Neuroprotection of Chrysanthemum indicum Linne against cerebral ischemia/reperfusion injury by anti-inflammatory effect in gerbils
2016-12-02KiYeonYooInHyeKimJeongHwiChoJiHyeonAhnJoonHaParkJaeChulLeeHyunJinTaeDaeWonKimJongDaiKimSeongkweonHongMooHoWonIlJunKang
Ki-Yeon Yoo, In Hye Kim, Jeong-Hwi Cho Ji Hyeon Ahn Joon Ha Park Jae-Chul Lee Hyun-Jin Tae, Dae Won Kim, Jong-Dai Kim, Seongkweon Hong, Moo-Ho Won, Il Jun Kang
1 Department of Oral Anatomy, College of Dentistry and Research Institute of Oral Science, Gangneung-Wonju National University, Gangneung, Republic of Korea
2 Department of Neurobiology, School of Medicine, Kangwon National University, Chuncheon, Republic of Korea
3 Department of Biomedical Science, Research Institute of Bioscience and Biotechnology, Hallym University, Chuncheon, Republic of Korea
4 Department of Biochemistry and Molecular Biology, College of Dentistry and Research Institute of Oral Science, Gangneung-Wonju National University, Gangneung, Republic of Korea
5 Division of Food Biotechnology, School of Biotechnology, Kangwon National University, Chuncheon, Republic of Korea
6 Department of Surgery, School of Medicine, Kangwon National University, Chuncheon, Republic of Korea
7 Department of Food Science and Nutrition, Hallym University, Chuncheon, Republic of Korea
RESEARCH
Neuroprotection of Chrysanthemum indicum Linne against cerebral ischemia/reperfusion injury by anti-inflammatory effect in gerbils
Ki-Yeon Yoo1,#, In Hye Kim2,#, Jeong-Hwi Cho2, Ji Hyeon Ahn2, Joon Ha Park2, Jae-Chul Lee2, Hyun-Jin Tae3, Dae Won Kim4, Jong-Dai Kim5, Seongkweon Hong6, Moo-Ho Won2,*, Il Jun Kang7,*
1 Department of Oral Anatomy, College of Dentistry and Research Institute of Oral Science, Gangneung-Wonju National University, Gangneung, Republic of Korea
2 Department of Neurobiology, School of Medicine, Kangwon National University, Chuncheon, Republic of Korea
3 Department of Biomedical Science, Research Institute of Bioscience and Biotechnology, Hallym University, Chuncheon, Republic of Korea
4 Department of Biochemistry and Molecular Biology, College of Dentistry and Research Institute of Oral Science, Gangneung-Wonju National University, Gangneung, Republic of Korea
5 Division of Food Biotechnology, School of Biotechnology, Kangwon National University, Chuncheon, Republic of Korea
6 Department of Surgery, School of Medicine, Kangwon National University, Chuncheon, Republic of Korea
7 Department of Food Science and Nutrition, Hallym University, Chuncheon, Republic of Korea
In this study, we tried to verify the neuroprotective effect of Chrysanthemum indicum Linne (CIL) extract, which has been used as a botanical drug in East Asia, against ischemic damage and to explore the underlying mechanism involving the anti-inflammatory approach. A gerbil was given CIL extract for 7 consecutive days followed by bilateral carotid artery occlusion to make a cerebral ischemia/reperfusion model. Then, we found that CIL extracts protected pyramidal neurons in the hippocampal CA1 region (CA1) from ischemic damage using neuronal nucleus immunohistochemistry and Fluoro-Jade B histofluorescence. Accordingly, interleukin-13 immunoreactivities in the CA1 pyramidal neurons of CIL-pretreated animals were maintained or increased after cerebral ischemia/reperfusion. These findings indicate that the pre-treatment of CIL can attenuate neuronal damage/death in the brain after cerebral ischemia/reperfusion via an anti-inflammatory approach.
nerve regeneration; transient cerebral ischemia; delayed neuronal death; pyramidal neurons; inflammatory cytokines; neural regeneration
# These authors contributed equally to this work.
orcid: 0000-0002-7178-6501 (Moo-Ho Won) 0000-0003-0314-5195 (Il-Jun Kang)
Accepted: 2015-09-28
Introduction
Cerebral ischemic injury results from the interruption of cerebral blood flow that causes cell damage/death in the brain. The oxygen and glucose deprivation in ischemic state can trigger molecular pathways, including oxidative stress, glutamate excitotoxicity and inflammation, and ultimately neuronal death (Buffo et al., 2008; Sutherland et al., 2012).
Transient ischemic attack can cause neuronal death selectively in several brain areas, such as the cerebral cortex, hippocampus and striatum (Crain et al., 1988; Butler et al., 2002). Especially, the hippocampal CA1 region is highly susceptible to transient ischemic attack that can result in the death of pyramidal neurons in the stratum within several days (Kirino, 1982; Pulsinelli et al., 1982). This neuronal death is called “delayed neuronal death” (Kirino, 2000).
Chrysanthemum indicum Linne (CIL) which is used as a botanical drug in East Asia has been prescribed to cure inflammation, hypertension, respiratory diseases, headache, ulcerative colitis, vertigo, and eye irritation (Yu et al., 1992; Matsuda et al., 2002; Cheng et al., 2005; Shunying et al., 2005; Lee do et al., 2009; Wang et al., 2010). Chemical studies regarding CIL have identified major components of CIL such as 1,8-cineole, camphor, germacrene D, α-cadinol, camphene, β-caryophyllene, 3-cyclohexen-1-ol pinocarvone and γ-curcumene (Wang and Yang, 2006; Zhang et al., 2010). Recent studies regarding bioactivities of CIL such as anti-oxidative, anti-microbial and anti-inflammatory effects have been reported (Cheon et al., 2009; Pongjit et al., 2011).
Recently, many researchers have focused on neuroprotective effects of extracts from medicinal plants against transient focal/ global cerebral ischemia (Tang et al., 2010; Wu et al., 2010; Chen et al., 2012, 2013; Ghosh et al., 2014); however, little is reported regarding the neuroprotective effect of CIL. Therefore, the aim of this study was to examine the neuroprotective effect of CIL against neuronal death using a gerbil model of cerebral ischemia/reperfusion injury (Min et al., 2012; Shcherbak et al., 2013; Liu et al., 2014); furthermore, we examined changes in inflammatory factors to understand a part of the mechanisms underlying the neuroprotection of CIL against cerebral ischemia/reperfusion injury in the gerbil.
Materials and Methods
Experimental animals
Twenty-eight male Mongolian gerbils (body weight 65-75 g, 6 months of age) were provided by the Experimental Animal Center, Kangwon National University, Chunchon, Republic of Korea. These animals were housed conventionally at a temperature of 23°C and a relative humidity of 60%. All the experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Kangwon National University (approval no. KW-130424-1) and formulated in compliance with the Guide for the Care and Use of Laboratory Animals (the National Academies Press, 8thed., 2011).
Preparation of CIL extract
CIL was collected by Professor Jong Dai Kim from Division of Food Biotechnology, School of Biotechnology, Kangwon National University in Kangwon Province, Republic of Korea, in October 2013 and kept in a deep freezer (-70°C). The CIL was extracted with 70% ethanol at 70°C for 4 hours, which was repeated three times. After filtered via the Whatman filter paper (No. 2), the extracts were concentrated using a vacuum evaporator, and completely dried using a freeze-drier. Finally, the extraction yield was 14.5%.
CIL administration
Twenty-eight gerbils were equally randomized into four groups, with seven animals in each group: (1) vehicle-sham group, which was treated with vehicle (0.9% saline) and received sham operation; (2) vehicle-ischemia group, which was treated with vehicle and received ischemia operation; (3) CIL-sham group, which was treated with CIL and received sham operation; (4) CIL-ischemia group, which was treated with CIL and received ischemia operation.
CIL was dissolved in saline and administrated orally at doses of 25, 50 or 200 mg/kg per day, respectively, using a feeding needle for 7 days prior to transient cerebral ischemia; the last treatment was implemented at 30 minutes prior to cerebral ischemia. In previous studies, significant neuroprotective effects were found in animals treated with 200 mg/kg of CIL, and therefore, CIL at 200 mg/kg was preferred in this study.
Induction of transient cerebral ischemia
Transient cerebral ischemia was developed as described previously (Yu et al., 2012; Park et al., 2014a). Experimental animals were anesthetized with a mixture of 2.5% isoflurane, 33% oxygen and 67% nitrous oxide. Common carotid arteries were occluded bilaterally for 5 minutes. Then, the blood flow was restored under an ophthalmoscope. Body (rectal) temperature was maintained at 37 ± 0.5°C prior to, during and after the surgery until the animals were awakened completely. Except for common carotid artery occlusion, rats in sham groups were subjected to the same surgical procedures.
Histological observation
The animals (n = 7 at each time point in each group) were sacrificed with 30 mg/kg Zoletil 50 (Virbac, Carros, France) at 2 and 5 days after reperfusion. The animals were given intracardially perfusion with 4% paraformaldehyde (Yu et al., 2012). The brain tissues were cryoprotected by infiltration with 30% sucrose and serial coronal sections were cut on a cryostat (Leica, Wetzler, Germany). The sections were 30 μm in thickness.
Fluoro-Jade B (F-J B) histofluorescence
To investigate the neuronal death in the ischemic hippocampal CA1 region, F-J B histofluorescence staining as a high-affinity fluorescent marker for locating neuronal degeneration was conducted according to a modified method by Schmued and Hopkins (2000). The brain sections were immersed in 1% sodium hydroxide solution, then transferred to 0.06% potassium permanganate solution and finally to a 0.0004% F-J B staining solution (Histochem, Jefferson, AR, USA). After that, the sections were observed under an epifluorescent microscope (Carl Zeiss, Göttingen, Germany) with blue excitation light (450-490 nm) and a barrier filter.
Immunohistochemistry for neuronal nuclei (NeuN), glial fibrillary acidic portein (GFAP), ionized calcium-binding adapter molecule-1 (Iba-1), interleukin (IL)-2 and IL-13
As previously described (Park et al., 2014b), the sections were incubated with diluted mouse anti-NeuN (1:800, Chemicon International, Temecula, CA, USA), mouse anti-GFAP (1:800, Chemicon International), rabbit anti-Iba-1 (1:800, Wako, Osaka, Japan), rabbit anti-IL-2 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) as pro-inflammatory cytokines and rabbit anti-IL-13 (1:200, Santa Cruz Biotechnology) as anti-inflammatory cytokine overnight at 4° C. Thereafter, the sections were incubated with biotinylated horse anti-mouse and goat anti-rabbit IgG (Vector, Burlingame, CA, USA) Streptavidin peroxidase complex (1:200, Vector). Staining was developed by 3,3′-diaminobenzidine tetrahydrochloride in 0.1 M Tris-HCl buffer (pH 7.2). To establish the specificity of the immunostaining, a negative control test was performed with the pre-immune serum rather than the primary antibody. Absence of immunoreactivity in all structures occurred in the negative control test.
Western blot analysis
According to a method by Yoo et al. (2012), CA1 tissues (n = 7 at each time point in each group) were homogenized and centrifugalized, and a Micro BCA protein assay kit was used to detect the protein level in the supernatants. Bovine serum albumin acted as a standard (Pierce Chemical, Rockford, IL,USA). The gels were electrophoretically separated and transferred to nitrocellulose membranes (Pall Crop, East Hills, NY, USA). To reduce background staining, the membranes were incubated with PBS containing 5% non-fat dry milk and 0.1% Tween 20 for 45 minutes, followed by incubation with rabbit anti-IL-2 (1:1,000, Santa Cruz Biotechnology) or rabbit anti-IL-13 (1:1,000, Santa Cruz Biotechnology), peroxidase-conjugated goat anti-rabbit IgG (Sigma) and an ECL kit (Pierce Chemical).
Data analysis
In order to quantitatively analyze F-J B-positive and NeuN-immunoreactive cells, digital images of the hippocampus were captured with an AxioImager.A2 light microscope (Carl Zeiss) equipped with a digital camera (Axiocam, Carl Zeiss) connected to a PC monitor (Yoo et al., 2012; Park et al., 2014b). The positive cells were counted in a 250 × 250 μm2area approximately at the center of the CA1 region using Optimas 6.5 (CyberMetrics, Scottsdale, AZ, USA). According to the anatomical landmarks corresponding to AP -1.4 to -1.9 mm of the gerbil brain atlas, the tissue sections were selected with an interval of 300 μm, and the cell number calculated by averaging the counts from each animal: A ratio of the count was calibrated as %, with vehicle-sham group or vehicle-ischemia group designated as 100%.
In addition, images of all GFAP, Iba-1, IL-2 and IL-13-immunoreactive structures were taken from the CA1 region through an AxioImager to quantitatively analyze GFAP, Iba-1, IL-2 and IL-13 immunoreactivity (Yoo et al., 2012; Park et al., 2014b). An AxioImager. A2 light microscope (Carl Zeiss) equipped with a digital camera (Axiocam, Carl Zeiss) was connected to a PC monitor. Images were calibrated into an array of 512 × 512 pixels corresponding to a tissue area of 140 × 140 μm2(40-fold magnification) including the stratum pyramidale. The densities of all GFAP, Iba-1, IL-2 and IL-13-immunoreactive structures were evaluated based on the optical density (OD), which was obtained after the transformation of the mean gray level using the formula: OD value = log (256/ mean gray level). The OD value of background was taken from areas adjacent to the measured area. After the background density was subtracted, the OD value of image file was calibrated as % (relative optical density, ROD) using Adobe Photoshop version 8.0 and then analyzed using NIH Image 1.59 software. The ROD was calibrated as %, with vehicle-sham group designated as 100%.
Western blot results were scanned and quantified using Scion Image software (Scion Corp., Frederick, MD, USA), and then used to calculate the ROD that was calibrated as %.
Statistical analysis
Data are expressed as the mean ± SEM and were analyzed using SPSS 18.0 (IBM Corporation, New York, USA). Intergroup comparisons were made using parametric two-way analysis of variance. Further comparisons were assessed using Duncan’s multiple-range test. P < 0.05 was considered statistically significant.
Results
Neuroprotective effects NeuN-immunoreactive neurons
In the vehicle-sham group, NeuN-immunoreactive neurons were distributed through all the hippocampal sub-regions (Figure 1A), but mainly concentrated in the stratum pyramidale in the CA1 region (Figure 1B). In the CIL-sham group, the distribution pattern and population of NeuN-immunoreactive neurons in the hippocampus were similar to that in the vehicle-sham group (Figure 1D-E), and the mean number of NeuN-immunoreactive neurons was not altered (Figure 1M). In the vehicle-ischemia-group and CIL-ischemia group, 2 days after ischemic injury, NeuN immunoreactivity had no changes in the hippocampal CA1 region, and the mean number of NeuN immunoreactive neurons was similar to that in the vehicle-sham group (Figure 1M). In the vehicle-ischemia group, a few NeuN-immunoreactive neurons were found in the CA1 region at 5 days post-ischemia (Figure 1G-H), and the mean number of NeuN-immunoreactive neurons was reduced by about 92% compared to the vehicle-sham group (Figure 1M). However, in the CIL-ischemia group, many NeuN-immunoreactive neurons were found in the CA1 region compared with the vehicle-ischemia group (Figure 1J); the survival rate of NeuN-immunoreactive neurons was 63.6% of the vehicle-sham group (Figure 1K and M).
F-J B-positive cells
In the vehicle-sham and CIL-sham groups, F-J B positive cells of jade color disppeared in the CA1 region (Figure 1C and F). In the vehicle-ischemia group, a large number of F-J B positive cells were observed in the stratum pyramidale of the CA1 region (Figure 1I). In the CIL-ischemia group, however, the number of F-J B positive cells was 22.2%, which was lower than that in the vehicle-ischemia group (Figure 1L and N).
Glial activation GFAP-immunoreactive astrocytes
GFAP, an astrocyte marker, is one of the major intermediate filament proteins of mature astrocytes (Cho et al., 2010). In this study, the change of astrocyte activation in the ischemic CA1 region was examined by GFAP immunohistochemistry. In the vehicle-sham group, GFAP-immunoreactive astrocytes showed a thread-like shape throughout the CA1 region; in the CIL-sham group, astrocytes were not changed compared with those in the vehicle-sham group (Figure 2A and B). Two days after ischemic injury, the cytoplasm and processes of GFAP-immunoreactive astrocytes became a little thicker in the vehicle-ischemia group, but were not significantly changed in the CIL-ischemia group (Figure 2E, F and M). At 5 days post-ischemia, GFAP-immunoreactive astrocytes showed severer cytoplasmic hypertrophy with thicker processes in the vehicle-ischemia group compared with the CIL-ischemia group (Figure 2I, J and M).
Iba-1-immunoreactive microglia
Iba-1 is a calcium-binding protein that is expressed in macrophage/microglia (Kolenda-Roberts et al., 2013). In this study, the change of microglia activation in the ischemic CA1 regionwas examined by Iba-1 immunoreactivity. In the vehicle-sham group, Iba-1-immunoreactive microglia with ramified thin processes were inactivated throughout the CA1 region, similar to those in the CIL-sham group (Figure 2C and D). At 2 days post-ischemia, both in the vehicle-ischemia group and CIL-ischemia group, Iba-1-immunoreactive microglia had highly branched processes (Figure 2G, H and N). In the vehicle-ischemia group, Iba-1-immunoreactive microglia were much more activated and stronger in their immunoreactivity at 5 days post-ischemia than those at 2 days post-ischemia (Figure 2K and N); especially, many activated microglia were aggregated in the stratum pyramidale. However, in the CIL-ischemia group, there were less activated Iba-1-immunoreactive microglia than those in the vehicle-ischemia group at 5 days after ischemic injury (Figure 2L and N).
Inflammatory cytokine expression IL-2 immunoreactivity
IL-2 immunoreactive neurons were highly expressed in the stratum pyramidale of the CA1 region of the vehicle-sham group (Figure 3A), as well as in the CA1 pyramidale of the CIL-sham group (Figure 3B). At 2 days post-ischemia, IL-2 immunoreactive neurons in the stratum pyramidale of the vehicle-ischemia group were decreased in number; however, IL-2 immunoreactive neurons of the CIL-ischemia group were not altered compared with the CIL-sham group (Figure 3E, F and M). At 5 days post-ischemia, IL-2-immunoreactive cells were significantly decreased (about 19% of the vehicle-sham group) in the CA1 region (Figure 3I and M); however, in the CIL-ischemia group, IL-2-immunoreactive cells in the stratum pyramidale were slightly decreased compared with the CIL-sham group (Figure 3J and M).
IL-13 immunoreactivity
In the vehicle-sham group, weak IL-13 immunoreactivity was found in the stratum pyramidale of the CA1 region (Figure 3C); however, IL-13 immunoreactivity was increased in the CA1 pyramidal neurons of the CIL-sham group (Figure 3D and N). At 2 days after ischemia, IL-13-immunoreactive neurons in the stratum pyramidale were slightly decreased in the vehicle-ischemia group; however, IL-13-immunoreactive neurons in the CIL-ischemia group were increased (Figure 3G, H and N). At 5 days post-ischemia, IL-2-immunoreactive cells were rarely observed in the CA1 region of the vehicle-ischemia group (Figure 3K); however, many IL-2-immunoreactive cells were detected in the stratum pyramidale of the CIL-ischemia group (Figure 3L and N).
IL-2 and IL-13 protein levels
Change patterns in IL-2 and IL-13 protein levels were generally similar to the immunohistochemical data (Figure 4). There was no difference in the protein level of IL-2 between the CIL-sham group and the vehicle-sham group. In the vehicle-ischemia group, the IL-2 level was significantly decreased with time after ischemia/reperfusion (P < 0.05); however, in the CIL-ischemia group, it was not significantly altered with time after ischemia/ reperfusion. Additionally, IL-13 level in the CIL-sham group was significantly higher than that in the vehicle-sham group (P < 0.05). In the vehicle-ischemia group, IL-13 level was very low at 5 days post-ischemia; however, the level of IL-13 in the CIL-ischemia group was significantly higher than that in the vehicle-ischemia group (P < 0.05).
Discussion
Transient cerebral ischemia leads to neuronal death in various brain regions such as the cortex, cerebellum, stratum and hippocampus. Of these brain regions, pyramidal neurons in the hippocampal CA1 region are particularly vulnerable to ischemic injury and die starting from 4 days after ischemia in gerbils (Kirino, 1982, 2000; Kirino et al., 1984). In the present study, neuronal death was evaluated by NeuN immunohistochemistry and F-J B histofluorescence 5 days after ischemic injury, and the administration of CIL protected CA1 pyramidal neurons (the survival rate was about 64% of the vehicle-sham group) from ischemic damage.
Although the exact mechanism of neuronal death is unclear, the delayed death of CA1 pyramidal neurons is associated with inflammation following transient cerebral ischemia (Wang et al., 2007). Ischemia/reperfusion activates and accumulates inflammatory cells such as microglia within ischemic tissue, thereby resulting in inflammatory injury (Benakis et al., 2014). In addition, molecular cues induced by ischemia/reperfusion injury activate components of innate immunity, increase inflammatory signaling, and develop tissue damage after ischemia (Iadecola and Anrather, 2012).
In this study, microglia and astrocytes were strongly activated in the CA1 region of the vehicle-ischemia group 5 days after ischemic injury because of the neuronal death of CA1 pyramidal neurons. However, in the CIL-ischemia group, the occurrence of reactive gliosis was significantly decreased with the attenuation of neuronal death in the CA1 region 5 days after ischemia.
Glial cells are involved in the modulation of immune response (microglia), the maintenance of homeostasis (astrocytes) and the myelination of axon (oligodendrocytes) in the central nervous system (Buffo et al., 2008) and they are increased in number and activated by central nervous system injuries (Giulian and Vaca, 1993; Sofroniew, 2005). This response is referred to as “reactive gliosis” and mainly involves activated microglia and astrocytes (Giulian, 1993; Sofroniew, 2005; Fitch and Silver, 2008). Although detrimental and beneficial effects of activated microglia and astrocyte are unclear yet, these cells in an activated state commonly produce and release inflammatory mediators and cytotoxic molecules (reactive oxygen species, nitric oxide synthesis, protease, etc.). These factors can lead to cell damage or death (Schubert et al., 2000; Nowicka et al., 2008; Ceulemans et al., 2010). Many reports have suggested that inflammatory reaction in the brain is related with a balance between pro- and anti-inflammatory cytokines and the breakdown of this balance by ischemic injuiry leads to inflammation or a recovery from inflammation (Pahan et al., 2000; Perini et al., 2001; Huang et al., 2006; Wong and Crack, 2008).
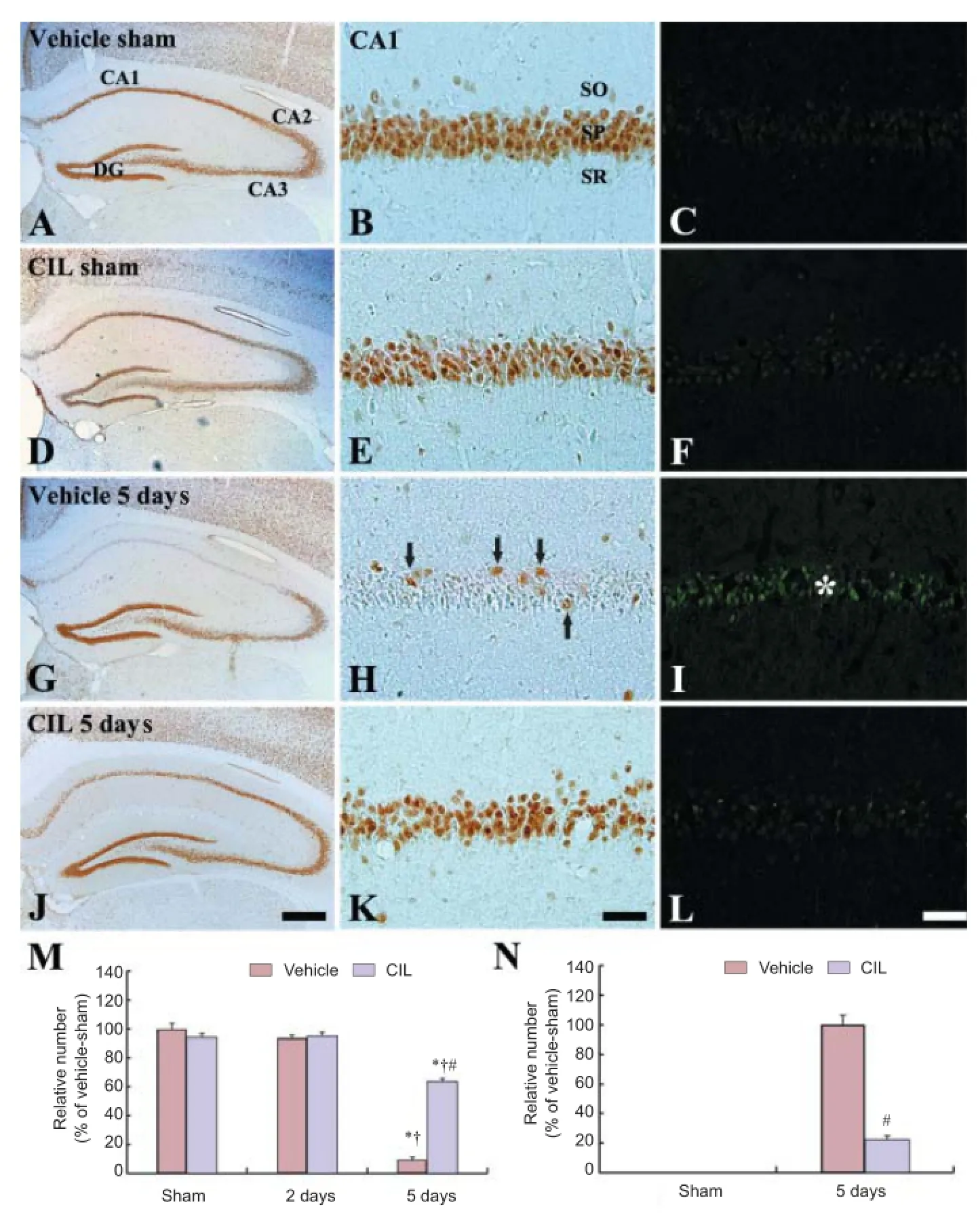
Figure 1 NeuN- (left and middle columns) and F-J B (right column)-positive cells in the vehicle-sham (A-C), CIL-sham (D-F), vehicle-ischemia (G-H) and CIL-ischemia (J-L) groups at 5 days post-ischemia.

Figure 2 GFAP and Iba-1 immunoreactivities in the CA1 region of the vehicle-sham (A and C), CIL-sham (B and D), vehicleischemia (E, I, G and K) and CIL-ischemia (F, J, H and L) groups.
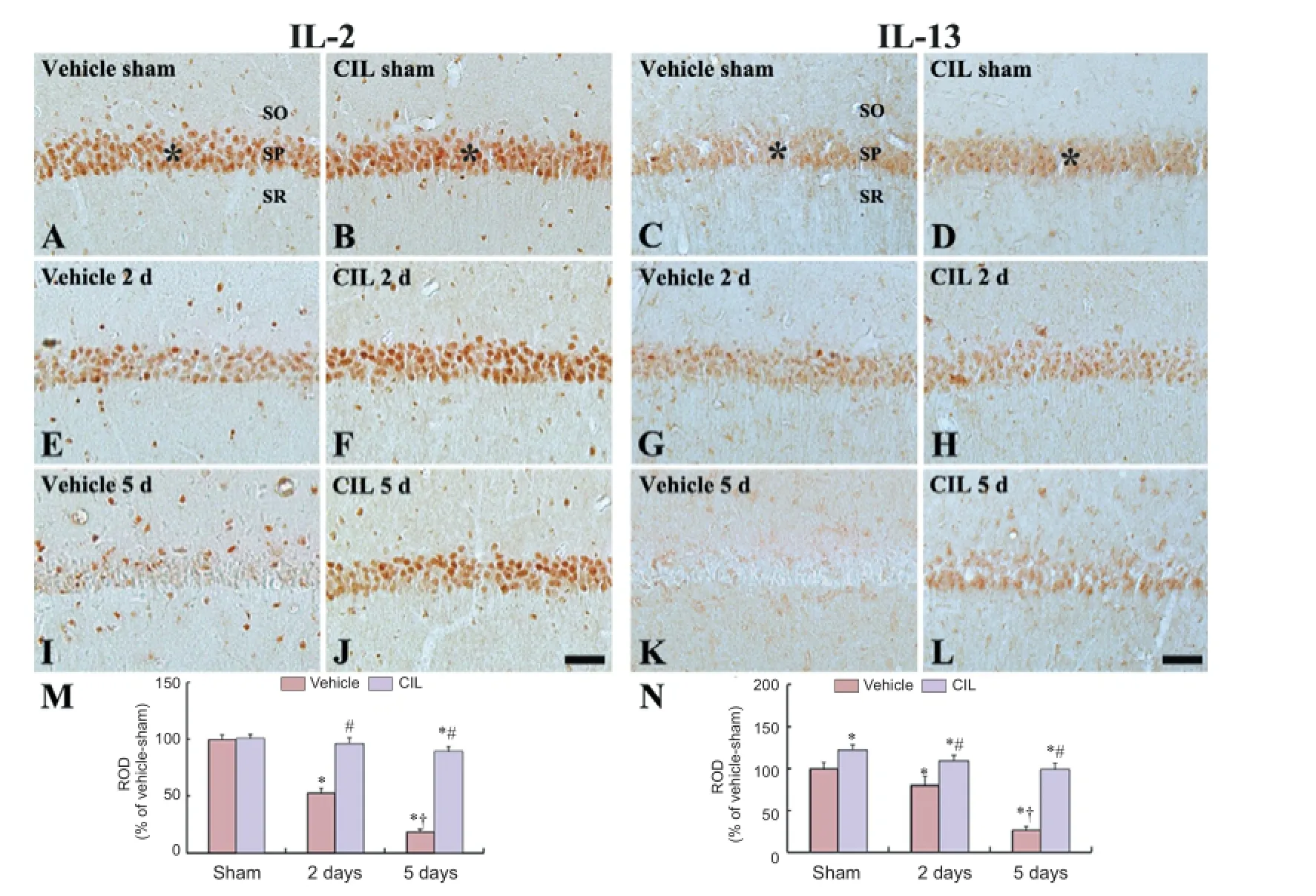
Figure 3 Interleukin (IL)-2 and IL-13 immunoreactivities in the CA1 region of the vehicle-sham (A and C), CIL-sham (B and D), vehicle-ischemia (E, I, G and K) and CIL-ischemia (F, J, H and L) groups.
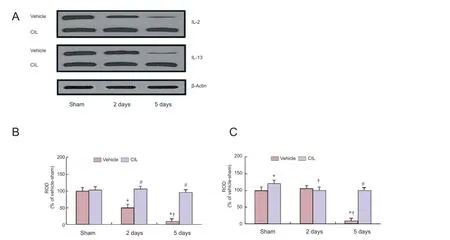
Figure 4 Western blot detection of interleukin (IL)-2 and IL-13 levels in the CA1 tissue derived from the vehicle-sham, CIL-sham, vehicleischemia and CIL-ischemia groups.
In the present study, IL-2 immunoreactive neurons werehighly expressed in the CA1 pyramidale of the vehicle-sham group. This finding is consistent with previous results that IL-2 is expressed in the hippocampus of the human, rat and gerbil (Araujo et al., 1989; Hwang et al., 2006). In addition, we found that IL-2 immunoreactivity in the CA1 pyramidal neurons was significantly decreased with time after ischemia in accordance with a previous report by Hwang et al. (2006); however, the immunoreactivity was maintained in the CIL-ischemia group after ischemic injury. Previous studies have reported that pro-inflammatory cytokines such as IL-2 are involved in the development of neuronal damage due to brain ischemia and that an abnormal expression of these cytokines raises a risk of neuronal damage induced by brain ischemia (Vila et al., 2000; Iadecola and Alexander, 2001; Yan et al., 2012). Therefore, our results indicate that the neuroprotective effects of CIL may be related to not increasing IL-2 immunoreactivity by cerebral ischemia.
IL-13 immunoreactivity, in the present study, was also significantly deduced in the CA1 pyramidal neurons of the vehicle-ischemia group (Yu et al., 2010; Yan et al., 2012); however, IL-13 immunoreactivity in the CIL-sham group was significantly higher than that in the vehicle-sham group, and the immunoreactivity was maintained in the CIL-ischemia group. IL-13 as an anti-inflammatory cytokine is related to a recovery from inflammation in the brain (Ledeboer et al., 2000; Pahan et al., 2000). Therefore, our present finings indicate that the increase and maintenance of IL-13 by the treatment of CIL may be associated with protective effect against ischemic injury.
Some recent studies have shown that CIL has anti-microbial, anti-oxidative and anti-inflammatory activities (Cheon et al., 2009; Pongjit et al., 2011). Especially, CIL can inhibit inflammatory mediators including nitric oxide, prostaglandin E2, IL-1β and tumor necrosis factor-α through suppressing mitogen-activated protein kinases and nuclear factor-κ B-dependent pathways (Yu et al., 1992; Cheng et al., 2005; Lee do et al., 2009). In addition, Cheon et al. (2009) demonsterated that CIL could inhibit the lipopolysaccharide-induced production of inflammatory cytokines via down-regulating nuclear factor-κB and mitogen-activated protein kinases in RAW264.7 macrophages, and Kim et al. (2012) reporetd that the inhibition of nuclear factor-κB was associated with suppressed activation of inhibitors of nuclear factor κB kinase α and β. Besides, CIL treatment decreased Bax expression and increased Bcl-2 expression dose-dependently (Kim et al., 2011). The overexpression of Bcl-2 is known to protect cells from the apoptosis mediated by reactive oxygen species; however, Bax, which is another member of Bcl-2 protein family, accelerates the rate of apoptosis (Hockenbery et al., 1993; Tsujimoto and Shimizu, 2002). On the basis of these papers, the protective effect of CIL is partly related to inhibition of apoptotic signaling pathways.
In conclusion, CIL treatment can protect CA1 pyramidal neurons from transient cerebral ischemia, which may be related to the increasing levels of anti-inflammatory cytokines.
Acknowledgments: We would like to thank Mr. Seung Uk Lee (Department of Physiology, College of Medicine, and Institute of Neurodegeneration and Neuroregeneration, Hallym University, Chuncheon, Republic of Korea) for his technical help in this study.
Author contributions: All authors were responsible for design, implementation and evaluation of the study and approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Araujo DM, Lapchak PA, Collier B, Quirion R (1989) Localization of interleukin-2 immunoreactivity and interleukin-2 receptors in the rat brain: interaction with the cholinergic system. Brain Res 498:257-266.
Benakis C, Garcia-Bonilla L, Iadecola C, Anrather J (2014) The role of microglia and myeloid immune cells in acute cerebral ischemia. Front Cell Neurosci 8:461.
Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M (2008) Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A 105:3581-3586.
Butler TL, Kassed CA, Sanberg PR, Willing AE, Pennypacker KR (2002) Neurodegeneration in the rat hippocampus and striatum after middle cerebral artery occlusion. Brain Res 929:252-260.
Ceulemans AG, Zgavc T, Kooijman R, Hachimi-Idrissi S, Sarre S, Michotte Y (2010) The dual role of the neuroinflammatory response after ischemic stroke: modulatory effects of hypothermia. J Neuroinflammation 7:74.
Chen L, Xiang Y, Kong L, Zhang X, Sun B, Wei X, Liu H (2013) Hydroxysafflor yellow A protects against cerebral ischemia-reperfusion injury by anti-apoptotic effect through PI3K/Akt/GSK3beta pathway in rat. Neurochem Res 38:2268-2275.
Chen Y, Wu X, Yu S, Fauzee NJ, Wu J, Li L, Zhao J, Zhao Y (2012) Neuroprotective capabilities of Tanshinone IIA against cerebral ischemia/ reperfusion injury via anti-apoptotic pathway in rats. Biol Pharm Bull 35:164-170.
Cheng W, Li J, You T, Hu C (2005) Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linne. J Ethnopharmacol 101:334-337.
Cheon MS, Yoon T, Lee do Y, Choi G, Moon BC, Lee AY, Choo BK, Kim HK (2009) Chrysanthemum indicum Linne extract inhibits the inflammatory response by suppressing NF-kappaB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol 122:473-477.
Cho W, Brenner M, Peters N, Messing A (2010) Drug screening to identify suppressors of GFAP expression. Hum Mol Genet 19:3169-3178.
Crain BJ, Westerkam WD, Harrison AH, Nadler JV (1988) Selective neuronal death after transient forebrain ischemia in the Mongolian gerbil: a silver impregnation study. Neuroscience 27:387-402.
Fitch MT, Silver J (2008) CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol 209:294-301.
Ghosh N, Ghosh R, Bhat ZA, Mandal V, Bachar SC, Nima ND, Sunday OO, Mandal SC (2014) Advances in herbal medicine for treatment of ischemic brain injury. Nat Prod Commun 9:1045-1055.
Giulian D (1993) Reactive glia as rivals in regulating neuronal survival. Glia 7:102-110.
Giulian D, Vaca K (1993) Inflammatory glia mediate delayed neuronal damage after ischemia in the central nervous system. Stroke 24:I84-90.
Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ (1993) Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75:241-251.
Huang J, Upadhyay UM, Tamargo RJ (2006) Inflammation in stroke and focal cerebral ischemia. Surg Neurol 66:232-245.
Hwang IK, Yoo KY, Kim DW, Lee HJ, Kang HY, Lee HY, Kang TC, Choi SY, Kim YS, Won MH (2006) Transient ischemia-induced changes of interleukin-2 and its receptor beta immunoreactivity and levels in the gerbil hippocampal CA1 region. Brain Res 1106:197-204.
Iadecola C, Alexander M (2001) Cerebral ischemia and inflammation. Curr Opin Neurol 14:89-94.
Iadecola C, Anrather J (2012) The immunology of stroke: from mechanisms to translation. Nat Med 17:796-808.
Kim IS, Ko HM, Koppula S, Kim BW, Choi DK (2011) Protective effect of Chrysanthemum indicum Linne against 1-methyl-4-phenylpridinium ion and lipopolysaccharide-induced cytotoxicity in cellular model of Parkinson’s disease. Food Chem Toxicol 49:963-973.
Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239:57-69.
Kirino T (2000) Delayed neuronal death. Neuropathology 20 Suppl:S95-97.
Kirino T, Tamura A, Sano K (1984) Delayed neuronal death in the rat hippocampus following transient forebrain ischemia. Acta Neuropathol 64:139-147.
Kolenda-Roberts HM, Harris N, Singletary E, Hardisty JF (2013) Immunohistochemical characterization of spontaneous and acrylonitrile-induced brain tumors in the rat. Toxicol Pathol 41:98-108.
Ledeboer A, Breve JJ, Poole S, Tilders FJ, Van Dam AM (2000) Interleukin-10, interleukin-4, and transforming growth factor-beta differentially regulate lipopolysaccharide-induced production of pro-inflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia 30:134-142.
Lee do Y, Choi G, Yoon T, Cheon MS, Choo BK, Kim HK (2009) Anti-inflammatory activity of Chrysanthemum indicum extract in acute and chronic cutaneous inflammation. J Ethnopharmacol 123:149-154.
Liu YR, Li PW, Suo JJ, Sun Y, Zhang BA, Lu H, Zhu HC, Zhang GB (2014) Catalpol provides protective effects against cerebral ischaemia/reperfusion injury in gerbils. J Pharm Pharmacol 66:1265-1270.
Matsuda H, Morikawa T, Toguchida I, Harima S, Yoshikawa M (2002) Medicinal flowers. VI. Absolute stereostructures of two new flavanone glycosides and a phenylbutanoid glycoside from the flowers of Chrysanthemum indicum L.: their inhibitory activities for rat lens aldose reductase. Chem Pharm Bull (Tokyo) 50:972-975.
Min D, Mao X, Wu K, Cao Y, Guo F, Zhu S, Xie N, Wang L, Chen T, Shaw C, Cai J (2012) Donepezil attenuates hippocampal neuronal damage and cognitive deficits after global cerebral ischemia in gerbils. Neurosci Lett 510:29-33.
Nowicka D, Rogozinska K, Aleksy M, Witte OW, Skangiel-Kramska J (2008) Spatiotemporal dynamics of astroglial and microglial responses after photothrombotic stroke in the rat brain. Acta Neurobiol Exp (Wars) 68:155-168.
Pahan K, Khan M, Singh I (2000) Interleukin-10 and interleukin-13 inhibit proinflammatory cytokine-induced ceramide production through the activation of phosphatidylinositol 3-kinase. J Neurochem 75:576-582.
Park JH, Park OK, Yan B, Ahn JH, Kim IH, Lee JC, Kwon SH, Yoo KY, Lee CH, Hwang IK, Choi JH, Won MH, Kim JD (2014a) Neuroprotection via maintenance or increase of antioxidants and neurotrophic factors in ischemic gerbil hippocampus treated with tanshinone I. Chin Med J (Engl) 127:3396-3405.
Park JH, Park O, Cho JH, Chen BH, Kim IH, Ahn JH, Lee JC, Yan BC, Yoo KY, Lee CH, Hwang IK, Kwon SH, Lee YL, Won MH, Choi JH (2014b) Anti-inflammatory effect of tanshinone I in neuroprotection against cerebral ischemia-reperfusion injury in the gerbil hippocampus. Neurochem Res 39:1300-1312.
Perini F, Morra M, Alecci M, Galloni E, Marchi M, Toso V (2001) Temporal profile of serum anti-inflammatory and pro-inflammatory interleukins in acute ischemic stroke patients. Neurol Sci 22:289-296.
Pongjit K, Ninsontia C, Chaotham C, Chanvorachote P (2011) Protective effect of Glycine max and Chrysanthemum indicum extracts against cisplatin-induced renal epithelial cell death. Hum Exp Toxicol 30:1931-1944.
Pulsinelli WA, Brierley JB, Plum F (1982) Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol 11:491-498.
Schmued LC, Hopkins KJ (2000) Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res 874:123-130.
Schubert P, Morino T, Miyazaki H, Ogata T, Nakamura Y, Marchini C, Ferroni S (2000) Cascading glia reactions: a common pathomechanism and its differentiated control by cyclic nucleotide signaling. Ann N Y Acad Sci 903:24-33.
Shcherbak NS, Galagudza MM, Ovchinnikov DA, Kuzmenkov AN, Yukina GY, Barantsevich ER, Tomson VV, Shlyakhto EV (2013) Activity of succinate dehydrogenase in the neocortex and hippocampus of Mongolian gerbils with ischemic and reperfusion brain injury. Bull Exp Biol Med 155:14-17.
Shunying Z, Yang Y, Huaidong Y, Yue Y, Guolin Z (2005) Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J Ethnopharmacol 96:151-158.
Sofroniew MV (2005) Reactive astrocytes in neural repair and protection. Neuroscientist 11:400-407.
Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, Kleinschnitz C (2012) Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke 7:407-418.
Tang C, Xue H, Bai C, Fu R, Wu A (2010) The effects of Tanshinone IIA on blood-brain barrier and brain edema after transient middle cerebral artery occlusion in rats. Phytomedicine 17:1145-1149.
Tsujimoto Y, Shimizu S (2002) The voltage-dependent anion channel: an essential player in apoptosis. Biochimie 84:187-193.
Vila N, Castillo J, Davalos A, Chamorro A (2000) Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 31:2325-2329.
Wang Q, Tang XN, Yenari MA (2007) The inflammatory response in stroke. J Neuroimmunol 184:53-68.
Wang Y, Yang XW (2006) GC-MS analysis of essential oil of the flower of the Chrysanthemum morifolium by the different processing methods. Zhongguo Zhong Yao Za Zhi 31:456-459.
Wang ZD, Huang C, Li ZF, Yang J, Li BH, Liang RR, Dai ZJ, Liu ZW (2010) Chrysanthemum indicum ethanolic extract inhibits invasion of hepatocellular carcinoma via regulation of MMP/TIMP balance as therapeutic target. Oncol Rep 23:413-421.
Wong CH, Crack PJ (2008) Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem 15:1-14.
Wu PF, Zhang Z, Wang F, Chen JG (2010) Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacol Sin 31:1523-1531.
Yan BC, Kim SK, Park JH, Ahn JH, Lee CH, Yoo KY, Choi JH, Lee DS, Kim MJ, Kim YM, Won MH (2012) Comparison of inflammatory cytokines changes in the hippocampal CA1 region between the young and adult gerbil after transient cerebral ischemia. Brain Res 1461:64-75.
Yoo KY, Kwon SH, Lee CH, Yan B, Park JH, Ahn JH, Choi JH, Ohk TG, Cho JH, Won MH (2012) FoxO3a changes in pyramidal neurons and expresses in non-pyramidal neurons and astrocytes in the gerbil hippocampal CA1 region after transient cerebral ischemia. Neurochem Res 37:588-595.
Yu DK, Yoo KY, Shin BN, Kim IH, Park JH, Lee CH, Choi JH, Cho YJ, Kang IJ, Kim YM, Won MH (2012) Neuronal damage in hippocampal subregions induced by various durations of transient cerebral ischemia in gerbils using Fluoro-Jade B histofluorescence. Brain Res 1437:50-57. Yu DQ, Xie FZ, He WY, Liang XT (1992) Application of 2D NMR techniques in the structure determination of chrysanthetriol. Yao Xue Xue Bao 27:191-196.
Yu JT, Lee CH, Yoo KY, Choi JH, Li H, Park OK, Yan B, Hwang IK, Kwon YG, Kim YM, Won MH (2010) Maintenance of anti-inflammatory cytokines and reduction of glial activation in the ischemic hippocampal CA1 region preconditioned with lipopolysaccharide. J Neurol Sci 296:69-78.
Zhang C, Qin MJ, Shu P, Hong JL, Lu L, He DX (2010) Chemical variations of the essential oils in flower heads of Chrysanthemum indicum L. from China. Chem Biodivers 7:2951-2962.
Copyedited by Modi JP, Zhai L, Li CH, Wang L, Song LP, Zhao M
10.4103/1673-5374.177735 http://www.nrronline.org/
How to cite this article: Yoo KY, Kim IH, Cho JH, Ahn JH, Park JH, Lee JC, Tae HJ, Kim DW, Kim JD, Hong S, Won MH, Kang IJ (2016) Neuroprotection of Chrysanthemum indicum Linne against cerebral ischemia/reperfusion injury by anti-inflammatory effect in gerbils. Neural Regen Res 11(2):270-277.
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2013R1A2A2A01068190), and by Hallym University Specialization Fund (HRF-S-
*Correspondence to: Moo-Ho Won, DVM, Ph.D. or Il-Jun Kang, Ph.D., mhwon@kangwon.ac.k or ijkang@hallym.ac.kr.
杂志排行
中国神经再生研究(英文版)的其它文章
- Tissue-type plasminogen activator is a modulator of the synaptic vesicle cycle
- Impaired consciousness caused by injury of the lower ascending reticular activating system: evaluation by diffusion tensor tractography
- Considering calcium-binding proteins in invertebrates: multi-functional proteins that shape neuronal growth
- Cardiovascular dysfunction following spinal cord injury
- Practical application of the neuroregenerative properties of ketamine: real world treatment experience
- Exergames: neuroplastic hypothesis about cognitive improvement and biological effects on physical function of institutionalized older persons
