氰基桥联的Fe(Ⅱ)-Mn(Ⅲ)双金属链的可控组装和磁性作用的调控
2016-12-15矫成奇姜文静文雯任奕王佳良刘涛何成
矫成奇 姜文静 文雯 任奕 王佳良 刘涛何成
氰基桥联的Fe(Ⅱ)-Mn(Ⅲ)双金属链的可控组装和磁性作用的调控
矫成奇 姜文静 文雯 任奕 王佳良 刘涛*何成
(大连理工大学精细化工重点实验室,大连116024)
基于不同位阻的三氰基构筑单元和双齿配体,合成了2个氰基桥联的Fe(Ⅱ)-Mn(Ⅲ)链状化合物。利用不同的结构扭曲类型调控了它们的磁性相互作用。化合物{[Fe(Ⅲ)(Pz Tp)(CN)3][Mn(Ⅲ)(5,5′-dmbpy)2]ClO4}n(1;Pz Tp=tetrakis(pyrazolyl)borate;5,5′-dmbpy= 5,5′-dimethyl-2,2′-bipyridine)显示为左右手螺旋链的一维2,2-CC链状结构并且表现出亚铁磁行为。化合物{[Fe(Ⅲ)(Tp*)(CN)3]2 [Mn(Ⅱ)(dpqc)]·CH3OH·H2O}n(2;Tp*=hydridotris(3,5-dimethylpyrazolyl)borate;dpqc=dipyrido[3,2-a:2′,3′-c]-(6,7,8,9-tetrahydro) phenazine)具有一维4,2-带状的双链结构并且表现出典型的反铁磁性相互作用。
氰基桥联;链;亚铁磁;反铁磁
0 Introduction
Cyano-bridged molecule-based magnetic materials have been a research field of rapid expansion in recent decades,because the cyano-bridge not only directs the formation of predictable structure but also can efficiently transfer magnetic interactions[1-5]. Meanwhile,since the first experimental onedimensional(1D)system displaying slow magnetic dynamics was reported by Gatteschi and co-workers[6], single-chain magnets(SCMs)have attracted increasing interest because they exhibit the same slow magnetic relaxation as well as with possibly higher magnetic transition temperature than the single-moleculemagnets(SMMs)[7-10].Therefore,the rationaldesign and synthesis of the low dimensional cyanometallate compounds with the property of the SCMs has become a particularly important subject.A rational synthetic strategy is to use the capped building blocks [Fe(L)(CN)x]y-(x=2~5),where L isa variety ofbidentate, tridentate or tetradentate ligands and so on[4,5,11-15]. Based on these building blocks,a great number of low dimensional cyanide-bridged compounds have been prepared;some of them are SCMs[16-19].Among them, the use of fac-[Fe(TpR)CN)3]-as the cyanometallate building block has attracted our attention.The above building block exhibits a stable topology,and directs the formation of predictable structure through rational design.As a consequence,its use often leads to form the tetranuclear{Fe2M2}square systems[20-22],2,2-CT (C=cis;T=trans)single chain[23-25]and double 4,2-ribbon like bimetallic chain[16,26-27](Scheme 1).Most of the reported studies focused on Fe(Ⅲ)-M(Ⅱ)(M=Fe,Co, Ni and Cu)bimetallic assemblies.However,a few Fe (Ⅲ)-Mn(Ⅱ)bimetallic systems were reported based on the fac-[Fe(TpR)CN)3]-[28-33],and they all exhibited the antiferromagnetic behavior.Numerous papers dealing with Fe(Ⅲ)-M analogous systems showed that the magnetic interactions strength is highly sensitive to metal-ligand distances,M-C≡N and M′-N≡C angles and torsion angles[24-25,34-36].In the reported Fe(Ⅲ)-Mn(Ⅱ) systems,the bending of the Mn-N≡C bond angles is larger in chain systems than that of in polynuclear systems(Table 1).Therefore,if the bending of the Mn-N≡C bond angles becomes large,the decrease of the antiferromagnetic interactions could be realized, leading to the ferromagnetic or ferrimagnetic behaviors.The introduction of steric hindrance ligands may induce the distortion of the structure,realizing the control of the magnetic interactions.Herein,we selected different bulky[Fe(PzTp)CN)3]-and[Fe(Tp*) CN)3]-as the building blocks,5,5′-dmbpy(5,5′-dmbpy= 5,5′-dimethyl-2,2′-bipyridine)and dpqc(dpqc= dipyrido[3,2-a:2′,3′-c]-(6,7,8,9-tetrahydro) phenazine)as the second ligands.Two cyano-bridged chains{[Fe(Ⅲ)(PzTp)(CN)3][Mn(Ⅱ)(5,5′-dmbpy)2]ClO4}n (1)and{[Fe(Ⅲ)(Tp*)(CN)3]2Mn(Ⅱ)(dpqc)·CH3OH·H2O}n (2)were synthesized.Compound 1 exhibits a 1D 2,2-CC helix chain structure and shows the ferrimagnetic behavior.Compound 2 has a novel 4,2-ribbon double chain structure and displays the antiferromagnetic interactions.As far as we know,such a novel architecture has never been reported previously.

Scheme 1 Different topologies based on different synthetic strategies of cyanide building blocks fac-[Fe(TpR)CN)3]-in the Fe(Ⅱ)-Mn(Ⅲ)bimetallic chains
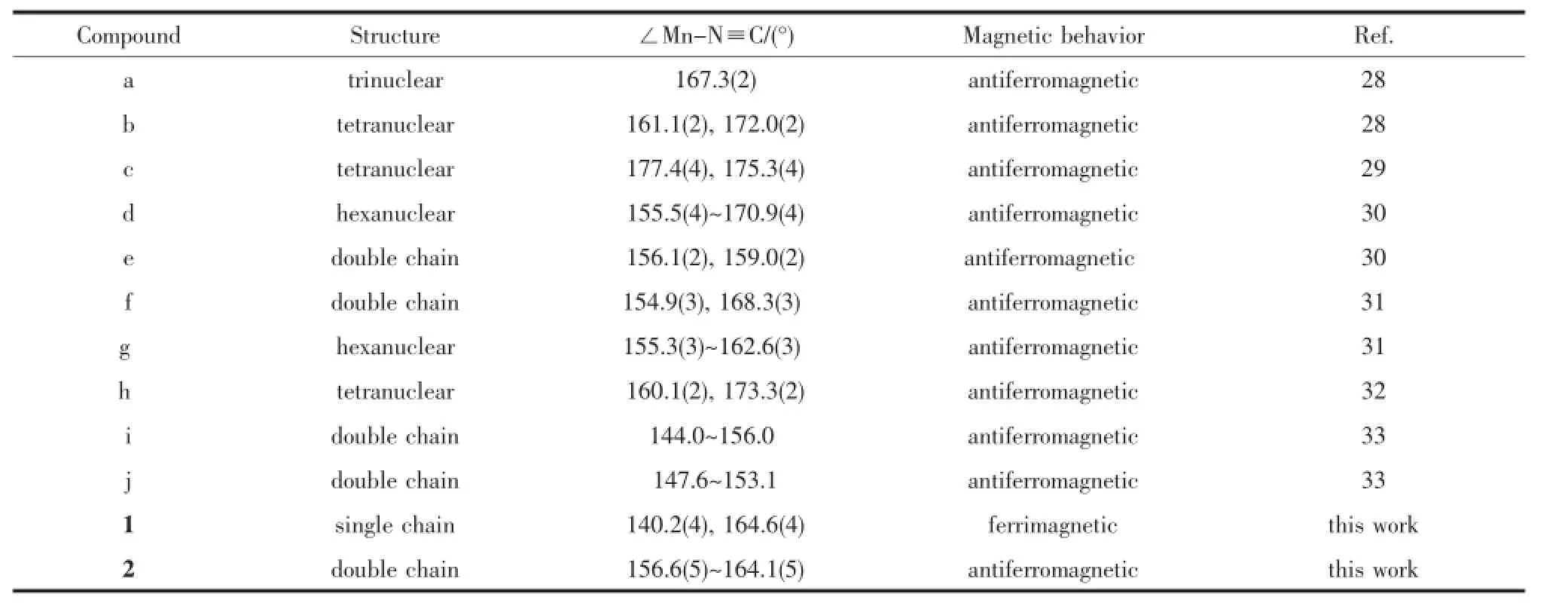
Table 1 Mn-N≡C bond angles and related magnetic behaviors for Fe(Ⅱ)-Mn(Ⅲ)systems constructed from the tricyanide precursors fac-[Fe(TpR)CN)3]-
1 Experimental
1.1 Materials and physical measurements
All chemical reagents were acquired from commercial sources and were used as received without further purification.Bu4N[Fe(PzTp)(CN)3],Bu4N[Fe (Tp*)(CN)3]and the ligand dpqc were synthesized according to the literature method[37-38].Elemental analyses were performed on an Elementar Vario ELⅢanalyzer.IR spectra were recorded on a Bruker AXS TENSOR-27 FTIR spectrometer with KBr pellets in the range of 400~4 000 cm-1.Magnetic measurements of the samples were performed on a Quantum Design SQUID(MPMSXL-7)magnetometer. Data were corrected for the diamagnetic contribution calculated from Pascal constants.
1.2 Synthesis of{[Fe(Ⅱ)(PzTp)(CN)3][Mn(Ⅲ)(5,5′-dmbpy)2]ClO4}n(1)
A 6.0 mL aqueous solution of Mn(ClO4)2·6H2O (0.03 mmol)was placed at the bottom of a test tube,a mixture of methanol and water(1∶2,V/V,6 mL)was gently layered on the top of the solution,and then a 6.0 mL methanol solution of Bu4N[Fe(PzTp)(CN)3] (0.03 mmol)and 5,5′-dmbpy(0.06 mmol)was carefully added as the third layer.After few weeks,red block crystals of 1 were collected,washed with water and dried in air.Yield:58%based on Mn(ClO4)2·6H2O. Anal.Calcd.for C39H36BClFeMnN15O4(%):C 50.05,H 3.88,N 22.45;Found(%):C 50.10,H 3.98,N 22.37. IR(KBr,cm-1):3 123(w),2 927(w),2 141(s),2 136(s), 1 610(m),1 572(m),1 504(m),1 482(s),1 436(m), 1 406(s),1 391(s),1316(s),1 241(m),1 211(s),1 106 (s),917(m),857(s),766(s),623(s),488(m),413(s).
1.3 Synthesis of{[Fe(Ⅱ)(Tp*)(CN)3]2Mn(Ⅲ)(dpqc) ·CH3OH·H2O}n(2)
The compound was obtained with a similar procedure to that of 1,except using Bu4N[Fe(Tp*) (CN)3](0.06 mmol)and dpqc(0.03 mmol)to replace Bu4N[Fe(PzTp)(CN)3]and 5,5′-dmbpy,respectively. The black red plate crystals of 2 were collected after several weeks,washed with water and dried in air. Yield:45%based on Mn(ClO4)2·6H2O.Anal.Calcd. for C55H64B2Fe2MnN22O2(%):C 52.70,H 5.15,N 24.58; Found(%):C 52.65,H 5.19,N 24.67.IR(KBr,cm-1): 3 416(br),2 927(m),2 859(w),2 528(m),2 142(s), 2128(w),1 632(m),1 542(s),1 452(m),1 414(m), 1 376(s),1 308(m),1 203(s),1 060(s),857(m),819 (m),789(m),736(m),691(m),638(m),570(w),435(m).
1.4 X-ray Crystallography
The data were collected on a Bruker Smart APEX(Ⅱ)X-diffractometer equipped with graphite monochromated Mo Kαradiation(λ=0.071 073 nm) using the SMART and SAINT[39]programs at298 K for compounds 1 and 2.Final unit cell parameters were based on allobserved reflections from integration ofall frame data.The structures were solved in the space group by direct method and refined by the full-matrix least-squares using SHELXTL-97 fitting on F2[40].For compounds 1 and 2,all non-hydrogen atoms were refined anisotropically.The hydrogen atoms of organic ligands were located geometrically and fixed isotropic thermal parameters.Attempts to add the hydrogen atoms for the solvent water molecules in the crystal structure of compound 2 through Fourier electron density were failed.The ClO4-group in compound 1 was disordered;therefore,large thermal displacement parameters were found for these atoms and refined with partial occupancy.In compound 2,the solvent water molecule(O1W)was disordered,which was splitover two sites and refined with partial occupancy.The crystal data and details of the structure refinement of compounds 1 and 2 are summarized in Table 2. Selected bond distances and angles ofcompounds 1 and 2 are listed in Table 3.
CCDC:1449127,1;1449128,2.
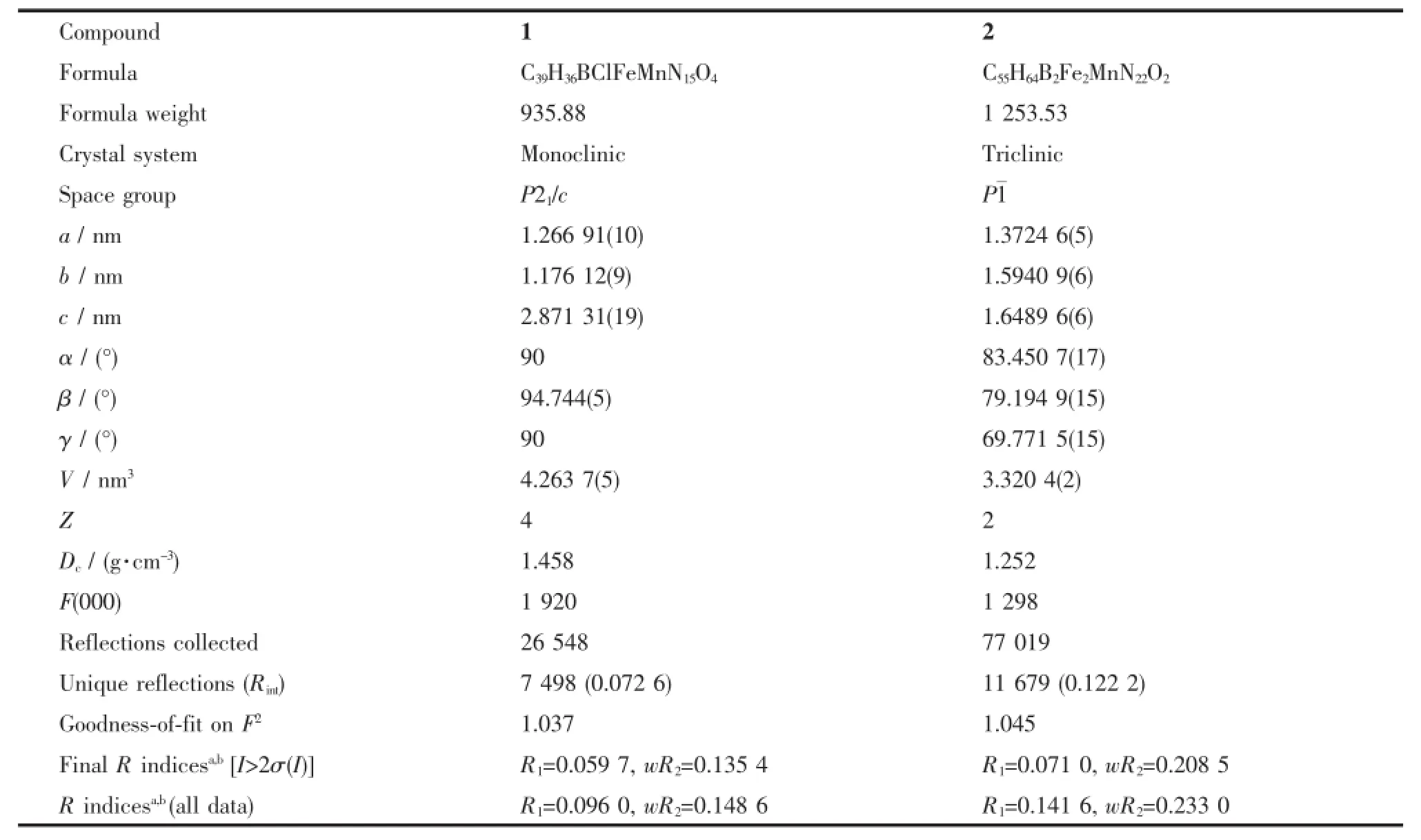
Table 2 Crystal data and structure refinements for compounds 1 and 2

Table 3 Selected bond lengths(nm)and angles(°)for compounds 1 and 2
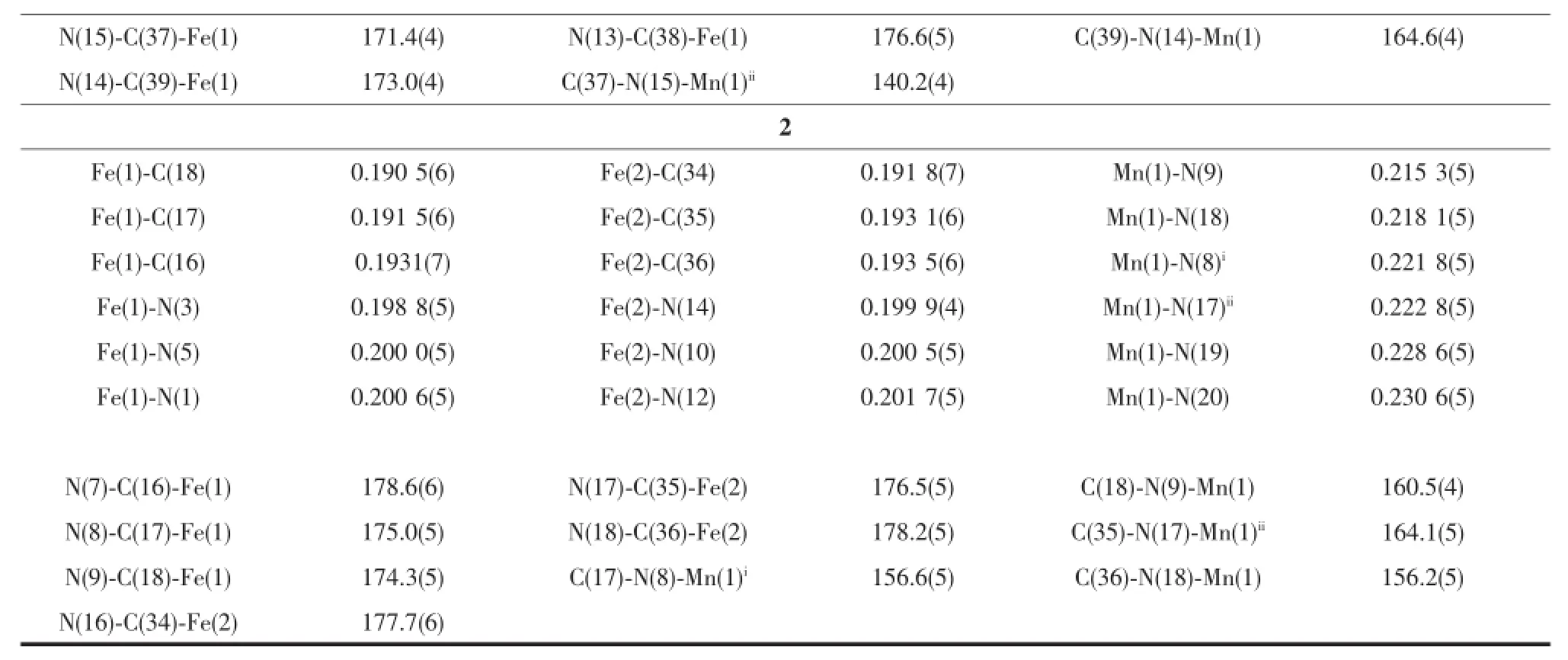
Continued Table 3

Fig.1(a)ORTEP representation of a selected unit of compound 1 with thermal ellipsoids drawn at the 30%probability level;(b)Side view ofa 1D single-zigzag chain ofcompound 1 along the b-axis;(c)Packing structure ofthe left-or right-handed helicalchains
2 Results and discussion
2.1 Crystal structural description
2.1.1 Crystal structure of 1
Single-crystal X-ray diffraction analysis revealed that 1 crystallized in the monoclinic space group P21/c.The crystal structure consists of a cyanobridged 2,2-CC zigzag chain(Fig.1b).The 2,2-CC chain is made up of a cyano-bridged alternating[Fe(Ⅱ)(PzTp)(CN)3]--[Mn(5,5′-dmbpy)2]2+fragment.Within the chain,each[Fe(Ⅱ)(PzTp)(CN)3]-entity connects two [Mn(5,5′-dmbpy)2]2+motifs with two ofits three cyanide groups in cis positions,and each[Mn(5,5′-dmbpy)2]2+unit links two[Fe(Ⅱ)(PzTp)(CN)3]-ions in cis modes. Interestingly,such connection mode results in forming a left-and right-handed helices along the b-axis(Fig. 1c).Because both left-and right-handed helices are alternatively arranged,the whole structure is mesomeric.Each centralFe(Ⅱ)environmentcan be described as a distorted octahedron,comprising three C atoms from terminal CN ligands and three N atoms from the tridentate ligand PzTp.The Fe-Ccyanide(0.192 8(5)~0.193 4(5)nm)and Fe-NPzTp(0.196 1(4)~0.197 4(4)nm) bond lengths are in good agreement with thoseobserved previously in the related LS Fe(Ⅱ)compounds[28].The Fe-C≡N bond angles in the range of 171.4(4)°~176.6(5)°depart slightly from linearity. In the[Mn(5,5′-dmbpy)2]2+unit,each Mn(Ⅲ)ion is also octahedral coordination.Four N atoms are from two bidentate 5,5′-dmbpy ligands,and the remaining coordination sites of each six-coordinated Mn(Ⅲ)ion are occupied by the bridging cyanide building blocks. The Mn-Ncyanidedistances(0.220 2(4)and 0.223 1(4) nm)are shorter than those of the Mn-Nbpy(0.225 4(3)~0.231 8(4)nm),which are comparable with related HS Mn(Ⅲ)compounds previously reported[32].The Mn-N≡C bond angles deviate significantly from linearity with the angles of C(39)-N(14)-Mn(1)164.6(4)°and C(37)-N(15)-Mn(1)ii140.2(4)°(Symmetry codes:ii-x,y+1/2, -z+1/2).The neighboring chains are linked together through C-H…πinteractions between methyl hydrogen and pyridine rings of 5,5′-dmbpy ligands (d=0.310 4 nm),resulting in a 2D supramolecular structure(Fig.2).The shortest intrachain Fe…Mn,Fe…Fe and Mn…Mn distances are 0.491,0.741 and 1.176 nm,respectively,while the nearest interchain Fe…Mn,Fe…Fe and Mn…Mn distances are 1.078, 1.021 and 0.969 nm,respectively,indicating that the interchain magnetic interactions are very weak.
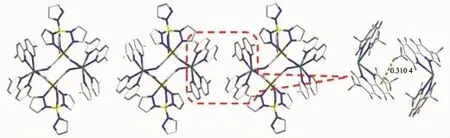
Fig.2 2D supramolecular structure of compound 1 via the C-H…πstacking interactions
2.1.2 Crystalstructure of 2
Single-crystal X-ray diffraction analysis revealed that 2 crystallized in the triclinic space group P1.The crystal structure comprises a neutral cyano-bridged 2, 4-ribbon like double-zigzag chain(Fig.3b).Within the chain,the basic structural unitis a Mn(Ⅲ)2(CN)4Fe(Ⅱ)2square with each Mn(Ⅲ)shared by two adjacent squares.Within each square,the[Fe(Ⅱ)(Tp*)(CN)3]-unit binds two Mn(Ⅲ)through two of its three cyanide groups,while each[Mn(Ⅲ)(dpqc)]2+unit links four [Fe(Ⅱ)(Tp*)(CN)3]-units.The square units exhibit two orientations of their mean planes(Fe(Ⅱ)2Mn(Ⅲ)2), showing an approximately perpendicular with the dihedral angle of 83.44°owing to the steric effect of the bulky[Fe(Ⅱ)(Tp*)(CN)3]-building block,which is rare for the double-zigzag chain reported in the literature[16].Each Fe(Ⅱ)center adopts a slightly distorted octahedral configuration consisting of three cyanide carbon atoms and three nitrogen atoms of Tp*-anion.The Fe-Ccyanidebond lengths range from 0.190 5(6) to 0.193 5(6)nm,and the Fe-NTp*distances are in the range of 0.198 8(5)~0.201 7(5)nm,respectively.The Fe-C≡N linkages are closer to linearity with bond angles of 174.3(5)°~178.6(6)°.Such characteristics of the bond lengths and bond angles indicate that the iron center is low-spin Fe(Ⅱ)[28].Each Mn(Ⅲ)center is located in a distorted N6octahedral coordination environment with four nitrogen atoms from four cyanide groups and two nitrogen atoms from a dpqc ligand.Similar to 1,the Mn-Ncyanidebond distances (0.215 3(5)~0.222 8(5)nm)are also shorter than the Mn-Ndpqcbond distances(0.228 6(5)and 0.230 6(5) nm).These values are in good agreement with the cyano-bridged Fe(Ⅱ)-Mn(Ⅲ)compounds[32].The Mn-N≡C bond angles range from 156.2(5)°to 164.1(5)°.The maximum deviation of the Mn-N≡C angles from linearity is smaller than that of 1(23.8°for 2 vs 39.2° for 1).The adjacent chains are linked to form a 3D supramolecular structure with assistance of C-H…π interactions between methyl hydrogen and pyrazole rings of Tp*ligands(d=0.300 3 nm and 0.310 5 nm) (Fig.3c).The shortest intrachain Fe…Mn,Fe…Fe andMn…Mn distances are 0.512,0.734 and 0.697 nm, respectively.Whereas,the shortest interchain Fe…Mn,Fe…Fe and Mn…Mn distances are 1.198,0.872 and 1.323 nm.
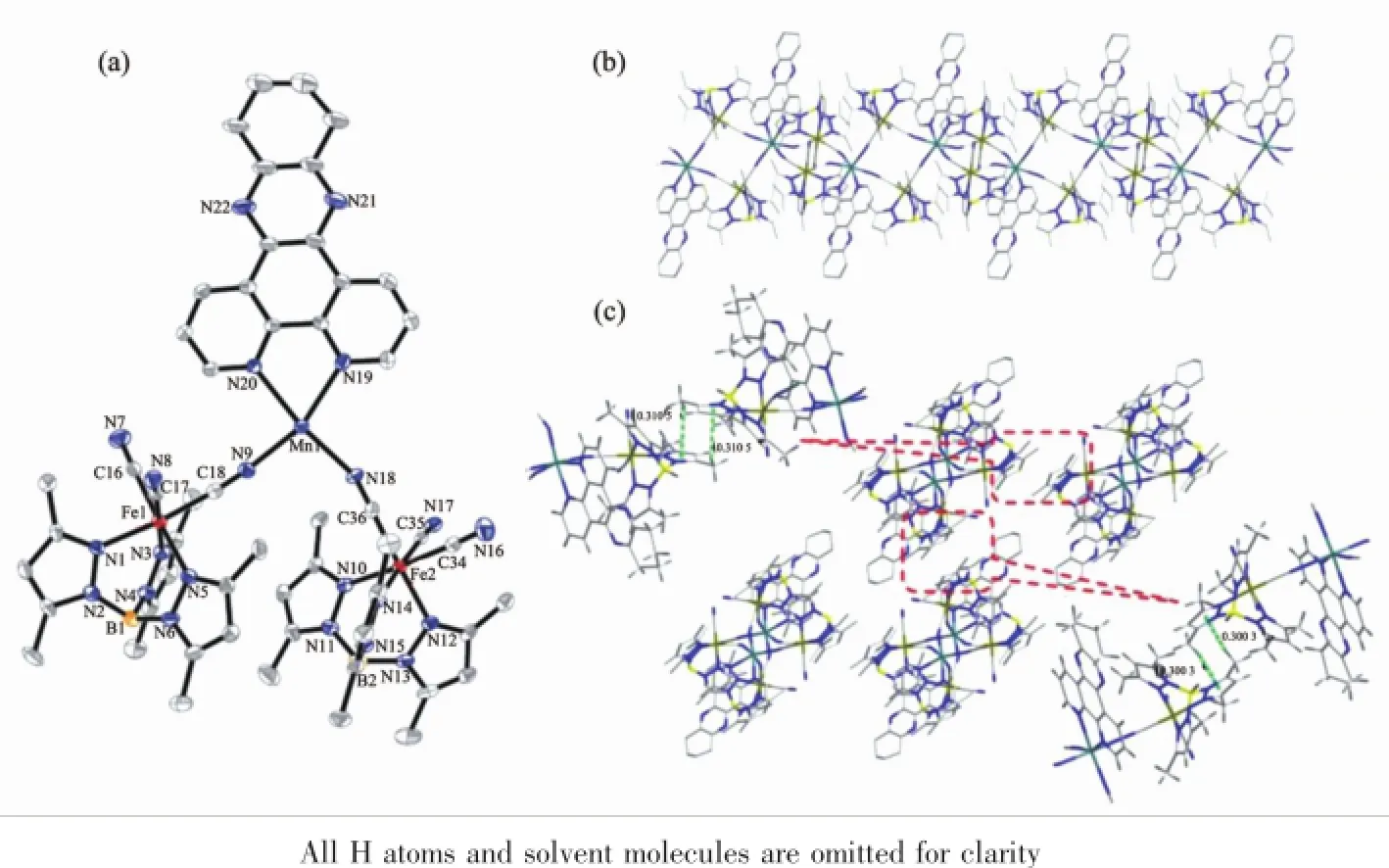
Fig.3(a)ORTEP representation of a selected unit of compound 2 with thermal ellipsoids drawn at the 30%probability level; (b)Side view of a 1D double-zigzag chain along the b-axis;(c)3D supramolecular structure of compound 2 via the C-H…πstacking interactions
It is worth noting that the structures of compounds 1 and 2 are quite different from the reported chains structures incorporating the anionic building block,fac-[Fe(TpR)CN)3]-.Self-assembly of the anionic building block,fac-[Fe(TpR)CN)3]-and fully solvated metal ions[M(S)6]2+or partially blocked metal cationic units[M(L)x(S)y]2+(L=monodentate, bidentate or tetradentate ligand;S=solvent molecule; x+y=6)frequently results in the formation of chains with two different topologies(Scheme 1):2,2-CT chain and 2,4-ribbon chain.In the reported 2,2-CT chain (Scheme 1a),the four coordination sites of the metal(Ⅱ)ion are occupied by two trans-positioned bidentate ligands or a tetradentate Schiff base ligand,and the remaining two sites are filled by two fac-[Fe(TpR)CN)3]-units in trans positions[23-25].In the 2,4-ribbon chain (Scheme 1c),each metal(Ⅱ)center is coordinated by fourcyanide nitrogen atoms from four fac-[Fe(TpR)CN)3]-units and two monodentate ligands or two solvent molecules[26-27,30].In the present work(Scheme 1b),in the[Mn(5,5′-dmbpy)2]2+unit,each Mn(Ⅲ)ion is coordinated by two bidentate ligands in cis positions, the remaining sites are occupied by the bridging cyanide building blocks in cis positions.Such similar structure was only reported by Oshio′s group incorporating a tetradentate N-donoring ligand and the [Fe(Tp)CN)3]-building block[19].Different from the classical 2,4-ribbon chain,each Mn(Ⅲ)ion in complex 2 is coordinated by a bidentate ligand in cis position instead of two monodentate ligands in trans-axial positions(Scheme 1d).As far as we know,such a novel architecture representing a new nettopology has never been reported previously.
2.2 Magnetic properties
2.2.1 Magnetic properties of 1
The magnetic susceptibility data of 1 were measured at 1 000 Oe in the temperature range of 2~300 K(Fig.4a).TheχT value is 5.10 cm3·mol-1·K at 300 K,which is slightly larger than the spin-only value of 4.75 cm3·mol-1·K expected for an uncoupled LS Fe(Ⅱ)(S=1/2)and one HS Mn(Ⅲ)(S=5/2)assuming that g=2.00.As the temperature is lowered,theχT value undergoes a gradual reduction,reaching a minimum value of 4.18 cm3·mol-1·K at 10 K.Below the temperature,it increases rapidly up to 9.41 cm3· mol-1·K at 2.0 K.The overall magnetic behaviorindicates the typicalofa ferrimagnetic situation within a chain.The magnetic susceptibility data are fitted by the Curie-Weiss law in the temperature range of 2~300 K,which give a Curie constant of 5.10 cm3· mol-1·K and a Weiss temperature of-3.64 K.The negative Weiss temperature demonstrates the antiferromagnetic coupling interactions between the paramagnetic centers.When the magnetic behavior of the 1D chain was simulated through the MAGPACK program[41],the 1D chain can be treated as a ring mode[42].Because of the presence of two different Mn-N≡C bridging angles in compound 1,2J coupling parameters were used to simulate the experimental magnetic susceptibility.Therefore,we consider the 1D zigzag chain as a 10-atom Fe5Mn5ring with the Hamiltonian H=-2J1(SFe1SMn1+SFe2SMn2+SFe3SMn3+SFe4SMn4+SFe5SMn5)-2J2(SMn1SFe2+SMn2SFe3+SMn3SFe4+SMn4SFe5+ SMn5SFe1),where SFe=1/2 and SMn=5/2.Using this model (Fig.5a),the magnetic susceptibility data were fitted by the MAGPACK program in the temperature range of 10~300 K,which gave J1=-1.68,J2=-2.42 and g= 2.07 with the agreement factor R=1.05×10-4.The coupling parameters also indicate the antiferromagnetic interactions.The field dependence of the magnetization at 1.8 K was measured in the field range from 0 to 50 kOe(Fig.4b).The magnetization value at 50 kOe is 4.48Nβ,which is a little larger than the ferrimagnetic results of 4.0Nβ calculated from MS=g(SMn-SFe)with g=2.00.The large saturation magnetization may originate from the existence of the significant orbital contributions of the LS Fe(Ⅱ)ions.

Fig.4(a)Temperature-dependent magnetic susceptibility for compound 1;(b)Field-dependent magnetization for compound 1

Fig.5 Schematic representations for the fitting models for 1(a)and 2(b)
2.2.2 Magnetic properties of 2
The temperature dependence of the magnetic susceptibility of 2 was measured in the range of 2~300 K at 1 000 Oe(Fig.6a).TheχT value per Fe2Mn unitat 300 K is 6.01 cm3·mol-1·K,which is close to but somewhat larger than the spin-only value of 5.13cm3·mol-1·K for the uncorrelated two LS Fe(Ⅱ)(S=1/2) and one HS Mn(Ⅲ)(S=5/2)with g=2.00.As the temperature is lowered,theχT value decreases very smoothly down to 5.23 cm3·mol-1·K at 60 K.Upon further lowing,theχT value abruptly decreases to reach a minimum value of0.43 cm3·mol-1·K at 2.0 K. The magnetic susceptibility data above 15 K obey the Curie-Weiss law,which give a Curie constant of 6.19 cm3·mol-1·K and a Weiss temperature of-12.00 K. To simulate the magnetic susceptibility of compound 2,the double chain also can be fitted with a 12-atom Fe8Mn4ring mode(Fig.5b).The MAGPACK program was used to fit based on the Hamiltonian H=-2J(SMn1SFe2+SMn1SFe3+SFe2SMn4+SFe3SMn4+SMn4SFe5+SMn4SFe6+SFe5SMn7+SFe6SMn7+SMn7SFe8+SMn7SFe9+SFe8SMn10+SFe9SMn10+SMn10SFe11+SMn10SFe12+SFe11SMn1+SFe12SMn1).The best-fit parameters with J=-8.61 and g=2.21 with the agreement factor R=3.00×10-5show a good curve match.The negative Weiss temperature and coupling parameters indicate the presence of intrachain antiferromagnetic coupling between neighbouring Fe(Ⅱ)and Mn(Ⅲ)ions.As shown in the Fig.6b,the fielddependent magnetization of 2 was measured at 1.8 K in the field(0~50 kOe).The magnetization increases linearly with the applied magnetic field,reaching a value of 2.48Nβat 50 kOe,which is lower than the saturation magnetization value of 3.0Nβexpected for MS=g(SMn-SFe)with g=2.00.The result further indicates that the dominant intrachain antiferromagnetic interactions were transmitted via the cyanide bridge.
Investigation of the magnetic properties of compounds 1 and 2 indicates that they have different magnetic behaviors.Compound 1 shows ferrimagnetic behavior in low temperature region,whereas compound 2 indicates the presence of the typically antiferromagnetic coupling.The different magnetic properties of compounds 1 and 2 may result from the different bent Mn-N≡C bond angles and interchain C-H…πstacking interactions.Generally,the bending ofthe Mn-N≡C bond angles diminishes the overlap of the spin-orbit coupling and then reduces the magnetic interactions.The maximum deviation of the Mn-N≡C angles from linearity in 1(39.2°)is the largest in comparison with the reported cyano-bridged Fe(Ⅱ)-Mn(Ⅲ)compounds(Table 1),therefore,the intrachain antiferromagnetic coupling of compound 1 is weak and then it exhibits the ferrimagnetic behavior.For compound 2,the Mn-N≡C bond angles are in the reported range of 144°~170°,thus it exhibits the typically antiferromagnetic coupling.

Fig.6(a)Temperature-dependent magnetic susceptibility for compound 2;(b)Field-dependent magnetization for compound 2
3 Conclusions
In summary,different steric hindrance ligands were introduced into Fe(Ⅱ)-Mn(Ⅲ)system to induce the distortion of the structures and finally realized the adjustment of the magnetic interactions.A cyanobridged 1D FeMn 2,2-CC helix single chain 1 and a 1D Fe2Mn 2,4-ribbon double chain 2 were successfully synthesized via tunable assembly,and the structure of compound 2 has never been reported in the previous literature.Investigation of the magnetic properties of compounds 1 and 2 indicates the bending of Mn-N≡Cbond angles and interchain C-H…πstacking interactions play important roles in adjusting the magnetic interactions.Therefore,compound 1 shows ferrimagnetic behavior because ofits largestbending of Mn-N≡C bond angles,whereas compound 2 exhibits the presence ofthe antiferromagnetic coupling.
[1]Sato O,Iyoda T,Fujishima A,et al.Science,1996,272:704 -705
[2]Shatruk M,Avendano C,Dunbar K R.Prog.Inorg.Chem., 2009,56:155-274
[3]Ohba M,kawa H.Coord.Chem.Rev.,2000,198:313-328
[4]Wang S,Ding X H,Li Y H,et al.Coord.Chem.Rev., 2012,256:439-464
[5]Wang S,Ding X H,Zuo J L,et al.Coord.Chem.Rev., 2011,255:1713-1732
[6]Caneschi A,Gatteschi D,Lalioti N,et al.Angew.Chem.Int. Ed.,2001,40:1760-1763
[7]Coulon C,Miyasaka H,Clerac R.Struct.Bond.,2006,122: 163-206
[8]Miyasaka H,Julve M,Yamashita M,et al.Inorg.Chem., 2009,48:3420-3437
[9]Sun H L,Wang Z M,Gao S.Coord.Chem.Rev.,2010,254: 1081-1100
[10]Lescouëzec R,Toma L M,Vaissermann J,et al.Coord. Chem.Rev.,2005,249:2691-2729
[11]Nihei M,Ui M,Yokota M,et al.Angew.Chem.Int.Ed., 2005,44:6484-6487
[12]Shen X P,Zhou H B,Yan J H,et al.Inorg.Chem.,2014, 53:116-127
[13]Liu T,Zhang Y J,Kanegawa S,et al.J.Am.Chem.Soc., 2010,132:8250-8251
[14]Wen H R,Wang C F,Zuo J L,et al.Inorg.Chem.,2006,45: 582-590
[15]Kim J I,Kwak H Y,Yoon J H,et al.Inorg.Chem.,2009, 48:2956-2966
[16]Dong D P,Liu T,Kanegawa S,et al.Angew.Chem.Int.Ed., 2012,51:5119-5123
[17]Liu T,Zheng H,Kang S,et al.Nat.Commun.,2013,4:2826 -2833
[18]Shao D,Zhang S L,Zhao X H,et al.Chem.Commun.,2015, 51:4360-4363
[19]Hoshino N,Iijima F,Newton G N,et al.Nat.Chem.,2012, 4:921-926
[20]Liu W,Wang C F,Li Y Z,et al.Inorg.Chem.,2006,45: 10058-10065
[21]Zhang Y Z,Mallik U P,Clérac R,et al.Polyhedron,2013, 52:115-121
[22]Zhang Y Z,Ferko P,Siretanu D,et al.J.Am.Chem.Soc., 2014,136:16854-16864
[23]Wen H R,Tang Y Z,Liu C M,et al.Inorg.Chem.,2009,48: 10177-10185
[24]Kwak H Y,Ryu D W,Lee J W,et al.Inorg.Chem.,2010, 49:4632-4642
[25]Dong D P,Zhang Y J,Zheng H,et al.Dalton Trans.,2013, 42:7693-7698
[26]Wen H R,Wang C F,Song Y,et al.Inorg.Chem.,2006,45: 8942-8949
[27]Mitsumoto K,Ui M,Nihei M,et al.CrystEngComm, 2010,12:2697-2699
[28]Kim J,Han S J,Cho I-K,et al.Polyhedron,2004,23:1333-1339
[29]Li D F,Parkin S,Wang G B,et al.Inorg.Chem.,2005,44: 4903-4905
[30]Jiang L,Feng X L,Lu T B,et al.Inorg.Chem.,2006,45: 5018-5026
[31]Gheorghe R,Kalisz M,Clérac R,et al.Inorg.Chem., 2010,49:11045-11056
[32]Pardo E,Verdaguer M,Herson P,et al.Inorg.Chem., 2011,50:6250-6262
[33]ZHENG Hui(郑慧),XU Yang(徐杨),DUAN Chun-Ying (段春迎).Chinese J.Inorg.Chem.(无机化学学报),2015,31 (7):1460-1466
[34]Wang S,Zuo J L,Zhou H C,et al.Eur.J.Inorg.Chem., 2004,3681-3687
[35]Ni Z H,Kou H Z,Zhang L F,et al.Angew.Chem.Int.Ed., 2005,44:7742-7745
[36]Costa V,Lescouëzec R,Vaissermann J,et al.Inorg.Chim. Acta,2008,361:3912-3918
[37]Gu Z G,Liu W,Yang Q F,et al.Inorg.Chem.,2007,46: 3236-3244
[38]Ma L L,Ge K,Zhang R,et al.Eur.J.Med.Chem.,2014, 87:624-630
[39]SMART,SAINT and XPREP,Area Detectr and Data Integration and Reduction Software,Bruker Analytical Instruments Inc.,Madison,WI,1995.
[40]Sheldrick G M.SHELXS-97,Program for X-ray Crystal Structure Solution and Refinement,University of Göttingen, Germany,1997.
[41]Borrás-Almenar J J,Clemente-Juan J M,Coronado E,et al. MAGPACK,J.Comput.Chem.,2001,22:985-991
[42]Kou H Z,Ni Z H,Liu C M,et al.New J.Chem.,2009,33: 2296-2299
Tuning Assembly and Magnetic Interactions of Cyano-bridged Fe(Ⅱ)-Mn(Ⅲ)Bimetallic Chains
JIAO Cheng-Qi JIANG Wen-Jing WEN Wen REN Yi WANG Jia-Liang LIU Tao*HE Cheng
(State Key Laboratory of Fine Chemicals,Dalian University of Technology,Dalian,Liaoning 116024,China)
Two cyano-bridged Fe(Ⅲ)-Mn(Ⅱ)chains were synthesized via using bidentate ligands and cyanometallate building blocks with different steric hindrance.The magnetic interactions were adjusted by the structural distortions of the two compounds.Compound{[Fe(Ⅲ)(PzTp)(CN)3][Mn(Ⅱ)(5,5′-dmbpy)2]ClO4}n(1;PzTp=tetrakis (pyrazolyl)borate;5,5′-dmbpy=5,5′-dimethyl-2,2′-bipyridine)shows a 1D 2,2-CC chain-like structure with a leftand right-handed helices chains and exhibits the ferrimagnetic behavior.Compound{[Fe(Ⅲ)(Tp*)(CN)3]2[Mn(Ⅱ) (dpqc)]·CH3OH·H2O}n(2;Tp*=hydridotris(3,5-dimethylpyrazolyl)borate;dpqc=dipyrido[3,2-a:2′,3′-c]-(6,7,8,9-tetrahydro)phenazine)has a novel 1D 4,2-ribbon double chain-like structure with dominant antiferromagnetic interactions.CCDC:1449127,1;1449128,2.
cyano-bridged;chain;ferrimagnetic;antiferromagnetic
O614.81+1;O614.7+11
A
1001-4861(2016)09-1637-10
10.11862/CJIC.2016.210
2016-05-25。收修改稿日期:2016-08-04。国家自然科学基金(No.21322103)资助项目。
*通信联系人。E-mail:liutao@dlut.edu.cn
