脑血红蛋白表达增高可减轻α-突触核蛋白引起的MES23.5细胞凋亡
2016-12-23杨巍巍李旭冉李旭颖
杨巍巍 李旭冉 李 昕 李旭颖 于 顺,3*
(1.首都医科大学宣武医院神经生物学研究室,北京 100053;2.帕金森病研究北京重点实验室,北京 100053;3.北京脑重大疾病研究院帕金森病研究所,北京 100053)
· 基础研究 ·
脑血红蛋白表达增高可减轻α-突触核蛋白引起的MES23.5细胞凋亡
杨巍巍1,2李旭冉1,2李 昕1,2李旭颖1,2于 顺1,2,3*
(1.首都医科大学宣武医院神经生物学研究室,北京 100053;2.帕金森病研究北京重点实验室,北京 100053;3.北京脑重大疾病研究院帕金森病研究所,北京 100053)
目的 观察脑血红蛋白(neuronal hemoglobin,nHb)在α-突触核蛋白(α-synuclein,α-syn)引起的细胞凋亡中的作用。方法 在MES23.5细胞中瞬时转染空载(myc)和nHb质粒,部分转染细胞外加α-syn,免疫荧光化学方法鉴定基因表达情况。Western blotting法检测α-syn与nHb表达水平。JC-1检测各组细胞线粒体膜电势水平,TUNEL染色和MTT法检测各组细胞死亡情况。结果 过表达nHb可与α-syn在胞质及线粒体均形成复合物,并显著降低游离α-syn在胞质及线粒体的水平,同时,nHb过表达可显著逆转α-syn所致线粒体膜电势下降及细胞凋亡。结论 nHb表达增高可缓解α-syn致细胞损伤。
α-突触核蛋白;血红蛋白;线粒体;凋亡
帕金森病(Parkinson’s disease,PD)是一种复杂的神经退行性疾病,以运动障碍为主要临床特征。目前,PD发病机制尚不明确,但研究[1-3]显示遗传因素与PD发生发展密切相关。
路易体的主要成分是α-突触核蛋白(α-synuclein,α-syn),其被认为是与散发性和遗传性PD发生发展紧密相关的蛋白[4-5]。近来文献[6-8]报道α-syn可以与其他蛋白如超氧化物歧化酶1(superoxide dismutase 1, SOD1)、 促微管聚集蛋白(tubulin polymerization promoting proteins, TPPP)及3,4-二羟基苯乙醛(3,4-dihydroxyphenylacetaldehyde, DOPAL)等发生相互作用从而参与了α-syn的病理机制。同时,文献[9]显示α-syn可引起线粒体损伤。
神经球蛋白属于氧结合蛋白家族,包括脑红蛋白,脑血红蛋白(neuronal hemoglobin,nHb),肌红蛋白,这些蛋白被普遍认为参与神经系统兴奋性、氧自由基清除及调节细胞存活[10]。但这些蛋白最主要的作用是维持线粒体正常生理功能[11-18]。神经球蛋白同时也参与了神经退行性病变的发生与发展。例如,在PD患者脑内发现nHb异常过表达[19]。但是nHb是否缓解α-syn所致的线粒体损伤及细胞凋亡尚未见报道。本文主要探讨nHb与α-syn的关系及nHb对MES23.5细胞的保护作用,可能有助于为PD的发病机制及药物作用靶点研究提供新思路。
1 材料和方法
1.1 仪器与试剂
DMEM/F12培养基、胎牛血清和胰蛋白酶(美国Gibco公司);actin抗体(美国Sigma公司);鼠抗人Hb单克隆抗体(美国Abcam公司);myc抗体(美国Clontech公司);鼠抗人α-syn单克隆抗体3D5由本室制备;生物素化的鼠单克隆抗体3D5(北京康为世纪生物科技有限公司);4-硝基磷酸二钠盐(美国Sigma公司);辣根过氧化物酶(horseradish peroxidase,HRP)标记山羊抗小鼠IgG多克隆抗体(北京中杉金桥生物技术有限公司);ELISA 96孔酶标板(美国Corning公司);酶标仪(瑞士Tecan公司);细胞凋亡检测试剂盒(美国Roche公司);MTT(北京欣经科生物公司);Lipofectamine 2000 (德国Qiagen公司);HRP标记的二抗(美国KPL公司);人源nHb质粒(pCMV-myc-nHb) 及其空载对照(pCMV-myc) 均由于顺教授实验室构建,其他试剂为分析纯。
1.2 方法
1)细胞培养及转染:MES23.5细胞采用含5%(体积分数)胎牛血清和Sato’s添加液、100 U/mL青霉素和100 U/mL链霉素的DMEM/F12培养基,置于37 ℃,5%(体积分数)CO2培养箱中培养。质粒转染按照Lipofectamine2000操作说明书进行。
2) MTT方法检测细胞活力:分组为正常对照组(Con),外加α-syn处理组(10mol/L,1、3、6 h)空载对照组(myc),空载对照组外加α-syn(10mol/L,1、3、6 h)单纯过表达nHb组,过表达nHb外加α-syn处理组(10mol/L,1、3、6 h)
上述各组MES23.5细胞按1×105个/孔接种于96孔板,每组细胞接种8个复孔。接种后24 h,向各孔加入MTT(5 g/L) 20 mL,37 ℃,5%(体积分数) CO2培养箱继续培养4 h。后每孔加入100 mL二甲基亚砜,振荡10 min。将孔板置于酶标仪中,取550 nm波长处的吸光度值统计分析。实验至少重复3次进行统计学分析。
3) Western blotting分析:实验分组为正常对照组(Con),外加α-syn处理组(10mol/L,6 h)空载对照组(myc),空载对照组外加α-syn(10mol/L,6 h)单纯过表达nHb组,过表达nHb外加α-syn处理组(10mol/L,6 h)。将上述每组细胞按2×108个接种于每个大培养皿中,每组细胞接种3个大皿。抽提上述各组细胞胞质、线粒体及胞膜蛋白,选用BCA法测定蛋白浓度,SDS-PAGE电泳,半湿转法将蛋白转移至PVDF膜,10%(质量分数)脱脂牛奶封闭1 h,分别入抗3D5单抗 (1∶5 000),Hb(1∶1 000),actin (1∶1 000),VDAC(1∶1 000),calnexin(1∶1 000)室温孵育3 h。后入相应HRP标记的羊抗兔(或鼠)免疫球蛋白抗体中(1∶5 000),室温孵育1 h。加入化学发光液,于暗室化学发光及显影、定影。实验至少重复3次进行统计学分析。
4)nHb检测:抽提上述各组细胞胞质、线粒体及胞膜蛋白,利用Western blotting分别检测上述各细胞组分中的nHb,利用肌动蛋白(actin)、电压依赖的选择性阴离子通道蛋白(voltage-dependent anion-selective channel, VDAC)、钙连接蛋白(calnexin)分别作为胞质、线粒体、膜组分的内参。以nHb条带灰度值比不同内参的灰度值作为标准化后不同组细胞各亚细胞组分nHb的水平。实验至少重复3次进行统计学分析。
5) 免疫细胞化学染色:上述各组细胞培养24 h后进行myc或者nHb质粒转染,转染24 h后做免疫荧光染色,1 ×PBS冲洗2次,加入4%(质量分数)多聚甲醛,室温固定20 min,10%(体积分数)山羊血清室温孵育1 h封闭,加入抗myc(1∶500)及3D5一抗(1∶1 000),室温避光孵育3 h,然后与Alexa Fluor标记山羊抗小鼠(或兔)荧光二抗(1∶5 000)室温孵育1 h。加入4,6-二氨基-2-苯基吲哚(DAPI)于37 ℃避光孵育5 min复染细胞核,甘油封片后用共聚焦扫描显微镜观察。
6) 与血红蛋白结合的α-syn的ELISA检测: 实验分组为正常对照组(Con),外加α-syn处理组 (10mol/L,6 h)空载对照组(myc),空载对照组外加α-syn(10mol/L,6 h)单纯过表达nHb组,过表达nHb外加α-syn处理组(10mol/L,6 h)。将上述每组细胞按2×108个接种于每个大培养皿中,每组细胞接种3个大皿。抽提每组细胞胞质、线粒体、胞膜组分,BCA定量后进行ELISA检测。用质量浓度为2 mg/L 血红蛋白单抗包被96孔酶标板,2.5%(质量分数)明胶封闭2 h。加入各组细胞样品100L(0.1g/L),37 ℃孵育2 h,再加入生物素标记3D5抗体 (终质量浓度1 mg/L),37 ℃孵育2 h。向各孔加入100 mL碱性磷酸酶标记的亲和素 (1∶5 000),37 ℃孵育1 h。最后加入100 mL对硝基酚磷酸显色液 (pNPP),37 ℃显色30 min。405 nm处测定吸光度值。实验至少重复3次进行统计学分析。
7)与血红蛋白结合的α-syn免疫共沉淀检测:实验所用细胞样本数同上,将上述细胞组织匀浆20 mg/L与7 mg/L的抗人红蛋白抗体于4 ℃孵育过夜,然后与protein G 反应,12 000 g离心5 min,弃上清,0.01 mol/L PBS缓冲液500L洗涤沉淀3次,弃上清,将沉淀用3D5抗体进行Western blotting检测,以分析各组细胞不同亚细胞部位与血红蛋白结合的α-syn的量。实验至少重复3次进行统计学分析。
8) TUNEL染色:实验分组为正常对照组(Con),外加α-syn处理组(10mol/L,6 h)空载对照组(myc),空载对照组外加α-syn(10mol/L,6 h)单纯过表达nHb组,过表达nHb外加α-syn处理组(10mol/L,6 h)。将上述每组细胞按1×103个接种于每个confocal培养皿中,每组细胞接种3个confocal皿。将上述各组细胞用1×PBS冲洗3次,后用4%(质量分数)多聚甲醛,室温固定20 min,5g/mL蛋白酶K处理细胞5 min,PBST冲洗3次,加入TdT酶反应液,37 ℃孵育1 h,避光。DAPI复染细胞核,甘油封片后用共聚焦扫描显微镜观察。实验至少重复3次进行统计学分析。
9) MES23.5细胞线粒体膜电势检测:JC-1是一种广泛应用于检测线粒体膜电势(ΔΨm)的染料。在ΔΨm 增高时,JC-1形成聚合物(J-aggregates)并聚集在线粒体基质中,可以产生红色荧光;在线粒体受损膜电位下降时,JC-1呈单体形式存在且不聚集在线粒体基质中,可以产生绿色荧光。通过红绿荧光强度的比值则可以判断线粒体膜电位的变化。MES23.5细胞转染myc/nHb质粒24 h后外加α-syn孵育1、3、6 h。收集上述各组细胞,以F12培养基稀释JC-1至工作质量浓度10g/mL,37 ℃,5%(体积分数) CO2培养箱内孵育10 min后,利用流式细胞仪进行检测。实验至少重复3次进行统计学分析。
1.3 统计学方法
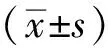
2 结果
2.1 在MES23.5细胞中nHb-α-syn复合物形成可减少线粒体游离nHb
Western blotting及免疫荧光结果显示,MES23.5细胞中均没有检测到内源性α-syn信号(图1A、B)。然而,在α-syn处理组细胞胞质、线粒体及胞膜组分中,均检测到外源性α-syn存在(图1A、B)。过表达nHb后,在细胞胞质及线粒体中nHb显著增高(P<0.05);而在α-syn处理组中,胞质以及线粒体中游离nHb分别较未处理组降低显著(P<0.05),详见图1A;而Co-IP及ELISA结果显示,细胞过表达nHb后,nHb-α-syn复合物在胞质及线粒体组分显著增高(P<0.05),详见图1C。
2.2 nHb可缓解α-syn所致MES23.5细胞线粒体膜电势降低
JC-1染色结果显示,α-syn可显著降低MES23.5细胞线粒体膜电势(P<0.05,图2A、B)。然而,过表达nHb后,MES23.5细胞线粒体膜电势较α-syn处理组增高显著(P<0.05),详见图2A、B。
2.3 nHb可缓解α-syn所致MES23.5细胞凋亡
TUNEL及MTT结果显示,α-syn可使细胞凋亡数目增加并使细胞活力降低(P<0.05),而nHb过表达则明显缓解α-syn所致细胞凋亡,并增加细胞活力(P<0.05),详见图3A~C。
3 讨论
本文在MES23.5多巴胺能神经细胞中外加人源性重组α-syn纯蛋白,在线粒体及胞质组分可明显观察到α-syn表达显著增高。这是由于α-syn加入细胞培养基后,可以通过被动扩散迅速进入到MES23.5细胞中[20]。但是在胞质及线粒体组分均发现游离nHb在α-syn细胞处理组降低显著。同时,笔者还观察到α-syn积聚可显著增加线粒体中nHb-α-syn复合物。原因目前尚不明确,但有可能是由于MES23.5细胞内源性α-syn极低,所以导致内源性nHb-α-syn复合物处于较低水平,而当添加外源性α-syn后,线粒体内α-syn浓度迅速增高并与nHb结合,因此,显示出较高的nHb-α-syn复合物形成,并在一定观察时间内没有降低到基线水平。更重要的是,nHb-α-syn复合物的形成可显著降低线粒体内游离nHb。
文献[12]显示,内源性nHb主要定位于线粒体,并且在减轻鱼藤酮致细胞线粒体损伤中起关键作用。线粒体nHb可提高线粒体膜电势并且维持线粒体ATP产量[21]。笔者进而观察了α-syn积聚所致线粒体nHb减低是否能使细胞线粒体膜电势降低并增加细胞凋亡。结果显示,加入外源性α-syn可使线粒体游离nHb降低并同时伴随线粒体膜电势降低。而过表达nHb可显著缓解α-syn积聚所致的线粒体损伤。由于线粒体介导的凋亡起始于线粒体膜电势降低,笔者进一步观察了nHb抵抗α-syn致细胞凋亡的保护作用。结果表明,nHb可明显减轻α-syn致细胞凋亡及细胞活力降低。以上结果提示nHb可能通过维持线粒体膜电势进而抵抗α-syn所介导的细胞凋亡。
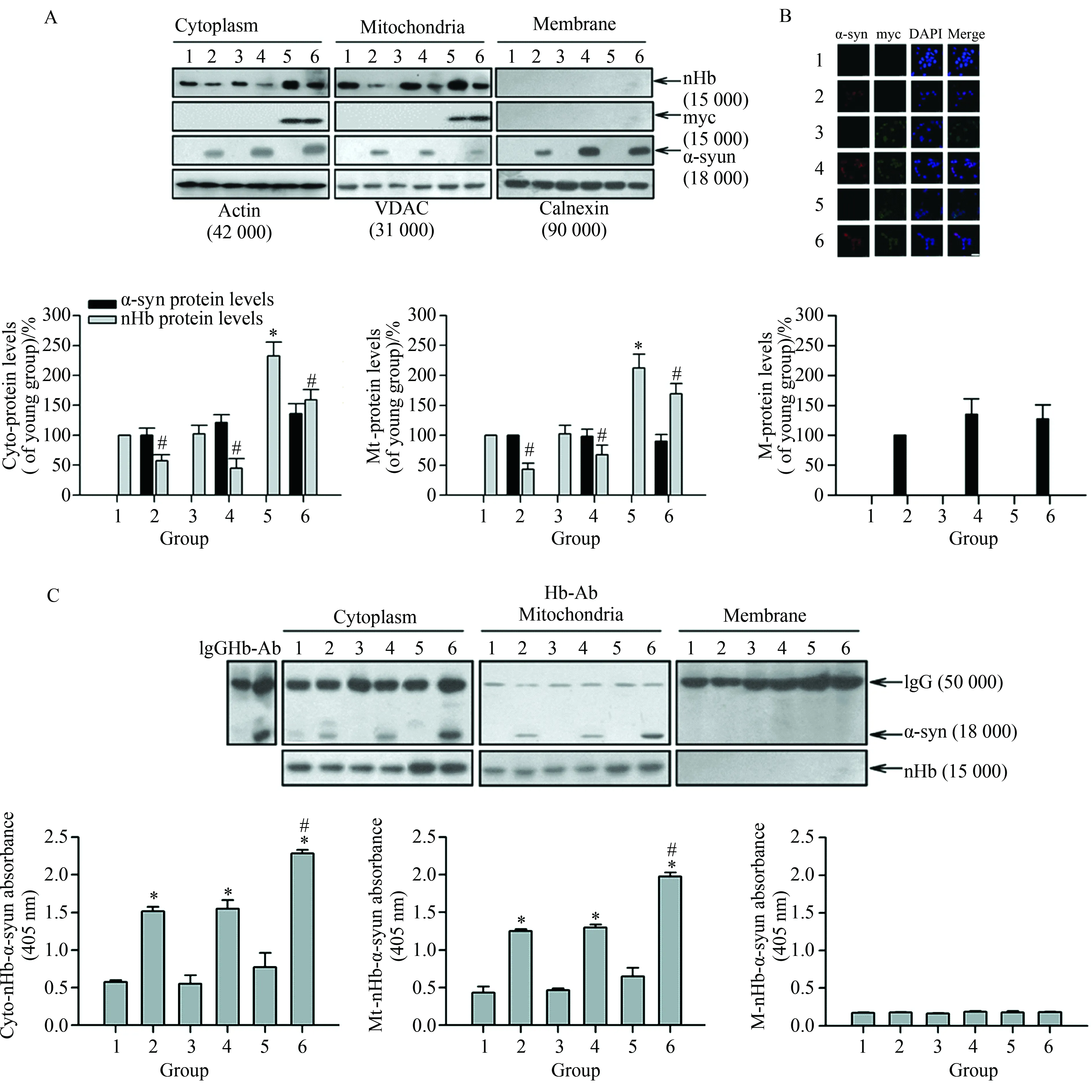
图1 在MES23.5细胞中nHb-α-syn复合物形成可减少线粒体游离nHb
Fig.1 Reduction of free mitochondrial nHb levels by formation of nHb-a-syn complex in MES23.5 cells
A:The amount of free nHb, myc, α-syn expressed indicated by Western blotting analysis of the protein samples before IP. Actin, VDAC, Calnexin were detected as a cytosol, mitochondria and membrane loading control, respectively.*P<0.05vs1 group (n=6);#P<0.05vs1, 3, 5 group, respectively (n=6);B: Cells were transfected with a myc or myc/nHb vector for 24 h with or without α-syn (10mol/L) treatment for 6 h. At 24 h after transfection, MES23.5 cells fixed and stained with myc and α-syn antibody, followed by a mouse Alexa 488- or rabbit Alexa 594-conjugated secondary antibody. The nucleus was counterstained by DAPI. Bar=50 μm;C: α-syn immunoprecipitated by an Hb antibody was detected in cytosolic- and mitochondrial-fraction in 2, 4, 6 lanes (upper panel). Levels of nHb-α-syn complex were detected in cytosol, mitochondria and membrane by ELISA (lower panel).*P<0.05vs1 group (n=6);#P<0.05vs2 or 4 group (n=6); Data were expressed as the mean±SD; Tukey’s multiple comparisons test after ANOVA; Hb-Ab: hemoglobin antibody; Cyto-nHb-α-syn: neuronal hemoglobin-α-syn complex in cytosolic fraction; M-nHb-α-syn: neuronal hemoglobin-a-syn complex in membrane fraction; Mt-nHb-α-syn: neuronal hemoglobin-α-syn complex in mitochondrial fraction; 1: control group; 2: α-syn (10mol/L) treatment for 6 h group; 3: myc vector transfection for 24 h group; 4: myc vector transfection for 24 h with α-syn (10mol/L) treatment for 6 h group; 5: myc/nHb vector transfection for 24 h group; 6: myc/nHb vector transfection for 24 h with α-syn (10mol/L) treatment for 6 h group,α-syn:α-synuclein; nHb:neuronal hemoglobin;VDAC:voltage dependent anion-selective channel.

图2 nHb可缓解α-syn致细胞线粒体膜电势降低
Fig.2 nHb alleviated α-syn induced reduction of mitochondrial membrane potential
MES 23.5 cells were transfected with a myc or myc/nHb vector for 24 h with or without α-syn (10mol/L) treatment for indicated times. At 24 h after transfection, MES23.5 cells were stained by JC-1 and detected the mitochondrial membrane potential. A: Representative images showed the J-aggregate (FL 2) and J-monomer (FL 1) fluorescence intensity. B: Statistical results showed the ratios of J-aggregate/J-monomer fluorescence intensity. Data were expressed as the mean ±SD. Tukey’s multiple comparisons test after ANOVA.*P<0.05vscontrol group (n=6);#P<0.05vsa-syn treatment groups (n=6); α-syn;α-synuclein; nHb:neuronal hemoglobin.

图3 nHb可缓解α-syn所致MES23.5细胞凋亡
Fig.3 nHb alleviated α-syn induced MES23.5 cells apoptosis
A: Cells were transfected with a myc or myc/nHb vector for 24 h with or without α-syn (10mol/L) treatment for 6 h. At 24 h after transfection, MES23.5 cells fixed and stained with TUNEL, the nucleus was counterstained by DAPI. Bar=50 μm; B: Statistical results show the TUNEL positive cells. C: MTT assay of the cell viability in 1 - 6 groups with or without addition of α-syn (10mol/L) for 1, 3, 6 h. Data were expressed as the mean ±SD. Tukey’s multiple comparisons test after ANOVA.*P<0.05vs1 group (n=6);#P<0.05vs2 or 4 group (n=6); 1: control group; 2: α-syn (10mol/L) treatment for 1, 3, 6 h group; 3: myc vector transfection for 24 h group; 4: myc vector transfection for 24 h with α-syn (10mol/L) treatment for 1, 3, 6 h group; 5: myc/nHb vector transfection for 24 h group; 6: myc/nHb vector transfection for 24 h with α-syn (10mol/L) treatment for 1, 3, 6 h group. α-syn:α-synuclein, nHb:neuronal hemoglobin.
综上所述,在MES23.5细胞中,nHb与α-syn可形成复合物,使线粒体游离nHb显著降低,并缓解α-syn致细胞凋亡及线粒体膜电势降低。这一发现,可能有助于为PD的发病机制及药物作用靶点研究提供新思路。
[1] Lin C H, Chen M L, Tai Y C, et al. Reaffirmation of GAK, but not HLA-DRA, as a Parkinson’s disease susceptibility gene in a Taiwanese population[J]. Am J Med Genet B Neuropsychiatr Genet, 2013,162(8): 841-846.
[2] Mizuta I, Takafuji K, Ando Y, et al. YY1 binds to alpha-synuclein 3’-flanking region SNP and stimulates antisense noncoding RNA expression[J]. J Hum Genet, 2013,58(11): 711-719.
[3] Brockmann K, Schulte C, Hauser A K, et al. SNCA: major genetic modifier of age at onset of Parkinson’s disease[J]. Mov Disord, 2013, 28(9):1217-1221.
[4] Chang C, Lang H, Geng N, et al. Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD[J]. Neurosci Lett, 2013, 548: 190-195.
[5] Shaltiel-Karyo R, Frenkel-Pinter M, Rockenstein E, et al. A blood-brain barrier (BBB) disrupter is also a potent alpha-synuclein (alpha-syn) aggregation inhibitor: a novel dual mechanism of mannitol for the treatment of Parkinson disease (PD)[J]. J Biol Chem, 2013, 288 (24): 17579-17588.
[6] Helferich A M, Ruf W P, Grozdanov V, et al. alpha-synuclein interacts with SOD1 and promotes its oligomerization [J]. Mol Neurodegener, 2015, 10: 66.
[7] Szunyogh S, Olah J, Szenasi T,et al. Targeting the interface of the pathological complex of alpha-synuclein and TPPP/p25 [J]. Biochim Biophys Acta, 2015, 1852(12): 2653-2661.
[8] Follmer C, Coelho-Cerqueira E, Yatabe-Franco D Y, et al. Oligomerization and membrane-binding properties of covalent adducts formed by the interaction of alpha-synuclein with the toxic dopamine metabolite 3,4-Dihydroxyphenylacetal -dehyde (DOPAL) [J]. J Biol Chem, 2015, 290(46): 27660-27679.
[9] Zhang Z, Hou L, Li X, et al. Neruoprotection of inositol hexaphosphate and changes of mitochondrion mediated apoptotic pathway and α-synuclein aggregation in 6-OHDA induced parkinson’s disease cell model [J].Brain Res,2016, 1633: 87-95.
[10]Ascenzi P, Gustincich S, Marino M. Mammalian nerve globins in search of functions [J]. IUBMB Life,2014, 66(4): 268-276.
[11]Biagioli M, Pinto M, Cesselli D, et al. Unexpected expression of alpha- and beta-globin in mesencephalic dopaminergic neurons and glial cells [J]. Proc Natl Acad Sci U S A, 2009, 106(36): 15454-15459.
[12]Richter F, Meurers B H, Zhu C, et al. Neurons express hemoglobin alpha- and beta-chains in rat and human brains [J]. J Comp Neurol, 2009, 515(5): 538-547.
[13]Wilson M T, Reeder B J. Oxygen-binding haem proteins [J]. Exp Physiol, 2008, 93(1): 128-132.
[14]Gorgun F M, Zhuo M, Singh S, et al. Neuroglobin mitigates mitochondrial impairments induced by acute inhalation of combustion smoke in the mouse brain [J]. Inhal Toxicol, 2014, 26(6): 361-369.
[15]Kawada N, Kristensen D B, Asahina K, et al. Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells [J]. J Biol Chem, 2001, 276(27): 25318-25323.
[16]Kawada N. Current situation and future prospect of cytoglobin research[J]. Nihon Rinsho,2013, 71(5): 927-935.
[17]Burmester T, Ebner B, Weich B, et al. Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues [J]. Mol Biol Evol, 2002, 19(4): 416-421.
[18]李维德,孙青,李松,等. 脑红蛋白在蛛网膜下腔出血大鼠脑组织中的表达[J]. 中国脑血管病杂志, 2013,10(8):416-420.
[19]Shephard F, Greville-Heygate O, Marsh O, et al. A mitochondrial location for haemoglobins--dynamic distribution in ageing and Parkinson’s disease [J]. Mitochondrion,2014(1), 14: 64-72.
[20]Cheng F, Li X, Li Y, et al. alpha-Synuclein promotes clathrin-mediated NMDA receptor endocytosis and attenuates NMDA-induced dopaminergic cell death [J]. J Neurochem, 2011, 119(4): 815-825.
[21]Schloesser A, Esatbeyoglu T, Piegholdt S, et al. Dietary tocotrienol/gamma- cyclodextrin complex Increases mitochondrial membrane potential and ATP concentrations in the brains of aged mice [J]. Oxid Med Cell Longev,2015, 2015: 789710.
编辑 孙超渊
Expression of neuronal hemoglobin alleviates α-synuclein-induced apoptosis in MES23.5 cells
Yang Weiwei1,2,Li Xuran1,2,Li Xin1,2,Li Xuying1,2,Yu Shun1,2,3*
(1.DepartmentofNeurobiology,XuanwuHospital,CapitalMedicalUniversity,Beijing100053,China;2.KeyLaboratoryofNeurodegenerativeDisease,MinistryofEducation,Beijing100053,China;3.CenterforParkinson’sDisease,BeijingInstituteforBrainDisorders,Beijing100053,China)
Objective To explore the effect of neuronal hemoglobin (nHb) on α-synuclein(α-syn)-induced cell apoptosis. Methods myc vector or nHb was transiently transfected into MES23.5 cells with or without addition of exogenous α-syn. As identification of gene expression by immunofluorescence, Western blotting was used to detect the expression of α-syn and nHb. JC-1 staining was used to detect the mitochondrial membrane potential. The cell injuries were observed by TUNEL staining and MTT assay. Results nHb gene overexpression in MES23.5 cells lead to formation the complexes with α-syn in cytosolic and mitochondrial fraction, and reduced free nHb levels. Meanwhile, nHb could reverse α-syn-induced reduction of mitochondrial membrane potential and cell apoptosis. Conclusion Increase of nHb could alleviate α-syn-induced cell injuries.
α-synuclein (α-syn);hemoglobin;mitochondria;apoptosis
国家重点基础研究发展计划(2011CB504101),国家自然科学基金(81071014, 81371200,81401042),国家科技支撑计划课题(2012BAI10B03),首都卫生发展科研专项课题(2011-4001-01),北京市自然科学基金(7122035)。 This study was supported by Major State Basic Research Development Program (“973” Program) of China (2011CB504101), National Natural Science Foundation of China (81071014, 81371200, 81401042), National Science and Technology Support Program (2012BAI10B03),The Capital Health Research and Development Special Fund (2011-4001-01), Natural Science Foundation of Beijing (7122035).
时间:2016-12-14 20∶10
http://www.cnki.net/kcms/detail/11.3662.r.20161214.2010.016.html
10.3969/j.issn.1006-7795.2016.06.016]
R 338
2016-02-14)
*Corresponding author, E-mail:yushun103@163.com
