Manual acupuncture at the SJ5 (Waiguan) acupoint shows neuroprotective effects by regulating expression of the anti-apoptotic gene Bcl-2
2016-12-02DongLinLiliLinKyleSutherlandChuanhaiCao
Dong Lin, Li-li Lin, Kyle Sutherland, Chuan-hai Cao,
1 College of Acupuncture, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian Province, China
2 Department of Neurosurgery and Brain Repair, Morsani College of Medicine, University of South Florida, Tampa, FL, USA
RESEARCH
Manual acupuncture at the SJ5 (Waiguan) acupoint shows neuroprotective effects by regulating expression of the anti-apoptotic gene Bcl-2
Dong Lin1, Li-li Lin1, Kyle Sutherland2, Chuan-hai Cao2,*
1 College of Acupuncture, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian Province, China
2 Department of Neurosurgery and Brain Repair, Morsani College of Medicine, University of South Florida, Tampa, FL, USA
Graphical Abstract
orcid: 0000-0002-5765-6401 (Chuan-hai Cao)
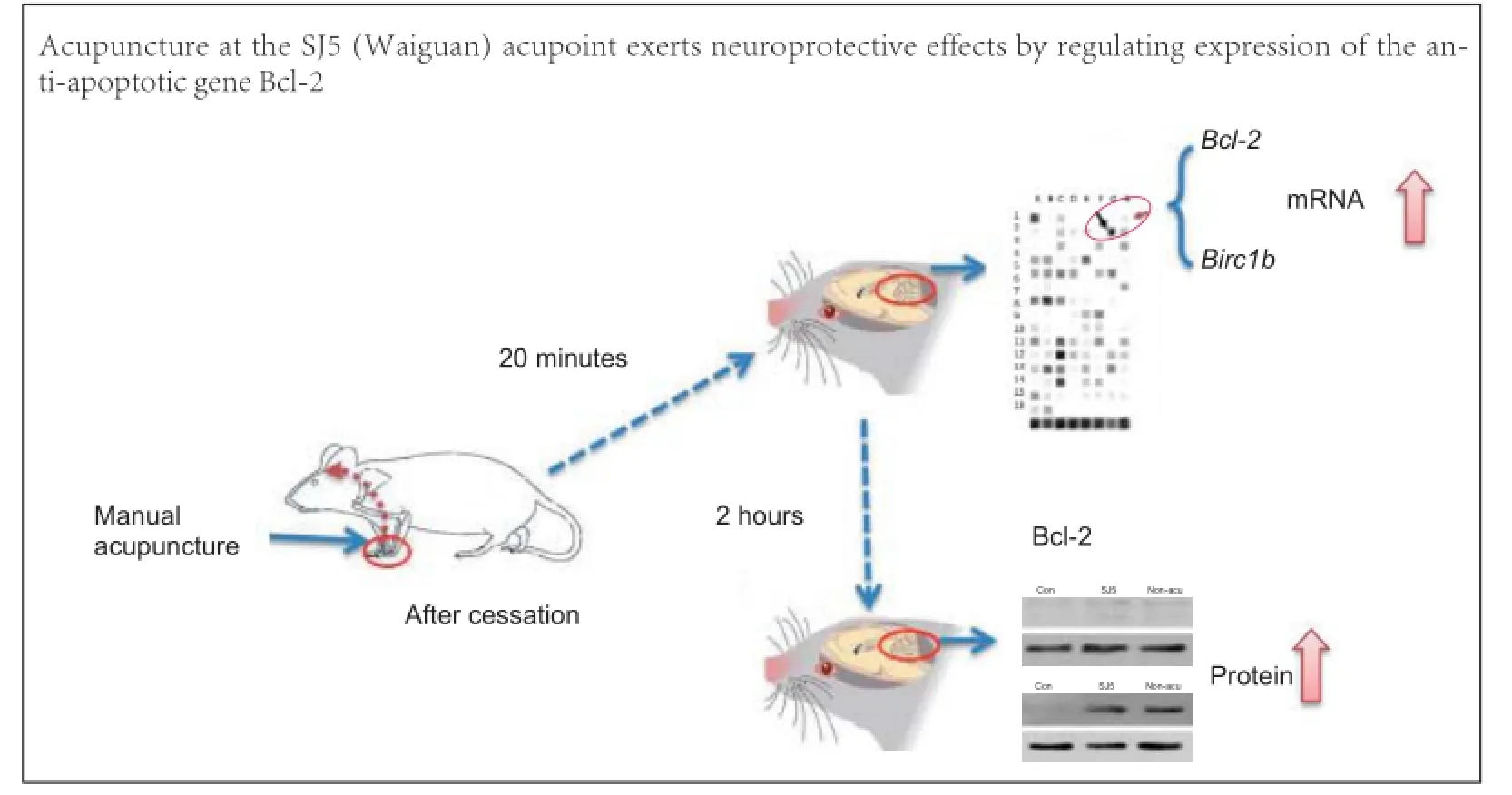
Acupuncture at the SJ5 (Waiguan) acupoint has neuroprotective effects in cerebral infarction, but the underlying mechanism remains poorly understood. Here, we analyzed gene expression in healthy rat cerebellum using a pathway-focused DNA microarray to screen 113 genes associated with 18 signal transduction pathways. After 20 minutes of acupuncture at SJ5, the expression of Bcl-2 and Birc1b mRNA was markedly increased. This was confirmed by real-time reverse transcription PCR. Furthermore, western blot analysis showed that Bcl-2 protein expression remained high in the cerebellum until at least 2 hours after cessation of acupuncture. These findings indicate that acupuncture at SJ5 exerts neuroprotective effects by regulating the expression of anti-apoptotic gene Bcl-2.
nerve regeneration; neuroprotection; acupuncture; SJ5; cerebellum; apoptosis; signal transduction; Bcl-2; neural regeneration
Introduction
Acupuncture is gaining widespread popularity. Its effects on brain function are well documented (Kim et al., 2011; Bai et al., 2014), and recent reports have demonstrated its therapeutic effects in Parkinson’s disease (Arankalle and Nair, 2013), epilepsy (Cakmak, 2006), stroke (Cho et al., 2013) and a number of neurological disorders (Soligo et al., 2013). Acupuncture has also shown promising effects in the cerebellum in experimental and clinical studies (Chen et al., 2013; Jin et al., 2014; Leung et al., 2014; Xie et al., 2014). A recent functional magnetic resonance imaging study showed that cerebellar activity was elevated after acupuncture (Bai et al., 2014). Furthermore, mounting evidence suggests that acupuncture stimulation affects brain activation by increasing blood oxygen level-dependent signal intensity in the cerebellum (Zhang et al., 2012; Lee et al., 2013). Early functional neuroimaging data indicate that acupuncture stimulation modulates specific motor-relatednetworks in different brain regions, and in particular activates the cerebellum (Quah-Smith et al., 2013). Acupuncture can significantly enhance sensorimotor integration, particularly by increasing the functional connectivity of the cerebellum (Chen et al., 2013; Leung et al., 2014; Romoli et al., 2014; Xie et al., 2014). Moreover, the cerebellum shows significant activation after manual acupuncture, even after local anesthesia at the acupoint (Jin et al., 2014). The impact of acupuncture on the functional connectivity between the cerebellum and the cerebrum might also be significant.
Despite mounting evidence pertaining to the benefits of acupuncture in different neurological and neurodegenerative diseases, few studies have addressed the link between acupuncture and biological action in specific brain regions in healthy animals (Manni et al., 2010). San Jiao 5 (SJ5), also known as Waiguan, is an important traditional acupuncture point commonly used in the treatment of stroke-related motor, neurological and autonomic nerve problems. Acupuncture at SJ5 specifically influences functional networks of the central nervous system, including sensorimotor areas, to improve sensorimotor function (Liu et al., 2009; Chen et al., 2014). In a recent study using18F-fluorodeoxyglucose positron-emission computed tomography (PET/ CT), needling at SJ5 in patients with cerebral infarction increased glucose metabolism in local cerebellar regions (Liu et al., 2013). Furthermore, continuous wavelet transform analysis of electroencephalography signals showed that during SJ5 acupuncture stimulation, θ power (4-8 Hz) was significantly elevated after 20 minutes of acupuncture (Hsu et al., 2011). Additionally, studies have shown a strong correlation between acupuncture at SJ5 acupoint and the function of corresponding brain regions (Small et al., 2002; Wang et al., 2010).
In the present study, we examined the effects of acupuncture on normal brain function, to elucidate the neural mechanisms underlying its therapeutic action. We hypothesized that acupuncture at SJ5 would modulate certain biological activities in the cerebellum, as suggested in a previous study (Liu et al., 2013). To investigate the effect of acupuncture on specific gene expression, we used a pathway-focused DNA microarray to identify signal transduction pathways activated after acupuncture, and real-time reverse transcription (RT)-PCR and western blot assays to determine mRNA and protein expression of Bcl-2 in the cerebellum.
Materials and Methods
Animals
All experiments were approved by the Institutional Animal Care and Use Committee of Fujian University of Traditional Chinese Medicine, China, and performed according to the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal discomfort and reduce the number of animals used. Thirty-nine adult male Sprague-Dawley rats (Shang Hai SLAC Laboratory Ltd., License No. SCXK (Hu) 2007-0005), weighing 180-220 g, were randomly assigned to three groups. The control group (9 animals; 3 for the microarray and 6 for western blot/RT-PCR) received no treatment, but the animals were immobilized by hand for 20 minutes. The SJ5 acupuncture group (15 animals; 3 for the microarray and 12 for western blot/RT-PCR) received acupuncture at SJ5. In the non-acupoint group (15 animals; 3 for the microarray and 12 for western blot/RT-PCR), a needle was inserted 3 mm medial to SJ5 (Figure 1). All rats were given free access to water and food, and were housed under a 12 hour light/dark cycle and standard vivarium conditions at 25 ± 3°C.
Acupuncture stimulation
To determine approximate acupoints in rats, we used the transpositional method, which transforms human acupoints onto animal anatomy (Yin et al., 2008). Acupuncture stimulation was performed by inserting a pair of stainless steel pins (0.3 mm in diameter, 13 mm in length; batch No. 120380, Suzhou HuaTuo Medicine Instrument Co., Ltd, China) into the SJ5 acupoint to a depth of 2-3 mm (No author listed, 2009). SJ5 is located at the midpoint between the ulna and the radius, about 2 cun (67 mm) above the crease of the wrist (Hsu et al., 2011) (Figure 1A). To rule out non-specific effects of acupuncture, the same stimulation was given at a “non-acupoint”, 3 mm medial to SJ5 (Chen et al., 2014) at the midpoint between the San Jiao (triple energizer) and small intestine meridians (Figure 1A). All rats were immobilized by hand during acupuncture to reduce stress. Acupuncture was performed manually by an experienced acupuncturist. The needles were twisted twice per second for 30 seconds, and the stimulation was applied every 5 minutes for a total of 20 minutes. The rats were observed while the acupuncture was performed, and if a rat looked uncomfortable, it was stroked gently on the back until it became calm again.
Gene expression profiling
Rats were sacrificed immediately after acupuncture stimulation, the brains removed, and the cerebellum dissected out and frozen rapidly. The frozen samples were homogenized in TRIzol Reagent (Invitrogen, Rockville, MA, USA), and total RNA was extracted using the ArrayGrade™ Total RNA Isolation kit (SABiosciences, Frederic, MA, USA) according to the manufacturer’s protocol. RNA integrity was assessed by electrophoresis in 1.5% agarose gel with ethidium bromide. Structural integrity, yield, and purity of RNA were confirmed using a spectrophotometer. For the conversion of RNA into cDNA and its further transcription, biotin-16-dUTP (Roche Cat. No. 1-093-070) labeling was performed using the True Labeling-AMP™ Linear RNA Amplification Kit (SABiosciences). The reaction mixture was incubated for 5 hours at 37°C. The cRNA obtained was purified with the SuperArray ArrayGrade cRNA Cleanup Kit (SABiosciences). After rapid amplification and labeling of the antisense RNA, we used the Oligo GEArray® DNA Microarray Rat Signal Transduction Pathway Finder™ (SABiosciences) to profile the expression of 113 genes, representative of 18 signal transduction pathways (Table 1). Gene expression profiling was then conducted from the pooled total RNA (5 μg) using an Oligo GEArray® (SABiosciences) according to the manufacturer’s instructions. Before prehybridization, the array membrane was wetted by adding 5 mL of deionized water to the hybridization tube, and the tube was allowed to sit inverted for at least 5 minutes. GEAhyb Hybridization Solution (SABiosciences) was warmed to 60°C and the bottle was inverted several times to allow complete dissolution of the buffer components. Hybridization solution was prepared by adding 4 μg cRNA target to a 2.0-mL aliquot of warm GEA-hyb Hybridization Solution. The mixture was left overnight at60°C with continuous agitation. A chemiluminescent array image was captured using X-ray film and a flatbed desktop scanner. The pictures were saved in grayscale 16-bit TIFF files and analyzed using the GEArray Expression Analysis Suite (SABiosciences, Frederic, MA, USA). The mean signal intensity was calculated from duplicates of each gene. Genes were considered to be differentially expressed if their expression level was at least twofold higher or lower than that in the control group.
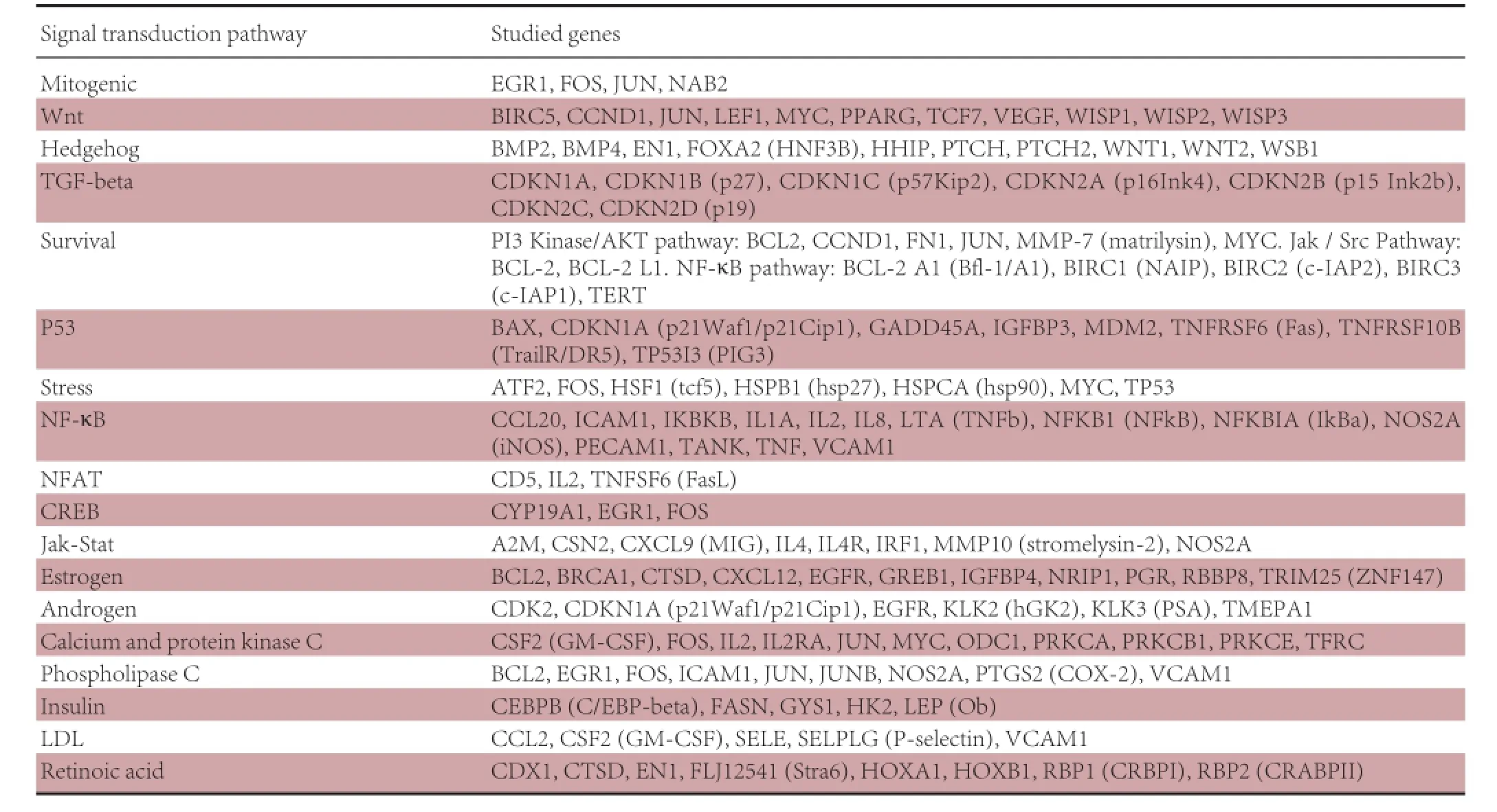
Table 1 Functional gene grouping in the Oligo GEArray® DNA Microarray: Rat Signal Transduction Pathway Finder™

Table 2 Oligo GEArray density analysis
Western blot assay
The frozen cerebellum samples were obtained after 20 minutes of acupuncture or 2 hours after acupuncture and lysed with RIPA buffer supplemented with a protease inhibitor cocktail. Protein concentrations were identified using the bicinchoninic acid assay method. Equivalent amounts of protein (30 mg/ lane) were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis, on a 10% gel, and transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 5% (w/v) bovine serum albumin in Tris-buffered saline with Tween 20 for 1 hour, then incubated with rabbit anti-rat Bcl-2 and mouse anti-rat GAPDH antibodies (Santa Cruz Biotechnology) overnight at 4 ° C (1:1,000; Thermo Fisher Scientific Inc, MA, USA). The immunoblots were then incubated with goat anti-rabbit/mouse horseradish peroxidase-conjugated IgG for 2 hours at room temperature (1:1,000), and labeled proteins were detected using the Electrochemiluminescence Plus Kit (Thermo Fisher Scientific Inc, MA, USA). Band intensities obtained by western blot assay were determined using ImageJ open source software (http://rsb.info.nih.gov/ij/) and protein levels were normalized to GAPDH.

Figure 1 Acupuncture treatment and experimental procedure.
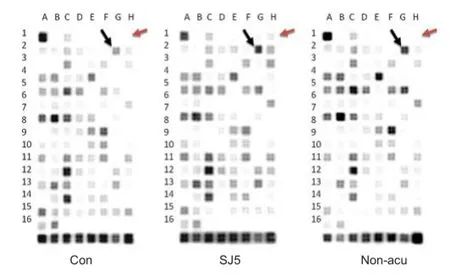
Figure 2 Oligo GEArray microarray.
Real-time RT-PCR
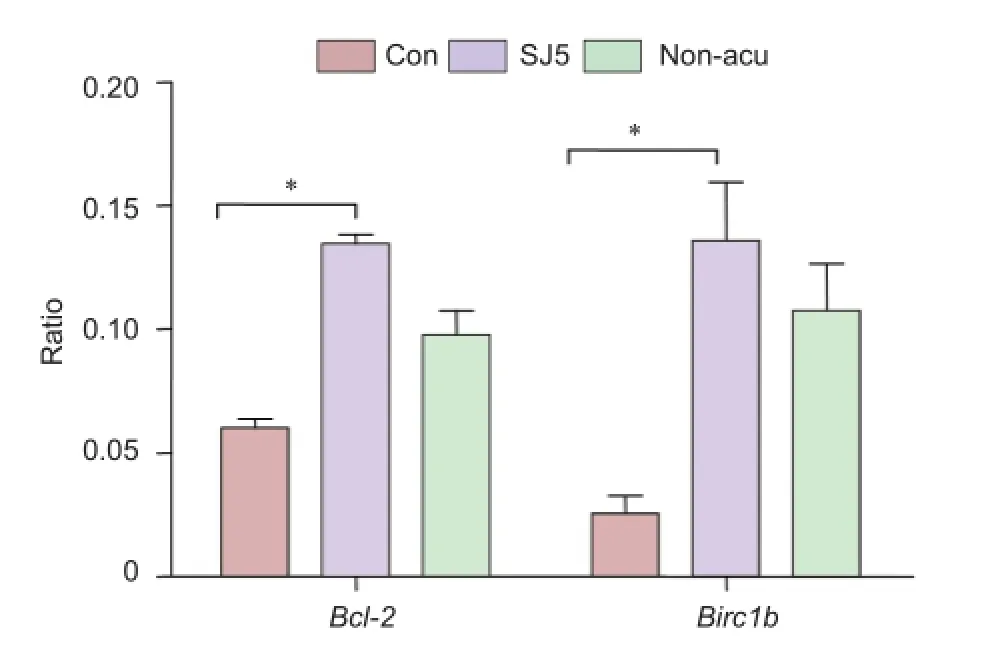
Figure 4 mRNA expression of Bcl-2 and Birc1b in the cerebellum 2 hours after acupuncture (RT-PCR).
Total RNA (500 ng) of the frozen cerebellum samples from animals in each group, obtained 20 minutes or 2 hours after acupuncture, was reverse-transcribed using the AMV First Strand cDNA Synthesis Kit, according to the manufacturer’s instructions (Thermo Fisher Scientific Inc, Waltham, MA USA; catalog No. LSK1200, USA). Complementary DNA was first synthesized from total RNA using reverse transcriptase (Takara Co., Shiga, Japan). Advanced relative expression levels of the representative genes, showing differential expressions, were monitored with the ABI StepOne Plus RT-PCR instrument (Applied Biosystems, Foster City, CA, USA) under the following conditions: 40 cycles of denaturation at 94° C for 10 seconds, annealing at 65°C for 30 seconds, and extension at 72°C for 30 seconds. Primers for Bcl-2, Birc1b and β-actin were designed with Primer Premier 5.0 software for the rat (Premier Biosoft International, Palo Alto, CA, USA) as follows: β-actin (229 bp), 5′-CGT AAA GAC CTC TAT GCC AAC A-3′ (forward) and 5′-CGG ACT CAT CGT ACT CCT GCT-3′ (reverse); Bcl-2 (219 bp), 5′-GCA AAG CAC ATC CAA TAA AAG C-3′ (forward) and 5′-CAT CTC CAG TAT CCC ACT CGT AG-3′ (reverse); Birc1b (110 bp), 5′-GGC TGA AGA CTT TTG TGA CCT AT-3′ (forward) and 5′-GCA GAA GCA CTG AAC CCC AT-3′ (reverse). For each reaction, 1 µL of each diluted cDNA sample was added to a mixture containing 10 µL of SYBR Green PCR Master Mix (2X) (Life Technologies, Carlsbad, CA, USA), 1 µL of each primer (10 µM), and 7 µL of ddH2O. Mean crossing point values were obtained, and the advanced relative expression level of the target gene in each experimental group was normalized by that of β-actin. The fold change of target gene cDNA relative to the internal control (β-actin) was determined as follows: fold change = 2-△△Ct, where△△Ct = (Cttarget- Ctβ-actin) test - (Cttarget-Ctβ-actin) control. Ct values were defined as the number of PCR cycles at which fluorescence signals were detected.
Statistical analysis
Scanned density differences on the Oligo GEArray® Rat Signal Transduction PathwayFinder™ Microarray between the SJ5 or non-acupoint group vs. control group were analyzed by nonparametric Student’s t-test. RT-PCR and western blot assay data are presented as the mean ± SEM and analyzed by oneway analysis of variance followed by Tukey’s honestly significant difference (HSD) post hoc test using Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA). P < 0.05 was consideredstatistically significant.
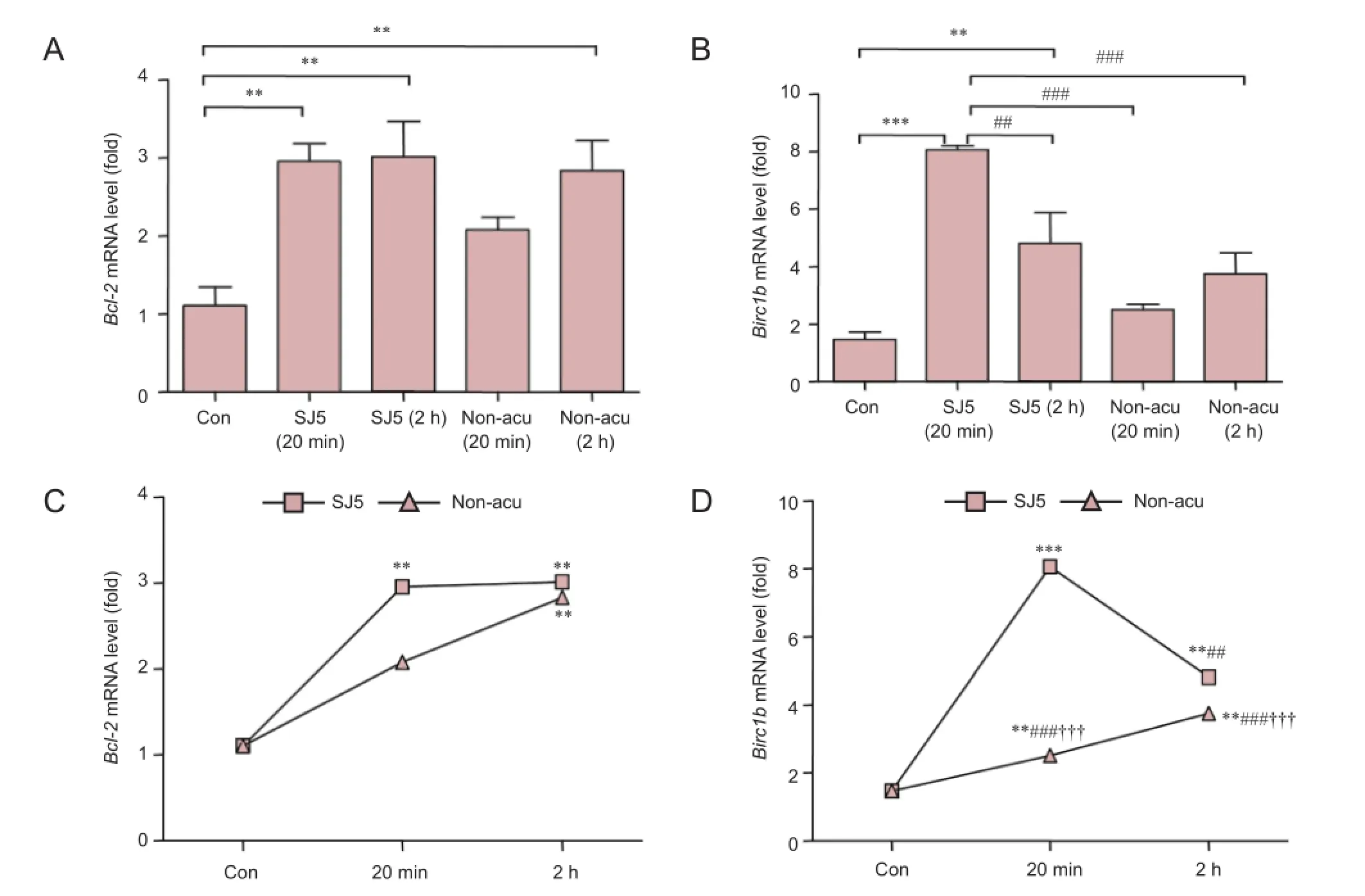
Figure 3 Bcl-2 and Birc1b mRNA expression in the cerebellum after acupuncture stimulation.
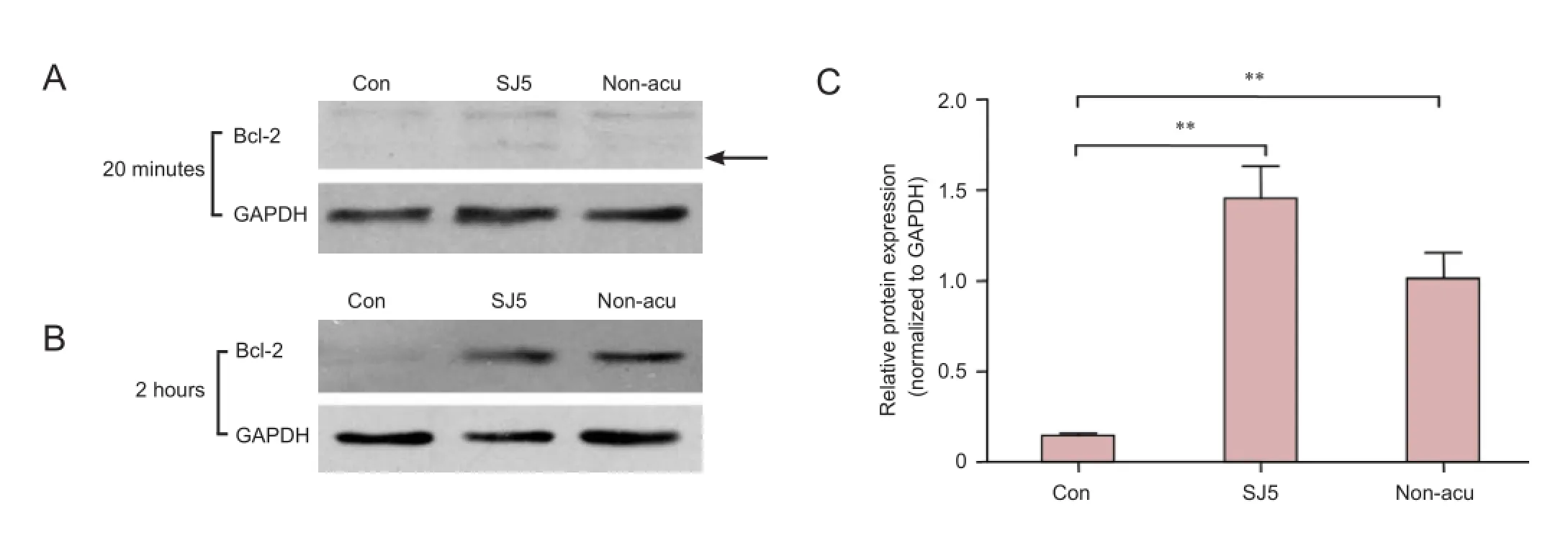
Figure 5 Bcl-2 protein expression in the cerebellum (western blot assay).
Results
Identification of differential gene expression between acupuncture at SJ5 and at the non-acupoint in rat cerebellum
113 genes associated with 18 signal transduction pathways were screened by the Oligo GEArray™ system (Table 1). The hybridized signal detected for each gene was normalized to the signal obtained for GAPDH in the same gene array to derive the expression value for each gene. Expression of Bcl-2 and Birc1b was elevated in distinct survival signaling pathways in the SJ5 acupuncture group compared with the control group (Figure 2; Table 2). The remaining candidate genes were excluded because the confirmatory analyses (n = 3) did not show a statistically significant difference in their expression levels between the two groups (SJ5 acupuncture vs. control, and non-acupoint vs. control), even if gene expression showed a twofold difference from the control group. These results suggest that after 20 minutes, stimulation at SJ5 and at the non-acupoint upregulated gene expression in distinct survival signaling pathways.
Acupuncture stimulation at SJ5 increased mRNA expression of Bcl-2 and Birc1b in the cerebellum 2 hours after cessation
To validate the gene expression data obtained from the microarray, we analyzed Bcl-2 and Birc1b mRNA expression using real-time PCR. Normalized mRNA levels of Bcl-2 and Birc1b in the SJ5 and non-acupoint groups were significantly higher than those in the control group (P < 0.01; Figure 3A, B). At 20 minutes, Bcl-2 and Birc1b expression was greater in the SJ5 and non-acupoint groups than in the control group (P< 0.001), confirming the microarray data. After 2 hours, Bcl-2 expression remained elevated (P < 0.01), but Birc1b expression had decreased. Interestingly, the non-acupoint group showed the same tendency as the SJ5 group (Figure 3C, D), but the expression of these two genes remained lower than that in the SJ5 group (P < 0.001). The relative expression of Bcl-2 and Birc1b mRNA, normalized against β-actin, are shown in Figure 4.
Acupuncture at SJ5 increased Bcl-2 protein expression in the rat cerebellum 2 hours after acupuncture
Western blot assay showed that, 2 hours after acupuncture, protein expression of Bcl-2 in the SJ5 acupuncture group was significantly greater than that in the control group (P < 0.01; Figure 5). Compared with the control group, needling at the non-acupoint upregulated Bcl-2 protein expression after 2 hours (P < 0.01). No significant difference was observed between the SJ5 and non-acupoint groups (P > 0.05).
Discussion
It was recently reported that the effects of acupuncture start 15 minutes after the treatment, and are maintained for at least 30 minutes after completion of the treatment (Jiang et al., 2014). A study of the post-stimulation effects of electroacupuncture at the Yintang (EX-HN3) and Baihui (GV20) acupoints revealed that the cerebellum was activated 15 minutes after stimulation by electroacupuncture, and that this level of brain activation was higher at 15 minutes after treatment than at 5 minutes after treatment (Zheng et al., 2012). In the present study, after needle withdrawal, acupuncture modulation lasted up to 20 minutes in the cerebellum, manifesting as elevated Bcl-2 and Birc1b expression. The Bcl-2 gene was maintained at this high level of expression for 2 hours after treatment, and was significantly higher in the SJ5 acupuncture group than in the control group. Our data provides further evidence that the effects of acupuncture start several minutes after treatment, and last beyond its cessation. At least a 30-minute treatment period might be needed for the full expression of the analgesic effects of acupuncture, and no less than 15 minutes may be needed to produce the initial treatment effect (Jiang et al., 2014). All of these findings are consistent with the conventional clinical practice of acupuncture (Han, 2011). A distinguishing aspect of acupuncture is its ability to trigger significant modulation in different areas of the brain, in addition to changes in blood pressure and cardiovascular excitatory reflex responses (Li et al., 2010, 2012). Our present findings suggest that acupuncture at SJ5 can initiate protein changes in the cerebellum related to neuroprotection, and this effect may last from 20 minutes to 2 hours after treatment. This result is consistent with previous clinical and experimental studies (Zhou et al., 2005; Li and Longhurst, 2010), such as the prolonged influence of acupuncture in hypertension (Li et al., 2010, 2012). Together, this evidence indicates that acupuncture stimulation has strong and lasting after-effects on cerebellar function.
As a consequence of acupuncture-induced variations in neural activity, the expression of Bcl-2 and Birc1b were elevated compared with the control group. Bcl-2 is the prototype member of the anti-apoptotic Bcl-2 family (Korsmeyer, 1999). Bcl-2 mRNA and protein are present at relatively high levels in the developing nervous system, and decline sharply in the postnatal brain (Abe-Dohmae et al., 1993; Merry et al., 1994; Akhtar et al., 2004). In the adult brain, neuronal overexpression of Bcl-2 in transgenic mice promotes neuronal survival in many brain regions by inhibiting naturally occurring cell death (Farlie et al., 1995). This means that by regulating cell death in the mature nervous system, Bcl-2 may be able to reverse neurodegenerative pathologies (Marshall et al., 1997). In the present study, microarray analysis revealed upregulation of the survival signaling pathway members Bcl-2 and Birc1b in the SJ5 acupuncture group, and of Bcl-2 in the non-acupoint group, compared with the control group. Just being consistent with the gene expression array, we also found that there was no significant difference between the SJ5 and the non-acupoint acupuncture. This suggests that acupuncture stimulation (including the insertion of a needle) may play an important role in the effects of acupuncture. Our data support a recent study in which it was shown that acupuncture at the Baihui (GV20) and Dazhui (GV14) acupoints increased Bcl-2 expression, as well as reducing neuronal loss in the hippocampus and frontal lobe, and attenuating ultrastructural damage in ischemic rat brain (Hou et al., 2014). Together, this evidence supports the theory that acupuncture stimulation has neuroprotective effects and can mediate anti-apoptosis.
In conclusion, our results indicate that acupuncture mediates brain function by regulating the expression of the anti-apoptotic factors Bcl-2 and Birc1b, in turn affecting the survival signal pathway. Our study suggests that acupuncture has a promising role in the protection of the brain and centralnervous system from injury.
Author contributions: DL performed acupuncture, data analysis, fundraising, and wrote the paper. LLL performed PCR and western blots. KS provided critical revision of the paper for intellectual content. CHC designed the study and performed parts of the experiment. All authors approved the final version of this paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Abe-Dohmae S, Harada N, Yamada K, Tanaka R (1993) Bcl-2 gene is highly expressed during neurogenesis in the central nervous system. Biochem Biophys Res Commun 191:915-921.
Akhtar RS, Ness JM, Roth KA (2004) Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta 1644:189-203.
Arankalle DV, Nair PM (2013) Effect of electroacupuncture on function and quality of life in Parkinson’s disease: a case report. Acupunct Med 31:235-238.
Bai L, Tao Y, Wang D, Wang J, Sun C, Hao N, Chen S, Lao L (2014) Acupuncture induces time-dependent remodelling brain network on the stable somatosensory first-ever stroke patients: combining diffusion tensor and functional MR imaging. Evid Based Complement Alternat Med 2014:740480.
Cakmak YO (2006) Epilepsy, electroacupuncture and the nucleus of the solitary tract. Acupunct Med 24:164-168.
Chen H, Dai J, Zhang X, Wang K, Huang S, Cao Q, Wang H, Liang Y, Shi C, Li M, Ha T, Ai L, Li S, Ma J, Wei W, You Y, Liu Z, Tian J, Bai L (2013) Hypothalamus-related resting brain network underlying shortterm acupuncture treatment in primary hypertension. Evid Based Complement Alternat Med 2013:808971.
Chen J, Wang J, Huang Y, Lai X, Tang C, Yang J, Wu J, Zeng T, Qu S (2014) Modulatory effect of acupuncture at Waiguan (TE5) on the functional connectivity of the central nervous system of patients with ischemic stroke in the left basal ganglia. PLoS One 9:e96777.
Cho SY, Kim M, Sun JJ, Jahng GH, Kim HJ, Park SU, Jung WS, Ko CN, Park JM (2013) A comparison of brain activity between healthy subjects and stroke patients on fMRI by acupuncture stimulation. Chin J Integr Med 19:269-276.
Farlie PG, Dringen R, Rees SM, Kannourakis G, Bernard O (1995) bcl-2 transgene expression can protect neurons against developmental and induced cell death. Proc Natl Acad Sci U S A 92:4397-4401.
Han JS (2011) Acupuncture analgesia: areas of consensus and controversy. Pain 152:S41-48.
Hou X, Zhang R, Lv H, Cai X, Xie G, Song X (2014) Acupuncture at Baihui and Dazhui reduces brain cell apoptosis in heroin readdicts. Neural Regen Res 9:164-170.
Hsu SF, Chen CY, Ke MD, Huang CH, Sun YT, Lin JG (2011) Variations of brain activities of acupuncture to TE5 of left hand in normal subjects. Am J Chin Med 39:673-686.
Jiang Y, Liu J, Han J, Wang X, Cui C (2014) Cerebral blood flow-based evidence for mechanisms of low- versus high-frequency transcutaneous electric acupoint stimulation analgesia: a perfusion fMRI study in humans. Neuroscience 268:180-193.
Jin LM, Qin CJ, Lan L, Sun JB, Zeng F, Zhu YQ, Yu SG, Yin HY, Tang Y (2014) Local anesthesia at ST36 to reveal responding brain areas to deqi. Evid Based Complement Alternat Med 2014:987365.
Kim SN, Doo AR, Park JY, Bae H, Chae Y, Shim I, Lee H, Moon W, Park HJ (2011) Acupuncture enhances the synaptic dopamine availability to improve motor function in a mouse model of Parkinson’s disease. PLoS One 6:e27566.
Korsmeyer SJ (1999) BCL-2 gene family and the regulation of programmed cell death. Cancer Res 59:1693s-1700s.
Lee SH, Jahng GH, Choe IH, Choi CB, Kim DH, Kim HY (2013) Neural pathway interference by retained acupuncture: a functional MRI study of a dog model of Parkinson’s disease. CNS Neurosci Ther 19:585-595.
Leung A, Zhao Y, Shukla S (2014) The effect of acupuncture needle combination on central pain processing--an fMRI study. Mol Pain 10:23.
Li M, Tjen-A-Looi SC, Longhurst JC (2010) Electroacupuncture enhances preproenkephalin mRNA expression in rostral ventrolateral medulla of rats. Neurosci Lett 477:61-65.
Li M, Tjen-A-Looi SC, Guo ZL, Longhurst JC (2012) Repetitive electroacupuncture causes prolonged increased met-enkephalin expression in the rVLM of conscious rats. Auton Neurosci 170:30-35.
Li P, Longhurst JC (2010) Neural mechanism of electroacupuncture’s hypotensive effects. Auton Neurosci 157:24-30.
Liu B, Liu X, Chen J, Long Y, Chen ZG, Shang XJ, Mo WZ, Li XF (2009) Study on the effects of acupuncture at acupoint and non-acupoint on functional connectivity of different brain regions with functional magnetic resonance imaging. Zhongguo Zhen Jiu 29:981-985.
Liu ET, Wang SX, Huang Y, Lai XS, Tang CZ, Cui SY (2013) Effect of needling at waiguan (SJ5) on brain glucose metabolism in patients with cerebral infarction. Zhongguo Zhong Xi Yi Jie He Za Zhi 33:1345-1351.
Manni L, Albanesi M, Guaragna M, Barbaro Paparo S, Aloe L (2010) Neurotrophins and acupuncture. Auton Neurosci 157:9-17.
Marshall KA, Daniel SE, Cairns N, Jenner P, Halliwell B (1997) Upregulation of the anti-apoptotic protein Bcl-2 may be an early event in neurodegeneration: studies on Parkinson’s and incidental Lewy body disease. Biochem Biophys Res Commun 240:84-87.
Merry DE, Veis DJ, Hickey WF, Korsmeyer SJ (1994) bcl-2 protein expression is widespread in the developing nervous system and retained in the adult PNS. Development 120:301-311.
No authors listed (2009) Neurochemical mechanisms of sleep regulation. Glas Srp Akad Nauka Med: 97-109.
Quah-Smith I, Suo C, Williams MA, Sachdev PS (2013) The antidepressant effect of laser acupuncture: a comparison of the resting brain’s default mode network in healthy and depressed subjects during functional magnetic resonance imaging. Med Acupunct 25:124-133.
Romoli M, Allais G, Airola G, Benedetto C, Mana O, Giacobbe M, Pugliese AM, Battistella G, Fornari E (2014) Ear acupuncture and fMRI: a pilot study for assessing the specificity of auricular points. Neurol Sci 35 Suppl 1:189-193.
Small SL, Hlustik P, Noll DC, Genovese C, Solodkin A (2002) Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain 125:1544-1557.
Soligo M, Nori SL, Protto V, Florenzano F, Manni L (2013) Acupuncture and neurotrophin modulation. Int Rev Neurobiol 111:91-124.
Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, Woodward TS, Zhu C (2010) Dynamic functional reorganization of the motor execution network after stroke. Brain 133:1224-1238.
Xie Z, Cui F, Zou Y, Bai L (2014) Acupuncture enhances effective connectivity between cerebellum and primary sensorimotor cortex in patients with stable recovery stroke. Evid Based Complement Alternat Med 2014:603909.
Yin CS, Jeong HS, Park HJ, Baik Y, Yoon MH, Choi CB, Koh HG (2008) A proposed transpositional acupoint system in a mouse and rat model. Res Vet Sci 84:159-165.
Zhang Y, Glielmi CB, Jiang Y, Wang J, Wang X, Fang J, Cui C, Han J, Hu X, Zhang J (2012) Simultaneous CBF and BOLD mapping of high frequency acupuncture induced brain activity. Neurosci Lett 530:12-17.
Zheng Y, Qu S, Wang N, Liu L, Zhang G, Jiang X, Chen J, Huang Y, Zhang Z (2012) Post-stimulation effect of electroacupuncture at Yintang (EX-HN3) and GV20 on cerebral functional regions in healthy volunteers: a resting functional MRI study. Acupunct Med 30:307-315.
Zhou W, Fu LW, Tjen-A-Looi SC, Li P, Longhurst JC (2005) Afferent mechanisms underlying stimulation modality-related modulation of acupuncture-related cardiovascular responses. J Appl Physiol (1985) 98:872-880.
Copyedited by Slone-Murphy J, Robens J, Li CH, Song LP, Zhao M
10.4103/1673-5374.177740 http://www.nrronline.org/
How to cite this article: Lin D, Lin LL, Sutherland K, Cao CH (2016) Manual acupuncture at the SJ5 (Waiguan) acupoint shows neuroprotective effects by regulating expression of the anti-apoptotic gene Bcl-2. Neural Regen Res 11(2):305-311.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81273672, 81373720; the Natural Science Foundation of Fujian Province of China, No. 2014J01353; and a grant from Fujian Province Health Planning Commission Project (2014) for Training Young Talents, No. 2014-ZQN-JC-28.
Accepted: 2015-07-30
*Correspondence to: Chuan-hai Cao, M.D., ccao@health.usf.edu.
杂志排行
中国神经再生研究(英文版)的其它文章
- Tissue-type plasminogen activator is a modulator of the synaptic vesicle cycle
- Impaired consciousness caused by injury of the lower ascending reticular activating system: evaluation by diffusion tensor tractography
- Considering calcium-binding proteins in invertebrates: multi-functional proteins that shape neuronal growth
- Cardiovascular dysfunction following spinal cord injury
- Practical application of the neuroregenerative properties of ketamine: real world treatment experience
- Exergames: neuroplastic hypothesis about cognitive improvement and biological effects on physical function of institutionalized older persons
