshan station is one of the core stations of the Chinese Ecological Research Network (CERN) of the Chinese Academy of Sciences (CAS). The climate is subtropical monsoon with a distinct wet season (from April to September, mean temperature 29.2℃) and dry season (from October to March, mean temperature 12.6℃). The mean annual temperature is 21.7℃, and the mean annual precipitation is 1 700 mm. The soil is an acrisol, with soil texture being 42.3% sand, 23.8% silt, and 18.8% clay.
In 2005, an ecological restoration project was initiated on a degraded hilly land with an area of 50 hm2. In March, on an area of 50 hm2, degraded Masson pine (Pinusmassoniana) plantation was cut and residues were burned on sites. We established the following five reforestation models: ① exotic tree speciesEucalyptusurophylla(EU); ② exotic tree speciesAcaciacrassicarpa(AC); ③ native tree speciesCastanopsishystrix(CH); ④ 10 mixed native tree species (MS); ⑤ natural recovering shrubland (SL). Each restoration model was replicated three times, forming 15 experimental plots in total. All 15 plots were randomly distributed in the 50 hm2of study area. As to planting, tree saplings in tube stocks with similar height (50~100 cm) were planted at 3 m×2 m spacing in each plot. In mixture treatments, different species were planted randomly.
1.2 Vegetation description of the five reforestation treatments
In October, 2011, the vegetation composition all over the site was investigated, and the results showed in Table 1.Dicranopterisdichotomais the most representative understory species on this site.
1.3 Soil fauna sampling and measurements
Soil fauna samples were taken in July 27~August 1, 2011, 6 years after the initiation of restoration treatments. 3 sites were randomly selected in each plot. One sample in each site was taken by compositing 3 soil cores (5 cm in diameter, 15 cm in depth). So, there were 9 mixed samples for each treatment, and 45 samples of soil fauna altogether were collected in this study.
The samples were brought to laboratory immediately and fauna were collected using Tullgren dry funnels. All specimens were sorted and counted by a dissecting microscope (Leika, German), and were identified by Nikon Biological Microscope Eclipse 80i (Nikon, Tokyo, Japan). All Collembola individuals were classified to species level, and the identification was referred to the keys in “Checklist of the Collembola of the world”[17], as well as those in POTAPOV[18], BRETFELD[19], POMORSKI[20]. All other soil fauna individual numbers were counted and identified to lower taxa, which were treated as a group in the analysis to provide a better understanding of the community response to disturbance.
1.4 Soil properties and microbial community
In October, 2011, samples were collected to determine physical and microbial characteristics. Similarily, 3 sites were chosen randomly in each site, and 3 soil cores in each site were taken and mixed to one sample. There are 9 samples for each treatment and 45 samples altogether. The samples were taken back to laboratory and were divided into half: one part was used for physical and the other was used for PLFAs analysis. The soil microbial community was characterized by using phospholipids fatty acids (PLFAs) analysis as described by BOSSIO, et al.[21]. Soil samples collected for physical analyses were ground to pass through a 2 mm sieve. Total nitrogen (TN) concentration was measured after micro-Kjeldahl digestion using a flow injection autoanalyser (FIA, Lachat Instruments, USA). Soil organic carbon (SOC) was measured with the Walkley-Black method[22]. Soil pH was determined by using a 1∶25(wt/vol) ratio of soil to deionized water. Soil water content (SWC %, g of water per 100 g dry soil) was measured by oven-drying for 48 h at 105℃.

Table 1 Vegetation description of the five reforestation treatments
1.5 Statistical analyses
It is a randomly chosen design with 3 replicated plots and 9 replicated samples. The effects of the plantations on soil fauna density, richness and diversity, and soil physical and microbial characteristics were tested by one-way ANOVA. Data were transformed (natural log, square root, or rank) to meet assumptions of normality and homogeneity of variance. LSD multiple comparison was used to test the differences among the treatments. All tests were considered to be significant atP<0.05 level. SPSS 13.0 was used for all analyses. The relationships between environmental variables and soil fauna properties were analyzed by CCA using CANOCO software for Windows 45 (Ithaca, NY, USA). Forward selection was based on Monte Carlo permutation (n=499).
2 Results
2.1 Vegetation coverage and soil properties
The vegetations were greatly changed by the forest managements (P<0.001). The fern coverage was significantly higher in the shrublands than in the other plantations (P<0.05). On the contrary, the fern coverage was significantly lower inA.crassicarpathan in other plantations (P<0.05). However, the woody coverage was significantly higher inA.Crassicarpathan that in shrubland and native species plantations (P<0.05), and it was significantly lower in shrubland than all other plantations (P<0.05).
The 6 years of rehabilitation also caused some changes in the soil properties. It was found that the average pH value in control was 4.14, which was significantly higher than all the other plantations and indicated positive changes in the degraded and acidifying area. On the other hand, the soil water content was significantly lower inA.crassicarpathan inC.hystrix(Table 2).

Table 2 Vegetation coverage and soil properties in the five rehabilitation vegetations
SWC: Soil water content.
2.2 Soil fauna density
There were significant effects of the restoration treatments on the density of total soil fauna community and the community of Collembola and mites(Fig.1). The individuals of total fauna community, and Oribatida in shrublands andC.hystrixwere significantly more than that inA.crassicarpaandE.urophysella. There were significant higher density of soil mite in shrublands andC.hystrixthan inA.crassicarpa. The density of Collembola inC.hystrixwere significant higher than that inA.crassicarpaandE.urophysella.
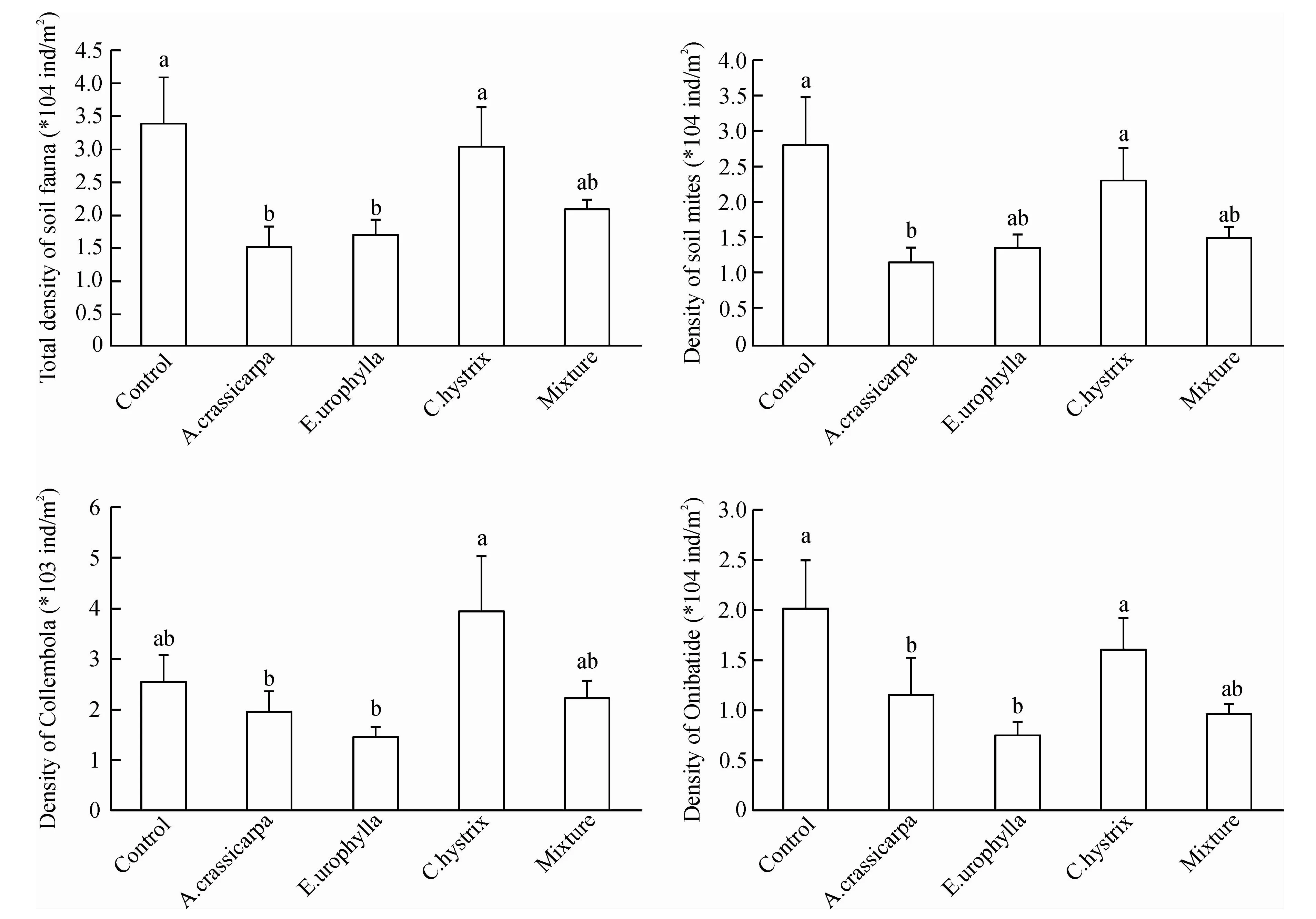
Fig.1 Effects of plantation type on soil fauna density
2.3 Soil fauna group richness and diversity
Soil fauna group richness and diversity was also significantly affected by the different restoration treatments(Fig.2). Soil fauna groups inE.urophysellawere significantly lower than in shrublands, native plantation ofC.hystrix, and the mixture plantation of native species. There were significant higher soil fauna groups inC.hystrixthan in exotic plantations ofE.urophysellaandA.crassicarpa. The diversity of Collembola in shrublands,C.hystrix, and the mixture plantation of native species was significant higher than that inA.crassicarpa.
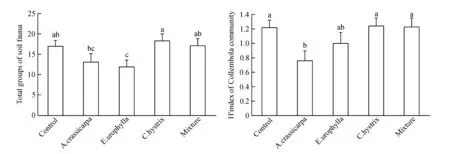
Fig.2 Effects of plantation type on the group richness and diversity of soil fauna
2.4 Effects of environmental factors on soil fauna
CCA on soil fauna data constrained by vegetation data was performed to quantify the effects of vegetation and soil on the variation in soil fauna community composition(Fig.3). All the measured vegetation and plant properties together could explain 34.7% of the soil fauna community community variation. Variables explaining the largest statistically significant amount of variation were herb cover, soil micorbial biomass carbon (unpublished data), soil water content and AM PLFAs (unpublished data).
In Fig.3, samples are labeled according to vegetation types. The symbols of circle, square, diamond, box and cross represent the samples from shrubland, plantations ofAcaciacrassicarpa,Eucalyptusurophylla,Castanopsishystrix, and Mixed native species.
3 Discussion
3.1 The positive responses of soil fauna in shrublands
Different reactions of soil fauna community to the rehabilitation treatments were detected, and it was astonishing to find significant advantages of soil fauna in control shrubland plots. They have the greatest variety of individuals, and almost all the indexes in shrublands were significantly higher than those in exotic plantationsE.urophyllaorA.crassicarpa. In the shrublands the understory vegetation was greatly flourishing, of which the fernDicranopterisedichotomadominated and was significantly more than that in all the other plantations. Understory vegetation was proved to have marked biomass, especially in younger stands, which can affects seed germination and it dispersers by constituting an adapted micoclimates and habits[23]. NILSSON and WARDLE reviewed recent researches in the boreal forest of northern Sweden to highlight the ecological role of understory vegetation as an ecosystem driver[24]. The ecological advantages of shrublands were also showed in other studies conducted in the site. It was found that theDicranopterisedominated understory had significant positive effects on soil microbial community, abundance of total, fungivorous and plantparasitic nematodes, and was proposed a major driver of intensive forest ecosystem in the humid subtropical and tropical regions[25].
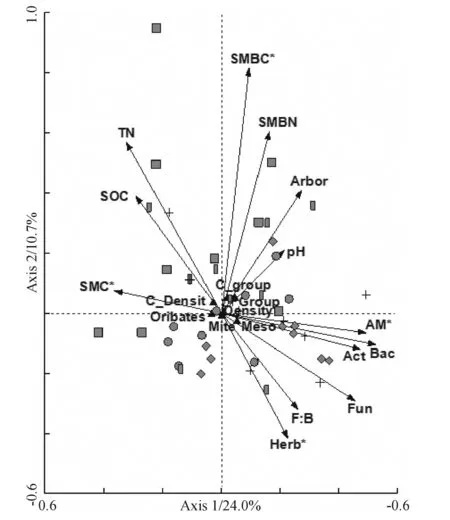
Fig.3 CCA of soil fauna community composition constrained by plant and soil properties in different vegetation types
According to the results of soil properties and CCA analysis, the fern coverage, soil water content and pH were closely related to the soil fauna community. They were all superior in the shrublands.D.dichotomais a dominant species of the understory in this site, which acted as early-stage colonizers in primary succession of acidic and oligotrophic soils distributing widely in humid subtropical and tropical regions of the world[25].D.dichotomain the area formed inconceivable indusaeform understory layer, which is in high-density and difficult to go through. At the same time, it produces also a thick layer of rhizomes and meshed roots on the soil surface occupying the whole humid layer, which constituted also complex econiches. Soil temperature would markedly increase 2~3℃ without the cover ofD.dichotoma[26]. The shrublands had also significantly higher soil water content due to the relative lower soil temperature, depression of evaporation by the dense rhizomes and fern canopy. Soil moisture has often been reported to be the most important environmental variable affecting both the structure and function of the soil fauna community[27]. In addition, we found that soil pH in the shrublands significantly increased compared with other plantations after 6 years restoration. pH is critical important to organisms, and would cause significant effects on the colony and survival of plants and invertebrates. For example, Collembola absorb free acidic water by mouth, skin and vertral tube, and the fecundity, egg production and hatchability were all adapted to only a certain pH environment[28]. In our experimental site, the lateritic soil is heavy acidified by vegetation degradation and acidic deposition. The improvement of soil pH was sure to be beneficial to the soil ecosystem. At last, the greatly complex and thick root sphere would logically stimulate the development of soil invertebrates in shrublands. Because the exudation and decomposition of fine roots supply energy and other resources to the soil biota, and most biotic activity in soil occurs in the rhizosphere[13], as was also confirmed by a isotope label experiment in Switzerland[29]. All the same, we thought, the amelioration of soil microclimates, soil condition and rhizosphere might produced more soil fauna individuals and diversity in shrublands than in the other treatments. This study might indicate the potentiality of forest restoration by nature in this area, and the simple protection from disturbance might be enough to facilitate the ecosystem regeneration.
3.2 Negative effects of exotic fast-growing species
In contrast to shrublands, we found disadvantages of soil fauna community in both exotic fast-growing plantations. All the indexes, including individuals and group numbers of the whole community, soil mites density, Collembola density and diversity were significantly lower inE.urophyllaandA.crassicarpathan in shrublands or nativeC.hystrixplantation.
Fast-growing and N-fixing legumes are widely used in afforestation. Because they may be the species easier to recolonize after disturbances, and they are though to be beneficial to the harsh soil condition through the production and decomposition of nitrogen-rich leaf litter[5]. However, it was inconsistent with this study. We found the negative effects ofA.crassicarpaon soil amelioration, at least in the early stages of reforestation. There were significantly lower individuals and group richness of soil fauna, and also soil pH and soil water content. It might be related to the significantly higher arboreal coverage, and the dense and large size of leaves. The canopy caused markedly less light puncture to the ground, and shaded out many of the understory plants, because light intensity may be the crucial environmental factor determining the composition and structure of understory vegetation[6]. There was significantly lower fern coverage inA.crassicarpathan that in all the other treatments. Absence of the advantages of shrubs on soil ecosystem mentioned above might caused the small soil fauna community. The negative effects ofA.crassicarpamight reflect the benefits of understory layer in the soil restoration on the contrary side.
Eucalyptuswas widely used in subtropical China in plantations. However, it was in great dispute because of the allelopathic effects, largely due to the biodiversity concern, for example plant community structure and composition[5]. There was significantly higher arboreal coverage inE.urophyllathat other plantations because of the fast-growth (Table 1). In contrast toA.crassicarpa, because of the high stem, small leaf and thin canopy, arboreal coverage did not obviously inhibit light puncturing. The fern coverage inE.urophyllawas relatively high and SWC was not different from shrublands andC.hystrix. The markedly negative responses of soil fauna inE.urophyllamight be due to the allelopathic effects. Although we could not find related studies on the effects ofEucalyptusallelochemicals on soil fauna, it was illustrated much in other biota.Eucalyptuscould dramatically alter the vegetation development through the release of allelochemicals[30]. The allelopathic effects ofEucalyptusspecies have been much reported in previous studies[30-31]. It was proved that certain phenolic acids and volatile oils released from the leaves, bark and roots ofEucalyptusspp. act as allelopathic agents inhibiting plants growth and mycorrhizal colonization[30-31]. In this study, soil microbial biomass carbon and AM fungi was found significantly related to the soil fauna data, and it was lower inE.urophyllathan that in shrublands. Similarly, TEDERSOO et al. reported thatEucalyptusspp. (i.e., E. robusta) could contract ectomycorrhizal associations in their introduction area with most of the native ectomycorrhizal symbionts[32], andE.urophyllaplanted soil significantly reduces the early growth and ectomycorrhization of native seedlings in Madagascar[5].
Most Collembola and Oribatida are mycophagous with preference for grazing the hyphae of mycorrhizal fungi[33-34]. For example, five needle-excavating oribatid species may need particular fungi to make oviposition possible[35]. Therefore, the negative responses of microbial might also contribute to the reduction of soil fauna. This study also implied the negative effects ofEucalyptuson biodiversity.
3.3 Potential value of revegetation of degraded lands in subtropical China
Varied restoration strategies have been suggested in many studies[1-2]. It is difficult to decide a standard model. However, an important consideration when planning a restoration project is the local condition. In our area, we found the natural recoverage mainly by fern developed very fast because of the adequate precipitation and sunshine. The flourishing fern community produced the biggest soil fauna community. It was consistent with the positive results under the shrubs in the same area for litter decomposition, fungal biomass and the ratio of fungal biomass to bacterial biomass, and plant-parasitic nematodes[25].
Plantation of native speciesC.hystrixalso showed significantly positive effects on soil fauna. However, XU et al. found the smallest soil fauna community in the native species plantation nearby after restoration for 20 years[36]. It was due to the slowly and same-step growing canopy which produced very thick and close crowns making light difficult to penetrate to the ground. The dark condition shaded out more understories than all other treatments. Therefore, we think, the positive effects of native speciesC.hystrixin this study might only be due to the slow growth in the early stage of plantation, which made the understory indifferent to the shrublands.
Although it is common to use exotic species during the revegetation of degraded lands, and it may be even necessary in some area to assist the restoration process where mining or severe overgrazing have resulted in loss of soil fertility to cause sterile exotic grasses to quickly establish cover. For example, Lugo provided observational evidence that exotic trees successfully ameliorated the degraded environmental conditions and stimulated the establishment of native tree species[37]. In addition, exotic fast-growing and N-fixing species were also considered to be nurse plants, whose higher levels of humidity and partial shade provide conditions suitable for seedling establishment, and can facilitate understory growing beneath their canopies[38]. However, the negative effects of exotic species should not be neglected, just as many studies proposed[5,8,39 ]. It was confirmed by our study. The results did not show the advantages of fast-growing species. In contrast, just because of the fast growth, it shaded out significantly the understory plants and then inhibited the soil improvement.
On the other hand, natural restoration is considered to be ecologically optimum among all kinds of restoration strategies[38]. Naturally recovered forest is superior to plantations in soil and water conservation, ecosystem stability, microclimates improvements, biodiversity increase, etc. VIEIRA and SCARIOT demonstrated that in seasonally dry tropical forests it was high potential to natural recovery because of the high proportion of small seeded, wind-dispersed species, the high ability of sprouting after disturbance, and the relatively simple community diversity and structure. In the eastern deciduous forests of the United States, natural regeneration after farmland abandonment produced widely forests succession largely similar (although younger) in structure to pre-disturbance forests. SKEEL, et al. suggested that physiological and vegetative performances of three native prairie grasses were all well adapted to the strip mine soils in Colorado and could act as viable alternative to nonnative plantings typically planted in these sites for restoration[40]. In the same area, consisting with this study, LIN, et al. proposed that degraded land could develop its understory very fast with fine ecological efficiency[41], and soil nutrients and water conservation by natural restoration could reach very fast to the level of zonal vegetation in a related short time only by area protection[38]. In conclusion, our study proved that natural restoration is valuable, because the fast development of understory provides more adapted microclimates for soil amelioration. At least, it was available in subtropical area with adequate precipitation and temperature.
[1] GAYNOR V. Prairie restoration on a corporate site[J]. Restor Reclam Rev, 1990, 1(1): 35-40.
[2] ZHAO P, PENG S L,ZHANG J W. Restoration ecology: An effective way to restore biodiversity of degraded ecosystems[J]. Chin J Ecol, 2000,19(1): 53-58.(in Chinese)
[3] BAO W K, LIU Z G, LIU Q. Ecological restoration and rehabilitation: Development, researching features and existing major problems[J]. The World’s Sci Tech Res Devel, 2001, 23(1):44-48.(in Chinese)
[4] HOLL K D, LOIK M E, LIN E H V, et al.Tropicalmontane forest restoration in Costa Rica: Overcoming barriers to dispersal and establishment[J]. Restor Ecol, 2000(8): 339-349.
[5] D′ANTONIO C, MEYERSON L A. Exotic plant species as problems and solutions in ecological restoration: A synthesis[J]. Restor Ecol, 2002(10): 703-713.
[6] DUAN W, REN H, FU S, et al. Community comparison and determinant analysis of understory vegetation in Six Plantations in South China[J]. Restor Ecol, 2010(18): 206-214.
[7] VITOUSEK P M, D’ANTONIOC M, LOOPEL L, et al. Introduced species: a significant component of human-caused global change[J]. New Zealand J Ecol, 1997(21):1-16.
[8] DEL-MORAL R, MULLER CH. The allelopathic effects ofEucalyptuscamaldulensis[J].Am Midl Nat, 1970(83):254-282.
[9] PAVAO-ZUCKERMAN M A. The nature of urban soils and their role in ecological restoration in cities[J]. Restor Ecol, 2008, 16(4): 642-649.
[10]HOOPER D U, BIGNELL D E, BROW N V K, et al. Interactions between aboveground and belowground biodiversity in terrestrialecosystems: patterns, mechanisms, and feedbacks[J].Bioscience, 2000(50): 1049-1061.
[11]WARDLE DA, BARDGETT RD, KLIRONOMOS JN, et al.Ecological linkages between aboveground and belowground biota[J]. Science, 2004(304): 1629-1633.
[12]RUSEK J. Biodiversity of Collembola and their functional role in the ecosystem[J]. Biodiv Conserv, 1998(7): 1207-1219.
[13]FITTER AH,GILLIGAN C A,HOLLINGWORTH K, et al. Biodiversity and ecosystem function in soil[J]. Funct Ecol, 2005(19): 369-377.
[14]ALEXANDRU M,STEPHAN P, REINHARDT L, et al. The response of decomposers (earthworms, springtails and microorganisms) to variations in species and functional group diversity of plants[J]. OIKOS, 2006(112): 513-524.
[15]CHAUVAT M, ZAITSEVA S, WOLTERS V.Successional changes of Collembola and soil microbiota during forest rotation[J].Oecologia, 2003(137):269-276.
[16]XU G L, SCHLEPPI P, LI M H, et al. Negative responses of Collembola in a forest soil (Alptal) under increased atmospheric N deposition[J]. Envir Poll, 2009(57): 2030-2036.
[17] JANSSENS F. Checklist of the Collembola of the world[EB/OL][2012-06-15]. http://www.collembola.org/.
[18]POTAPOV M. Isotomidae[C]∥DUNGER W. Synopses on Palaearctic Collembola. Görlitz, Germany, 2001(3): 603.
[19]BRETFELDG.Symphypleona[C]∥DUNGER W. Synopses on Palaearctic Collembola. Görlitz, Germany, 1999(2): 318.
[20]POMORSKI R J. Onychiurinae of Poland (Collembola: Onychiuridae)[M]. Wroclaw: Polish Taxonomical Society, 1998:1-201.
[21]BOSSIO D A, SCOW K M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns[J]. Microb Ecol, 1998(35): 265-278.
[22]LIU G S, JIANG N H, ZHANG L D, et al. Soil physical and chemical analysis and description of soil profiles[M]. Beijing: Standards Press of China, 1996.(in Chinese)
[23]ALABACK P B. Dynamics of understory biomass in Sitkasprucewestern hemlock forests of Southeast Alaska[J]. Ecology, 1982(63):1932-1948.
[24] NILSSON MC, WARDLE DA. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest[J]. Front Ecol Envir,2005(3): 421-428.
[25]ZHAO J, WANS Z, LI Z, et al. Dicranopteris-dominated understory as major driver of intensive forest ecosystem in humid subtropical and tropical region[J]. Soil Biol Bioch, 2012(49): 78-87.
[26]LIU Z F, WU J P, ZHOU L X, et al. Effect of understory fern (Dicranopterisdichotoma) removal on substrate utilization patterns of culturable soil bacterial communities in subtropicalEucalyptusplantations[J]. Pedobiologia, 2012(55): 7-13.
[27]XU G L, KUSTER TM, Günthardt-GOERG MS, et al. Seasonal exposure to drought and air warming affects soil collembola and mites[J]. PLOS ONE, 2012, 7(8): 1-6.
[28]RUSEK J, MARSHALL VG. Impacts of airborne pollutant on soil fauna[J]. Ann Rev Ecol System, 2000(31): 395-423.
[29]POLLIERER M M, LANGEL R, KÖRNER C, et al. The underestimated importance of belowground carbon input for forest soil animal food webs[J]. Ecol Lett, 2007(10): 729-736.
[30]DEL-MORAL R, MULLER CH. The Allelopathic effect ofEucalyptuscamaldulensis[J]. Amer Midl Natur, 1969, (83): 254-282.
[31]FORRESTER DI, BAUHUS J, COWIE AL, et al. Mixed-species plantations of eucalyptus with nitrogen-fixing trees: A review[J]. Forest Ecol Manag, 2006(233): 211-230.
[32]TEDERSOO L, SUVI T, BEAVER K, et al. Ectomycorrhizal fungi of the Seychelles: diversity patterns and host shifts from the nativeVateriopsisseychellarum(Dipterocarpaceae) andIntsiabijuga(Caesalpiniaceae) to the introducedEucalyptusrobusta(Myrtaceae), but notPinuscaribaea[J]. New Phytol, 2007(175):321-333.
[33]HOPKIN S P. Biology of the Springtails-Insecta: Collembola[D]. Oxford: Oxford University, 1997.
[34]MARAUN M, MIGGE S, SCHAEFER M, et al. Selection of microfungal food by six oribatid mite species (Oribatida, Acari) from two different beech forests[J]. Pedobiologia,1998(42): 232-240.
[36]XU G L, ZHOU G Y, MO J M. Changes of soil fauna with forest restoration in Subtropical China[J]. Zool Res, 2006, 27(1): 23-28.(in Chinese)
[37]LUGO A E. The future of the forest: ecosystem rehabilitation in the tropics[J]. Environment, 1988(30): 41-45.
[38]REN H,DU W B,WANG J, et al. The natural restoration of degraded rangeland ecosystem in Heshan hill land[J]. Acta Ecol Sin, 2007, 27(9): 3593-3600.(in Chinese)
[39]TEDERSOO L, SUVI T, BEAVER K, et al. Ectomycorrhizal fungi of the Seychelles: diversity patterns and host shifts from the nativeVateriopsisseychellarum(Dipterocarpaceae) andIntsiabijuga(Caesalpiniaceae) to the introducedEucalyptusrobusta(Myrtaceae), but notPinuscaribaea[J]. New Phytol, 2007(175):321-333.
[40]SKEEL V A, GIBSOND J. Physiological performance ofAndropogongerardii,PanicumvirgatumandSorghastrumnutanson reclaimed mine spoil[J]. Restor Ecol,1996(4):355-367.
[41]LIN Y B, REN H, PENG S L. The community structure of degraded grassland in Heshan City[J]. Ecol Sci, 2000, 19(3): 23-26.(in Chinese)
【责任编辑: 陈 钢】
Effects of forest rehabilitation managements on soil fauna community in southern subtropical Heshan, Guangdong of China
XUGuo-liang1,FANGBi-zhen1,ZHOULi-xia2,LIUZhan-feng2
(1.School of Geographical Sciences, Guangzhou University, Guangzhou 510006, China;2.South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China)
Restoration ecology has focused mainly on the vegetations aboveground, but few belowground, especially on soil fauna. Responses of soil fauna to 6 years of rehabilitation experiment in Heshan, subtropical China, 50 hm2area were reported in this study.It was found that the soil ecosystems were greatly changed by the forest managements. Because of the large canopy density of the plantations and the negative effects of exotic species, most of the results showed positive responses in the control shrublands during the experimental period.The density of total community and soil mites in shrublands were significantly higher than those inAcaciacrassicarpaandEucalyptusurophylla, and Collembola individuals inCastanopsishystrixwere significantly more than those inA.crassicarpaandE.urophylla.Soil fauna group richness was also significantly affected by the restoration treatments. Soil fauna groups inE.urophysellawere significantly lower than those in shrublands andC.hystrix, and species richness and diversity of Collembola inA.crassicarpawere significantly lower than that in shrublands andC.hystrix.There was no obvious effect of the mixture plantation on soil fauna community.The environmental analysis showed that the fern coverage and the average soil pH value were significantly higher in the shrublands than in the other plantations. Soil water content was negative inA.crassicarpa.The study indicated the ecological advantages of natural restoration in subtropical China and negative ecological effects of exotic fast-growing species on soil ecosystem in different ways.
ecological restoration; soil fauna; southern subtropical zone; Heshan
ET 471 Document code: A
Foundation items: This work was supported by Natural Science Foundation of China(41571247),Guangdong Natural Science Foundation (2014A030313532),and High Level University Construction Project of Guangdong Province.
1671- 4229(2016)05-0056-10
Q 151.2
A
Received date: 2016-07-15; Revised date: 2016-07-23
Biography: XU Guo-liang (1975-),male,Ph. D. E-mail: xugl@gzhu.edu.cn
