Effects of microalgal polyunsaturated fatty acid oil on body weight and lipid accumulation in the liver of C57BL/6 mice fed a high fat diet
2016-12-13RyeoEunGoKyungHwangGeonTaeParkHaeMiruLeeGeumLeeChoWonKimSoYeJeonJeongWooSeoWonKyungHongKyungChulChoi
Ryeo-Eun Go, Kyung-A Hwang, Geon-Tae Park, Hae-Miru Lee, Geum-A Lee, Cho-Won Kim, So-Ye Jeon, Jeong-Woo Seo, Won-Kyung Hong, Kyung-Chul Choi,✉
1Laboratory of Biochemistry and Immunology, College of Veterinary Medicine, Chungbuk National University, Cheongju, Chungbuk ASI/KR/KS001, Republic of Korea;
2Korea Research Institute of Bioscience & Biotechnology, Jeongup, Jeonbuk ASI/KR/KS004, Republic of Korea;
3LED Agri-bio Fusion Technology Research Center, College of Environmental & Bioresource Science, Chonbuk National University, Iksan Campus, Jeonbuk ASI/KR/KS004, Republic of Korea.
Effects of microalgal polyunsaturated fatty acid oil on body weight and lipid accumulation in the liver of C57BL/6 mice fed a high fat diet
Ryeo-Eun Go1, Kyung-A Hwang1, Geon-Tae Park1, Hae-Miru Lee1, Geum-A Lee1, Cho-Won Kim1, So-Ye Jeon1, Jeong-Woo Seo2, Won-Kyung Hong3, Kyung-Chul Choi1,✉
1Laboratory of Biochemistry and Immunology, College of Veterinary Medicine, Chungbuk National University, Cheongju, Chungbuk ASI/KR/KS001, Republic of Korea;
2Korea Research Institute of Bioscience & Biotechnology, Jeongup, Jeonbuk ASI/KR/KS004, Republic of Korea;
3LED Agri-bio Fusion Technology Research Center, College of Environmental & Bioresource Science, Chonbuk National University, Iksan Campus, Jeonbuk ASI/KR/KS004, Republic of Korea.
Dietary polyunsaturated fatty acids (PUFAs), which are abundant in marine fish oils, have recently received global attention for their prominent anti-obesogenic effects. Among PUFAs, eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3), which are n-3 long-chain PUFAs widely referred to as omega-3 oils, were reported to prevent the development of obesity in rodents and humans. In the present study, we evaluated the anti-obesity effects of microalgal oil on high-fat induced obese C57BL/6 mice, compared with commercial omega-3 fish oil and vegetable corn oil. Microalgal oil is an inherent mixture of several PUFAs, including EPA, DHA and other fatty acids produced from a marine microalgal strain of Thraustochytriidae sp. derived mutant. It was found to contain more PUFAs (>80%) and more omega-3 oils than commercial omega-3 fish oil (PUFAs >31%) and corn oil (PUFAs 59%). All three types of oils induced weight loss in high-fat-induced obese mice, with the loss induced by microalgal oil being most significant at 9 weeks (10% reduction). However, the oils tested did not improve blood lipid levels, although microalgal oil showed an apparent inhibitory effect on lipid accumulation in the liver. These findings may be attributed to the higher PUFA content, including omega-3 oils of microalgal oil than other oils. Collectively, these findings suggest that microalgal oil, derived from Thraustochytriidae sp. derived mutant, is a prominent candidate for replacement of omega-3 fish oils based on its apparent anti-obesity effect in vivo.
anti-obesity, polyunsaturated fatty acid, saturated fatty acid, omega-3 oil, microalgae
Introduction
Obesity is caused by a prolonged energy imbalance; namely, high energy intake exceeding energy expenditure. It leads to circulatory and metabolic disorders such as arteriosclerosis, hypertension, coronary heart disease, and type 2 diabetes. The rise in obesity has become a widespread social issue worldwide that poses a health threat and leads to serious socio-economic losses. Even though considerable efforts have beendevoted to the discovery of anti-obesity drugs, dietary therapy is considered to be more important and is still the first choice in the treatment of disorders resulting from obesity[1].
During dietary therapy, anti-obesity healthy foods derived from natural substances are generally preferred for safe long-term treatment over drugs which may have side effects. Foods rich in dietary fiber are well-known to have anti-obesity effects that occur by facilitating a reduction in body fat and body weight loss[2-4]. In addition, recent studies have shown that dietary polyphenols, including green tea catechins and epigallocatechin gallates, resveratrol, and curcumin have preventative effects on obesity and obesity-related chronic diseases[5-7].
Dietary polyunsaturated fatty acids (PUFAs), which are abundant in marine fish oils, have recently received global attention for their prominent anti-obesogenic effects. Among PUFAs, eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3), which are n-3 long-chain (LC) PUFAs widely referred to as omega-3 oils, have been shown to prevent development of obesity in rodents and humans[8-10]. The anti-obesity effects of EPA in high fat/high sucrose-induced obesity were reported to be associated with the suppression of hepatic lipogenesis and steatosis, a process referring to the abnormal retention of lipids within a cell[11]. Moreover, DHA was shown to exert its anti-obesity effects by inhibiting differentiation to adipocytes, inducing apoptosis in post-confluent preadipocytes, and promoting lipolysis[12].
The commercial production of omega-3 oils such as EPA and DHA has primarily depended on marine fish oils to date. but the application of fish oils is often hampered by difficulties including seasonal variations, marine pollution, and the high processing cost[13]. In addition, mass-scale fishing to match the increasing demand for fish oils is no longer sustainable[14]. As a result, microalgae have gained attention as a more reliable and healthy source of PUFAs, including omega-3 oils. A number of microalgal groups have been reported to possess the ability to accumulate lipids in high amounts and to present elevated levels of n-3 LC-PUFA[15]. Among them, thraustochytrids have attracted much attention because some strains contain large amounts of PUFAs in their lipids and grow well, to relatively high cell densities, in heterotrophic culture without the need for light[13,16]. Although the actual dominant PUFAs derived from thraustochytrids depend on the genera and habitats of the organism, they are composed of a good balance of PUFAs, including EPA, DHA, arachidonic acid (AA; 20:4n-6), docosapentaenoic acid (DPA; 22:5n-3), and linoleic acid (LA; 18:2n-6)[13]. In the present study, we demonstrated the anti-obesity effect of an inherent mixture of several PUFAs and other fatty acids produced from Thraustochytriidae sp. derived mutant, which is a mutant strain improved to increase lipid production, on high-fat induced obese mice. This study is the first to demonstrate an anti-obesity effect of microalgal oil produced from a Thraustochytriidae sp. derived mutant.
Materials and methods
Materials
We compared the anti-obesity effects of corn oil (Sigma-Aldrich, St. Louis, MO, USA) and commercial omega-3 fish oil with microalgal oil. The microalgal oil, derived from Thraustochytriidae sp. derived mutant, was produced and provided by the Jeonbuk Branch of the Korea Research Institute of Bioscience and Biotechnology (KRIBB). The microalgal oil was prepared as described below.
Preparation of microalgal oil
Thraustochytriidae sp. derived mutant was precultivated in 5 L baffled flasks containing 50 mL of basal medium [glucose (food grade), 60 g/L; yeast extract (food grade), 10 g/L; dried natural sea salt (CJ Co., Korea), 10 g/L] at 28°C, shaken at 120 rpm for 3 days. A pre-culture aliquot of which volume was 2.5% (v/v) of main fermentation medium volume was transferred into a 300 L bioreactor. The main fermentation was conducted at 28°C while stirring at 100 rpm with 1 v/v/min aeration in a 300 L bioreactor for 2.5 days. After fermentation, cell paste was harvested by centrifugation and sonicated for 5 minutes. Lipid extraction was performed by supercritical fluid (SCF) extraction at 400 bar and 50°C for 60 minutes (SCF; NATEX/5L 100 bar pilot plant). There were at least three independent experiments for each endpoint.
Analysis of fatty acid composition
The fatty acid composition was analyzed by gas chromatography (GC; Hewlett Packard 6890 N; Ramsey, MN, USA) as previously described[17]. The instrument was equipped with a flame-ionization detector (FID) and an HP-5 column (30 m × 0.32 mm; 0.25 mm; Agilent Technologies; Santa Clara, CA, USA). The column temperature was increased from 150°C (after 2 minutes of holding) to 270°C (with a further 2 m of holding) at 7°C per minute. The fatty acid composition of commercial omega-3 fish oil was also analyzed by the same procedure.
Animal care
Five-week-old male C57BL/6 mice were purchased from the Korea Research Institute of Bioscience & Biotechnology (Ochang, Republic of Korea). The micewere housed in a conventional animal facility at a constant temperature of (23 ± 3)°C, relative humidity of (55 ± 10)%, and a 12 hours light/dark cycle at the Laboratory Animal Research Center of Chungbuk National University (Cheongju, Republic of Korea). Animals were allowed to acclimate for 1 week after arrival. All animals were used for in vivo experiments in accordance with the approved institutional guidelines of Chungbuk National University.
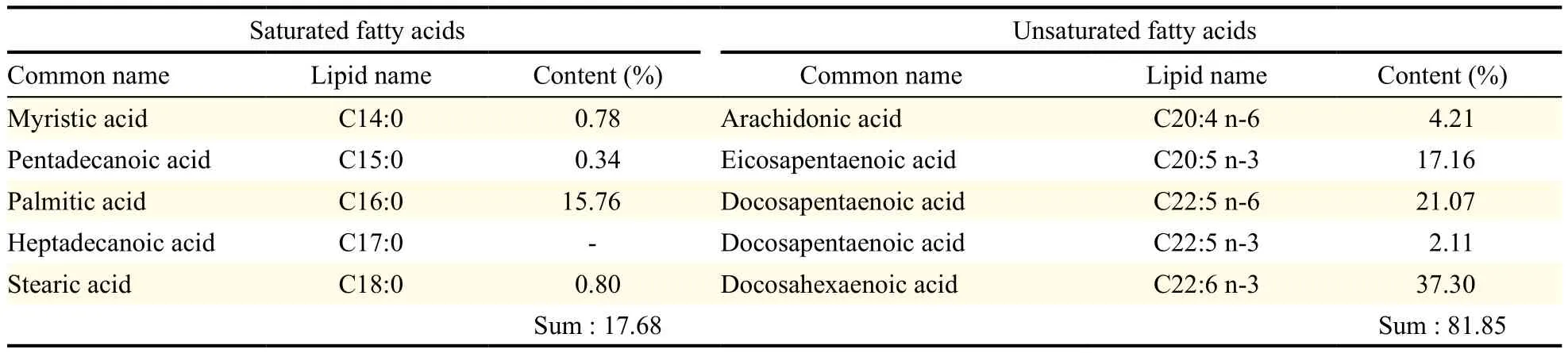
Table 1 Fatty acid composition of microalgal oil derived from Thraustochytriidae sp. derived mutant
Administration of diets and oils
Before administration of oils prepared for the test, mice were fed a high-fat diet (D12492 formula; Research Diets, Inc., New Brunswick, NJ, USA) for 4 weeks to induce obesity. The high-fat diet (773.85 g) contained 200 g casein, 3 g L-cystine, 125 g maltodextrin, 68.8 g sucrose, 50 g cellulose, 25 g soybean oil, 245 g lard, 10 g mineral mix, 13 g dicalcium phosphate, 5.5 g calcium carbonate, 16.5 g potassium citrate, 10 g vitamin mix, 2 g choline bitartrate, and 0.05 g blue dye. This diet contained 26% protein, 26% carbohydrate and 35% fat. The obese mice were then divided into four groups, and each group (n = 10) was also administered PBS [control, non-(oil) diet (ND) group], microalgal oil (MO group), commercial omega-3 fish oil (positive control, OM group), and corn oil (CO group) while taking in a normal diet. PBS and each oil were orally administered via a Zonde needle at a dose of 5 g/kg body weight every other day for 9 weeks. Commercial omega-3 oil and corn oil were employed to compare the anti-obesity effect of microalgal oil.
Analysis of body weight
The body weights of the mice were measured before and after administration of oils once a week within the experimental period.
Analysis of serum lipids
Blood samples were collected from each mouse on the last day of the experimental period. On the day of blood collection, all mice were fasted for 18 hours and blood samples were then collected from the tail vein after anesthesia using ether. About 2 cc of blood were collected using a Vacuum Serum Separation Tube (SST; Green Cross Corp., Yongin, Gyeonggi, Republic of Korea) and left at room temperature for 1 hour. Serum was isolated from the blood samples by centrifugation at 1977 g and 4°C for 20 minutes, then stored at -20°C. A Hitachi Clinical Analyzer 7080 (Hitachi Korea Ltd., Seoul, Republic of Korea) was used to measure the serum concentrations of various lipid components including TG, T-CHO, HDL cholesterol, and LDL cholesterol.
Histological analysis by Oil Red O staining
At 24 hours after the final oral administration of the experimental diets, liver tissues were harvested from the sacrificed rats and immediately frozen at -80°C. Frozen liver tissues were cryo-sectioned (6 μm thick), fixed in a 10% formalin solution (OCI Company Ltd.) at 4°C for 5 minutes, and then rinsed three times with distilled water. A 5% Oil Red O working solution was prepared by dissolving Oil Red O powder (Sigma-Aldrich) in propylene glycol (OCI Company Ltd.), and then used to stain the sectioned tissues according to the manufacturer’s instructions. Counter-staining was conducted with hematoxylin (Sigma-Aldrich), and the sections were then mounted in glycerine (OCI Company Ltd.). Lipid-containing cells were detected as those containing red inclusions using a light microscope (BX51 U-LH100HGWIG, Olympus, Tokyo, Japan; 100× and 200× magnification).
Statistical analysis
All data were analyzed using GraphPad Prism software (San Diego, CA, USA). In vitro data are presented as the means ± SEM. A one-way ANOVA was conducted followed by Dunnett's multiple comparison test. P-values < 0.05 were considered to be statistically significant.
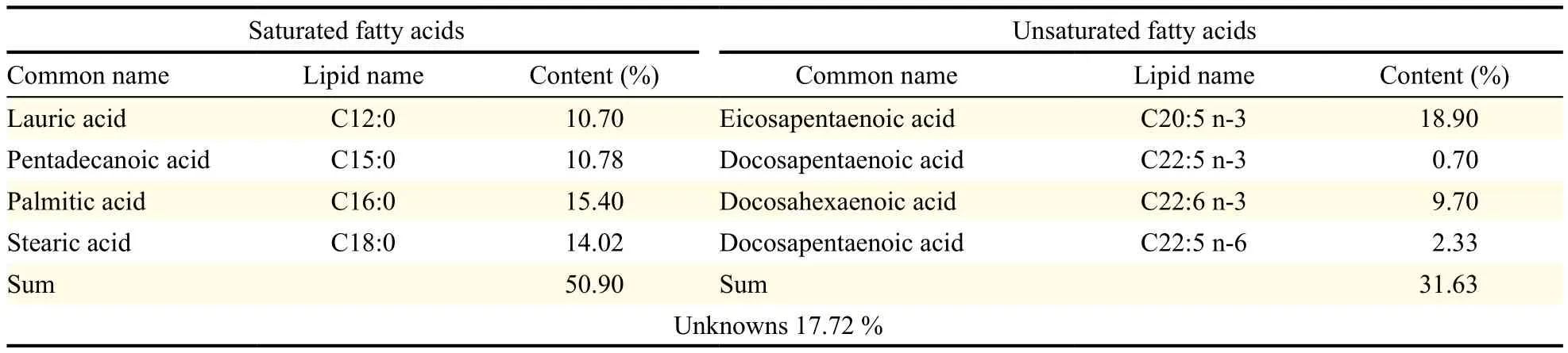
Table 2 Fatty acid composition of commercial omega-3 fish oil
Results
Fatty acid composition of microalgal oil and commercial fish oil
The microalgal oil produced by Thraustochytriidae sp. derived mutant was found to have diverse PUFAs (Table 1). Moreover, the microalgal oil was found to have more PUFAs than saturated fatty acids and more omega-3 oils than omega-6 oils. Specifically, the ratio of n-6 oils to n-3 oils (n-6:n-3) was 0.45. Conversely, commercial fish oil labeled with omega-3 oil was found to have more saturated fatty acids than PUFAs and more omega-3 oils than omega-6 oils, with a n-6 oils to n-3 oils ratio (n-6:n-3) of 0.08 (Table 2). Comparison of the amount of PUFAs revealed that microalgal oil contained more PUFAs than the commercial omega-3 fish oil and that this commercial fish oil contained much more saturated fatty acids than PUFAs, contrary to expectations from its label as omega-3 oil. The commercial fish oil was not found to be authentic omega-3 oil because it only contained omega-3 oils in part.
Body weight
The average body weight of mice at the beginning of the experiment was (21.2 ± 0.94) g, and this increased to (31.18 ± 1.94) g after receiving the high-fat diet for 4 weeks. When the diet was switched to the normal diet, the body weights decreased, even in the control group. The mice administered with oils showed greater weight loss than those in the control group for 9 weeks (Fig. 1A & Table 3). Among the oils, the weight loss effect of microalgal oil was revealed to be most significant than any other oils at the end of experiment (9thweek) (Fig. 1B). Specifically, the body weight of the MO group greatly decreased from the second week and was maintained at a reduced state until the end of the test. The weight loss effect of omega-3 fish oil was strongly apparent from the first week, but steadily decreased with time after week 6. Corn oil also induced weight loss compared to the control, although to a lesser degree than microalgal oil and commercial omega-3 fish oil.
Serum lipid concentrations
To examine the effects of the diets on blood lipids of mice, we analyzed the blood levels of triglyceride, total cholesterol LDL-cholesterol, and HDL-cholesterol (Fig. 2A-D). There were no significant differences in any lipids between diet groups, although slight differences were observed. These findings indicate that the diets tested did not improve blood lipid levels.
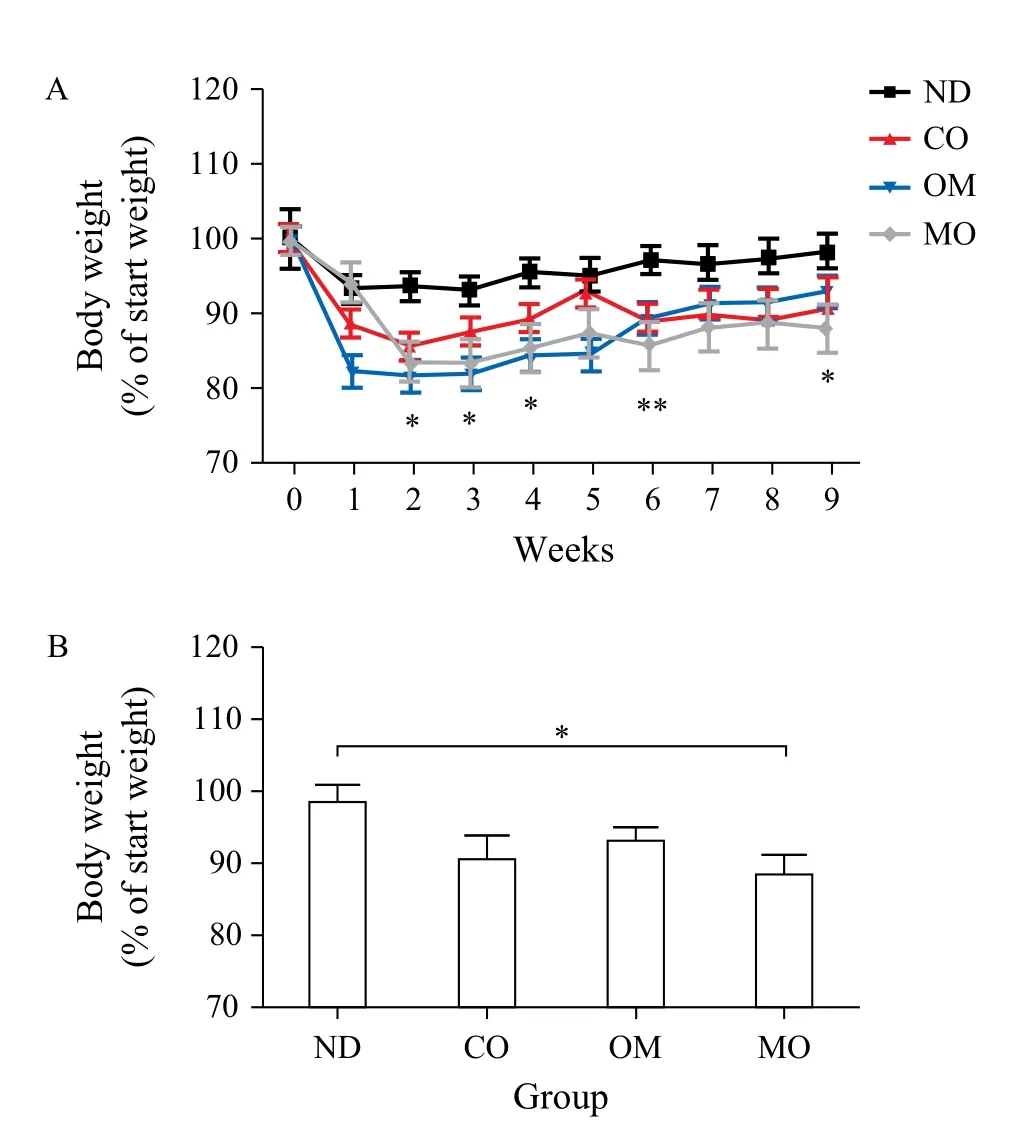
Fig. 1 Body weights of animals during the experimental period. A: Before the administration of oils prepared for the test, C57BL/6 mice were fed a high-fat diet for 4 weeks to induce obesity. The obese mice were then divided into 4 groups, each of which was administered PBS [control, non-diet (ND) group], microalgal oil (MO group), commercial omega-3 fish oil (positive control, OM group), or corn oil (CO group), in conjunction with normal diet. The body weights of mice of each group were measured once a week within the experimental period of 9 weeks. B: The final weights of the mice in each group were measured at week 9. Values represent the mean ± SEM. *P < 0.05 (Dunnett's multiple comparison test).
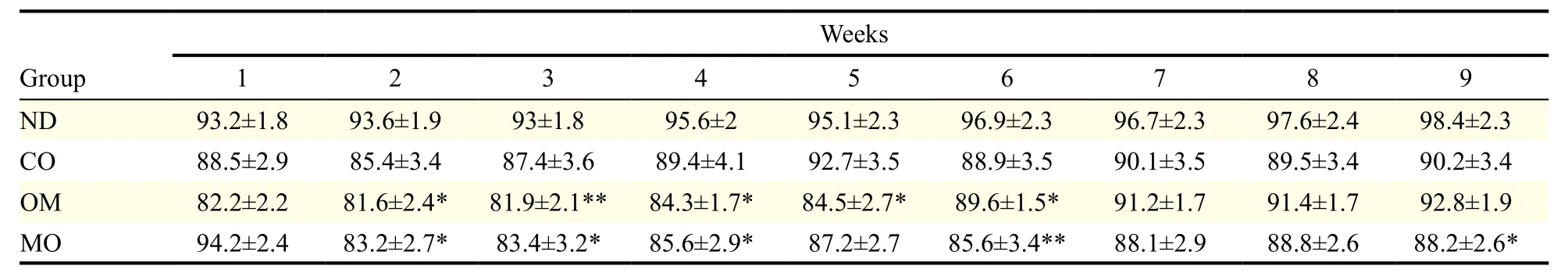
Table 3 Body weights of animals during the experimental period of 9 weeks
Lipid accumulation in liver tissue
The lipids accumulated in mouse liver tissues obtained from each experimental group were detected as red spots upon histological analysis by Oil-red-O staining. The red spots show the lipid components, which were digested from high-fat diet by lipase, absorbed into the intestine, and then accumulated in the liver (Fig. 3). The red spot areas containing lipids were abundantly observed in the ND and CO groups, but found in small quantities in the OM group or rarely found in the MO group (Fig. 3). The preventive effect of microalgal oil on lipid accumulation in liver tissue was shown to correspond to its weight loss effect to some degree. An obvious difference in lipid accumulation in the liver tissue between groups was observed at 200× magnification relative to 100× (Fig. 3B).
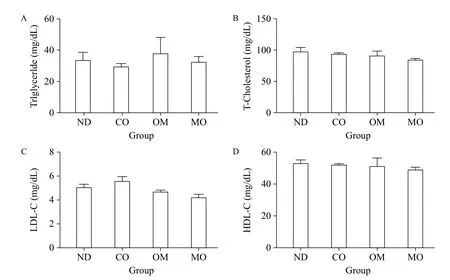
Fig. 2 Serum lipid analysis. After the induction of obesity, obese mice were divided into four groups, each of which was administered PBS (control, non-diet (ND) group), microalgal oil (MO group), commercial omega-3 fish oil (positive control, OM group), or corn oil (CO group), in conjunction with normal diet. On the last day of the experimental period, blood samples were collected from each mouse. Serum was isolated from the blood samples, and the serum concentrations of triglyceride (A), total-cholesterol (B), LDL-cholesterol (C), and HDL-cholesterol (D) were measured. Values represent the mean ± SEM. *P < 0.05 (Dunnett's multiple comparison test) vs. ND group.
Discussion
The anti-obesity effect of n-3 LC-PUFAs has been recognized as credible based on a number of animal and human studies. In animal studies using obesity-induced animals fed high-fat diets, co-administration of n-3 LC-PUFA induced an anti-obesity effect by reducing body fat accumulation[8,11,18]. During the development of obesity in C57BL/6J mice, EPA/DHA reduced the accumulation of body fat by limiting both hypertrophy and hyperplasia of fat cells[8]. EPA also exerted an anti-obesity effect via the suppression of hepatic lipogenesis and steatosis of high-fat/high-sucrosefed C57BL/6J mice[11]. In rats fed a high-fat diet, EPA reduced body-weight gain and retroperitoneal adipose tissue weight by increasing adiposity-corrected adiponectin plasma levels and TNF-α gene expression[18].
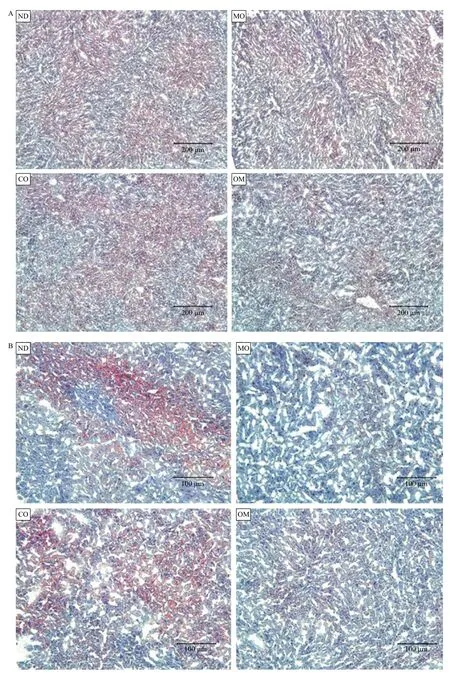
Fig. 3 Histological analysis of liver tissues. After oral administration of PBS (ND), microalgal oil (MO), corn oil (CO), and commercial omega-3 fish oil (OM), liver tissues were harvested from the mice, frozen, cryo-sectioned, and stained with an Oil Red O working solution. Counter-staining was conducted with hematoxylin, and the tissue sections were then mounted with glycerine. Lipids in the cells appeared as red inclusions under a light microscope with increasing magnifications, 100× (A) and 200× (B).
In addition, there is relatively little data available regarding the effects of n-3 LC-PUFAs on human obesity from well-controlled studies, even though their impact is less certain than in animal studies[19]. In a previous study in which volunteers consumed 6 g/d of visible fat replaced with DHA-rich fish oil, resting energy expenditure and resting fat oxidation increased, while body fat accumulation decreased[20]. Moreover, when combined with energy restriction and exercise, n-3 LC-PUFA intake led to greater weight loss and reduction of body mass index (BMI) in severely obese women[21].
Conversely, saturated fatty acids are generally considered a risk factor for dyslipidemia, which means an abnormal amount of lipids (e.g. cholesterol and/or fat) in the blood and more often hyperlipidemia, and in turn a risk factor for some types of cardiovascular disease[22-23]. There have been strong and consistent relationships between saturated fat intake and blood cholesterol levels and the mass occurrence of cardiovascular disease[24-26]. Quantitative meta-analyses of metabolic ward studies also revealed a significant relationship between saturated fat and serum cholesterol levels[27]. Hooper et al. demonstrated that reducing saturated fat reduces serum cholesterol levels, and advised that all those at risk of cardiovascular disease continue to include permanent reduction of dietary saturated fat and partial replacement by unsaturated fats[28].
As described above, marine fish oils have been used as the main source of n-3 LC-PUFAs to date. However, with the increasing concerns about a substantial decline in fish populations and the possibility of extinction due to the increasing consumption of omega-3 fish oils, microalgae have been exploited as an alternative to fish oils with the purpose of protecting fish species and the oceans' ecosystems[14,29]. Microalgae are the primary producers of the oceanic food chain. Because microalgae are easy to grow on a large scale due to their small size, they are a more reliable and healthy source of PUFAs[2,30]. In addition to PUFAs, superfluous lipid and protein collected from microalgae may be used as biodiesel and biomass for oil sources and animal feed, respectively[31].
In the present study, we investigated the effects of microalgal PUFA oil derived from Thraustochytriidae sp. derived mutant on body weight and lipid accumulation in the livers of C57BL/6 mice fed a high fat diet compared with omega-3 fish oil and vegetable corn oil. All three oils induced weight loss in highfat-induced obese mice, with the weight loss effect of microalgal oil being greatest. However, the tested oils did not improve blood lipids. Although omega-3 oil slightly reduced the blood levels of total cholesterol and LDL-cholesterol, there were no significant changes relative to the control. Serum lipid levels can be employed to calculate the atherogenic index (AI) and cardiac risk factor (CRF), which are useful to forecast the potential risks of atherosclerosis and cardiovascular diseases[32]. However, we did not provide these indexes in the present study due to lack of an obvious distinction between serum levels of triglyceride, total cholesterol, LDL-cholesterol, and HDL-cholesterol. Conversely, the inhibitory effect of lipid accumulation in the liver by omega-3 oil and microalgal oil was observed upon histological analysis. Specifically, the liver tissues obtained from mice in the control and corn oil groups (ND & CO) showed a great deal of red spots, while few red spots were observed in liver tissue samples from mice treated with omega-3 oil and microalgal oil. Microalgal oil showed more apparent inhibition of lipid accumulation in the liver than omega-3 oil. These findings may be attributed to the higher contents of PUFAs, including omega-3 oils of microalgal oil. As shown in the results of the fatty acid analysis in this study, microalgal oil contained more PUFAs and omega-3 oils than commercial omega-3 fish oil, while commercial fish oil included much more saturated fatty acids than microalgal oil. Corn oil induced weight loss and led to a slight reduction in lipid accumulation in the liver. According to Dupont et al., refined corn oil is composed of 99% triacylglycerols, with PUFAs accounting for 59%, monounsaturated fatty acid 24%, and saturated fatty acids 13%. The main PUFA of corn oil is linoleic acid (C18:2n-6), while there is a small amount of linolenic acid (C18:3n-3) giving a n-6/n-3 ratio of 83. This ratio is much higher than the n-6/n-3 ratio of 9 in common diets of Western countries[33-34]. Although there is a controversy regarding the role of omega-6 oils in human health, a high omega-6 intake can be considered to be related to excessive adipose tissue development and obesity[35-37]. In a human study, a higher percentage of arachidonic acid (C20:4 n-6) was found in adipose tissue from obese children relative to normal weight children[38].
Taken together, these results demonstrate the potential antiobesity effect of microalgal oil produced from a species of marine microalgae, Thraustochytriidae sp. derived mutant, for the first time. The significant weight loss and reduction of lipid accumulation in the liver in response to microalgal oil may have occurred due to its high content of PUFAs and omega-3 oils. Although more intense studies elucidating its antiobesity efficacy are needed, the microalgal oil tested in this study can be considered a prominent candidate for replacement of omega-3 fish oils.
Acknowledgements
This work was supported by a grant from the KRIBB Research Initiative Program (KGM2211531). In addition, this work was also supported by Priority Research Centers Program through NRF funded by the Ministry of Education, Science and Technology (2015R1A6A1A04020885).
References
[1] Bae JM, Yang YJ, Li ZM, et al. Low cholesterol is associated with mortality from cardiovascular diseases: a dynamic cohort study in Korean adults[J]. J Korean Med Sci, 2012,27(1):58-63.
[2] Liu S. Intake of refined carbohydrates and whole grain foods in relation to risk of type 2 diabetes mellitus and coronary heart disease[J]. J Am Coll Nutr, 2002,21(4):298-306.
[3] Slavin JL. Dietary fiber and body weight[J]. Nutrition (Burbank, Los Angeles County, Calif), 2005,21(3):411-418.
[4] Hu X, Gao J, Zhang Q, et al. Soy fiber improves weight loss and lipid profile in overweight and obese adults:a randomized controlled trial[J]. Mol Nutr Food Res, 2013,57(12):2147-2154.
[5] Jurgens TM, Whelan AM, Killian L, et al. Green tea for weight loss and weight maintenance in overweight or obese adults[J]. Cochrane Database Syst Rev, 2012, 12(CD008650).
[6] Jeon SM, Lee SA, Choi MS. Antiobesity and vasoprotective effects of resveratrol in apoE–deficient mice[J]. J Med Food, 2014,17(3):310-316.
[7] Wang S, Moustaid-Moussa N, Chen L, et al. Novel insights of dietary polyphenols and obesity[J]. J Nutr Biochem, 2014,25(1):1-18.
[8] Ruzickova J, Rossmeisl M, Prazak T, et al. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue[J]. Lipids, 2004,39(12):1177-1185.
[9] Woodman RJ, Mori TA, Burke V, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension[J]. Am J Clin Nutr, 2002,76(5):1007-1015.
[10] Rossmeisl M, Jelenik T, Jilkova Z, et al. Prevention and reversal of obesity and glucose intolerance in mice by DHA derivatives[J]. Obesity (Silver Spring, Md), 2009,17(5):1023-1031.
[11] Sato A, Kawano H, Notsu T, et al. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: importance of hepatic lipogenesis[J]. Diabetes, 2010,59(10):2495-2504.
[12] Kim HK, Della-Fera M, Lin J, et al. Docosahexaenoic acid inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 preadipocytes[J]. J Nutr, 2006,136(12):2965-2969.
[13] Jiang Y, Fan KW, Wong RT, et al. Fatty acid composition and squalene content of the marine microalga Schizochytrium mangrovei[J]. J Agric Food Chem, 2004, 52(5):1196-1200.
[14] Lenihan-Geels G, Bishop KS, Ferguson LR. Alternative sources of omega-3 fats: can we find a sustainable substitute for fish?[J] Nutrients, 2013,5(4):1301-1315.
[15] Martins DA, Custodio L, Barreira L, et al. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae[J]. Mar Drugs, 2013,11(7):2259-2281.
[16] Hong WK, Heo SY, Park HM, et al. Characterization of a squalene synthase from the thraustochytrid microalga Aurantiochytrium sp. KRS101[J]. J Microbiol Biotechnol, 2013,23(6):759-765.
[17] Hong WK, Rairakhwada D, Seo PS, et al. Production of lipids containing high levels of docosahexaenoic acid by a newly isolated microalga, Aurantiochytrium sp. KRS101[J]. Appl Biochem Biotechnol, 2011,164(8):1468-1480.
[18] Perez-Matute P, Perez-Echarri N, Martinez JA, et al. Eicosapentaenoic acid actions on adiposity and insulin resistance in control and high-fat-fed rats: role of apoptosis, adiponectin and tumour necrosis factor-alpha[J]. Br J Nutr, 2007,97(2):389-398.
[19] Buckley JD, Howe PR. Anti-obesity effects of longchain omega-3 polyunsaturated fatty acids[J]. Obes Rev, 2009,10(6):648-659.
[20] Couet C, Delarue J, Ritz P, et al. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults[J]. Int J Obes Relat Metab Disord, 1997,21(8):637-643.
[21] Kunesova M, Braunerova R, Hlavaty P, et al. The influence of n-3 polyunsaturated fatty acids and very low calorie diet during a short-term weight reducing regimen on weight loss and serum fatty acid composition in severely obese women[J]. Physiol Res / Academia Scientiarum Bohemoslovaca, 2006,55(1):63-72.
[22] Lin J, Yang R, Tarr PT, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP[J]. Cell, 2005,120(2):261-273.
[23] Siri-Tarino PW, Sun Q, Hu FB, et al. Saturated fat, carbohydrate, and cardiovascular disease[J]. Am J Clin Nutr, 2010,91(3):502-509.
[24] Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts)[J]. Eur Heart J, 2007,28(19):2375-2414.
[25] DiNicolantonio JJ, Lucan SC, O’Keefe JH. The Evidence for Saturated Fat and for Sugar Related to Coronary Heart Disease[J]. Prog Cardiovasc Dis, 2015. Epub ahead of print.
[26] Ebbesson SO, Voruganti VS, Higgins PB, et al. Fatty acids linked to cardiovascular mortality are associated with risk factors[J]. Int J Circumpolar Health, 2015,74:28055.
[27] Clarke R, Frost C, Collins R, et al. Dietary lipids and blood cholesterol: quantitative meta-analysis of metabolic ward studies[J]. BMJ (Clinical research ed), 1997,314(7074):112-117.
[28] Hooper L, Martin N, Abdelhamid A, et al. Reduction in saturated fat intake for cardiovascular disease[J]. Cochrane Database Syst Rev, 2015,6(CD011737).
[29] Reynolds JD, Dulvy NK, Goodwin NB, et al. Biology of extinction risk in marine fishes[J]. Proc Biol Sci, 2005,272(1579):2337-2344.
[30] Adarme-Vega TC, Lim DK, Timmins M, et al. Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production[J]. Microb Cell Fact, 2012,11:96.
[31] Subhadra B. Algal biorefinery-based industry: an approach to address fuel and food insecurity for a carbon-smart world[J]. J Sci Food Agric, 2011,91(1):2-13.
[32] Kang NH, Lee WK, Yi BR, et al. Modulation of lipid metabolism by mixtures of protamine and chitooligosaccharide through pancreatic lipase inhibitory activity in a rat model[J]. Lab Anim Res, 2012,28(1):31-38.
[33] Dupont J, White PJ, Carpenter MP, et al. Food uses and health effects of corn oil[J]. J Am Coll Nutr, 1990,9(5):438-470.
[34] Strandvik B. The omega-6/omega-3 ratio is of importance! [J] Prostaglandins, Leukot Essential Fatty Acids, 2011,85(6):405-406.
[35] Ailhaud G, Massiera F, Weill P, et al. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity[J]. Prog Lipid Res, 2006,45(3):203-236.
[36] Ailhaud G. Omega-6 fatty acids and excessive adipose tissue development[J]. World Rev Nutr Diet, 2008,98:51-61.
[37] Muhlhausler BS, Ailhaud GP. Omega-6 polyunsaturated fatty acids and the early origins of obesity[J]. Curr Opin Endocrinol Diabetes Obes, 2013,20(1):56-61.
[38] Savva SC, Chadjigeorgiou C, Hatzis C, et al. Association of adipose tissue arachidonic acid content with BMI and overweight status in children from Cyprus and Crete[J]. BR J Nutr, 2004,91(4):643-649.
✉ Kyung-Chul Choi, D.V.M., Ph.D., Laboratory of Biochemistry and Immunology, College of Veterinary Medicine, Chungbuk National University, Cheongju, Chungbuk, 28644, Republic of Korea, Tel: +82-43-261-3664, Fax: +82-43-267-3150, E-mail: kchoi@cbu.ac.kr.
23 January 2016, Revised 3 February 2016, Accepted 4 February 2016, Epub 10 April 2016
R714.25, Document code: A
The authors reported no conflict of interests.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Lipid transport to avian oocytes and to the developing embryo
- Role of stromal cell-derived factor 1α pathway in bone metastatic prostate cancer
- A pilot exome-wide association study of age-related cataract in Koreans
- Dynamic monitoring of menopause hormone therapy and defining the cut-off value of endometrial thickness during uterine bleeding
- Polycystic ovary syndrome patients with high BMl tend to have functional disorders of androgen excess: a prospective study
- Effect of vitamin D3 on production of progesterone in porcine granulosa cells by regulation of steroidogenic enzymes
