Polyethylene glycol-fusion retards Wallerian degeneration and rapidly restores behaviors lost after nerve severance
2016-12-02GeorgeD.Bittner,MichelleMikesh,CameronL.Ghergherehchi
PERSPECTIVE
Polyethylene glycol-fusion retards Wallerian degeneration and rapidly restores behaviors lost after nerve severance
Some biological uses of polyethylene glycol (PEG): The use of PEG as a membrane fusogen was first reported in 1976 with the creation of cell hybrids, formed by suspending two cell lines in a 50% w/w solution of PEG in water. More recently, direct application of PEG has been found to seal off small holes in axons after complete transections of cultured neurites in a procedure referred to as “PEG-sealing” (Spaeth et al., 2012). The term PEG-sealing is also used to describe intravenous injection of PEG or micelles/nano-particles associated with PEG (reviewed by Jin, 2014). The latter “PEG-sealing” in vivo procedures typically produce low concentrations of PEG in body fluids associated with modest increases in behavioral recovery many weeks post injury, presumably by neuroprotective effects that rescue damaged neurons from cell death, rather than by preventing or retarding Wallerian degeneration (Jin, 2014).
What is PEG-fusion technology? In contrast, “PEG-fusion”is a nerve repair technology typically consisting of micro-sutures and a well-specified sequence of solutions, one of which contains a high concentration of PEG in small volumes briefly applied directly to a lesion site (Lore et al., 1999; Bittner et al., 2012, 2015a,b; Ghergherehchi et al., 2015; Riley et al., 2015). PEG-fusion has recently been reported to retard Wallerian degeneration and rapidly restore behaviors lost after completely transecting or removing segments of rat sciatic nerves (Bittner et al., 2012, 2015b; Ghergherehchi et al., 2015).
A complete transection of a rat sciatic nerves causes the severed axonal ends to separate by 1-3 mm; an ablation of the entire sciatic nerve typically removes a 5-10 mm segment from a host animal followed by insertion of a slightly longer allograft from a donor animal. For successful PEG-fusion, axons and their sheaths are carefully trimmed so that cut ends of host and/or donor nerve axons form smooth flat planes that can be closely apposed with minimal gaps or axonal protrusions. Severed nerves with carefully-trimmed ends are closely re-apposed with micro-sutures. A Ca2+-free hypotonic saline to open cut axonal ends and expel vesicles by increased osmotic pressure and an anti-oxidant (e.g., methylene blue: MB) to inhibit formation of new vesicles is directly applied to the injury site for 1-2 minutes. PEG (2-5 kDa) in 50% w/w sterile, Ca2+-free, double distilled water (ddH2O) is then directly applied to the lesion site for 1-2 minutes to non-specifically join (connect, fuse) closely apposed membranes. Finally, axons are bathed in isotonic Ca2+-containing physiological saline to initiate sealing of any remaining axolemmal disruptions by naturally occurring, Ca2+-induced formation and accumulation of vesicles that plug axolemmal holes (Spaeth et al., 2012). For a detailed schematic of procedural steps in the PEG-fusion technology, see Figure 4 of Bittner et al. (2015b). Results of successful PEG-fusion: Within minutes post-operatively, successful PEG-fusion restores gross morphological/ anatomical continuity across cut-severed or ablation-allograft lesion site(s) of sciatic nerves in the upper thigh, as measured by conduction of action potentials or intracellular diffusion of dye across sites of PEG-fusion (Lore et al., 1999; Bittner et al., 2012; Ghergherehchi et al., 2015; Riley et al., 2015). At 6 post-operative weeks as illustrated in Figure 1, many successfully PEG-fused axons proximal (Figure 1B and D) and distal (Figure 1G and H) to a successful PEG-fusion site are morphologically similar to intact axons (Figure 1A). That is, PEG-fused axons do not undergo Wallerian degeneration even after 6 weeks post-severance because distal axonal segments are rapidly (but nonspecifically) connected to intact proximal axonal segments that survive. Data at 6 post-operative weeks show significantly greater survival in distal stumps of motor, sensory and total axons in allograft segments (Bittner et al., 2015b; Riley et al., 2015b) and in distal segments following simple cuts (Bittner et al., 2015b; Ghergherehchi et al., 2015). At 6 post-operative weeks, successfully PEG-fused allograft distal segments have axonal diameters and g-ratios (axonal diameter/ myelin + axon diameter) similar to non-severed axons (Figure 1H); singly cut PEG-fused axons have similar diameters, but higher g-ratios (less myelin: Figure 1G; Ghergherehchi et al., 2015). Soleus muscles distal to single-cut severance sites at 6 post-operative weeks have endplates of normal gross morphology (Ghergherehchi et al., 2015). These data are consistent with the hypothesis that many axons in PEG-fused nerves do not undergo Wallerian degeneration and remain connected to a nerve cell body. Morphological assessments of PEG-fused sciatic autograft axons at 3 post-operative days show similar retardation of Wallerian degeneration (Sexton et al., 2012). Survival of successfully PEG-fused axons is associated with (and almost-certainly responsible for) return within 1-4 weeks of many lost behavioral functions (Figure 2, curves 2-3) that are consistently recorded from rats with intact or sham-operated sciatic nerves (Figure 2, curve 1). This behavioral recovery is maintained for 6-12 postoperative weeks (Bittner et al., 2015b; Ghergherehchi et al., 2015; Riley et al., 2015) - or even 16 post-operative weeks (Bittner et al., 2015b). Similar morphological results are correlated with significant behavioral recovery at 3 post-operative days (Sexton et al., 2012).
In contrast, rapid Wallerian degeneration (Figure 1F and I) is always observed in animals with negative control nerves that receive micro-sutures and all PEG-fusion solutions except PEG. Proximal segments of such nerves (Figure 1C) appear similar to intact nerves (Figure 1A) or proximal segments of successfully PEG-fused nerves (Figure 1B and D). Similar results are observed at 3 post-operative days (Sexton et al., 2012). At 6 weeks, sciatic nerves with no PEG-fusion (or unsuccessful PEG-fusion) have small-diameter, relatively poorly myelinated axons; soleus muscles distal to severance sites are atrophied and endplates are rarely seen. These and other data are consistent with a hypothesis that these nerves have newly regenerating axons that often do not successfully re-innervate denervated synaptic targets. Wallerian degeneration in the absence of successful PEG-fusion is consistently associated with no or minimal recovery of lost behavioral functions within6-12 post-operative weeks (Figure 2, curves 4-6) (Bittner et al., 2015b; Ghergherehchi et al., 2015; Riley et al.,2015) -or even 16 post-operative weeks (Bittner et al., 2015b). Similar results showing little behavioral recovery are reported for animals with cut-severed sciatic nerves that receive all PEG-fusion procedures but are not micro-sutured. That is, cut axonal ends that are not closely apposed before adding PEG do not PEG-fuse, but rather the membrane leaflets at the ends of a cut axonal end collapse and “PEG-seal” making PEG-fusion almost impossible (Lore et al., 1999; Bittner et al., 2015b).

Figure 1 Typical morphology of plastic embedded, osmiumferrocyanide stained cross sections of sciatic axons in sciatic nerves that are (A) intact (unoperated), or lesioned 6 weeks previously (B-I).
Variables affecting successful PEG-fusion as assessed behaviorally: PEG-fusion success depends in part on the following variables and their interactions: (1) crush- vs cut-severance vs ablation lesions that are made in (2) Ca2+-containing vs Ca2+-free salines in which (3) cut ends are trimmed vs not trimmed and (4) more vs less stretch/tension is used to closely appose cut ends that is required for successful PEG-fusion (Ghergherehchi et al., 2015). These variables are manifested in the following ways.
PEG-fusion of 1-2 mm long crushes or cut-severances with axonal ends closely apposed by micro-sutures made in hypotonic Ca2+-free salines are typically successful (Figure 2, curve 3) because the hypotonic Ca2+-free salines cause crush or cut-severed nerve ends to swell open, increase axonal length, expel existing vesicles at cut ends, and reduce vesicle formation (Lore et al., 1999; Bittner et al, 2012, 2015b).
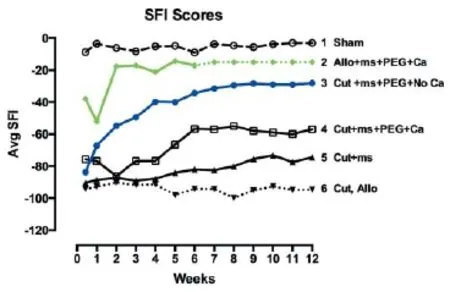
Figure 2 Schematic representation of behavioral recovery as measured by the Sciatic Function Index (SFI) for 12 weeks postoperatively after various surgical procedures to rat sciatic nerves as abbreviated in key.
Crushes greater than 3 mm in length made in isotonic Ca2+-containing saline (extracellular fluids) are not well-repaired because Ca2+influx into crush-severed ends induces vesicles to seal off the severed ends and thereby reduce PEG-fusion success (Spaeth et al., 2012). In such cases, the application of PEG to vesicle-filled ends that are not in close apposition causes the ends to seal off (“PEG-seal”) rather than rejoin to become a continuous axon (“PEG-fuse”) (Spaeth et al., 2012).
Trimming cut axonal ends increases PEG-fusion success by creating better axonal apposition for PEG-fusion, but also decreases PEG-fusion success because additional tension (stretch) must be applied during micro-suturing to bring the cut ends in tight apposition required for PEG-fusion (Ghergherehchi et al., 2015). These results are expected because clinical studies report that the stretch-tension required to micro-suture cut ends for gaps > 3 mm causes many axons to be irreversibly damaged and subsequent behavioral recovery is very poor or non-existent (Green and Wolfe, 2011).
Ablation lesions made in Ca2+-containing saline can be repaired by autografts (Sexton et al., 2012) or by donor allografts that are successfully PEG-fused at their proximal interface with severed proximal host axonal ends and their distal interface with severed distal host axonal ends (Riley et al., 2015). Successfully PEG-fused donor allografts are associated with the greatest retardation of Wallerian degeneration (Figure 1E and H) and best restoration of lost behaviors observed to date (Figure 2, curve 2), with some animals recovering to sham or un-operated levels within 3-14 days. If successfully PEG-fused, donor allografts are not rejected by 6 post-operative weeks. Allografts can be carefully sized so that the proximal and distal diameters of the sciatic donor nerve match the diameter of the host sciatic nerve and the length of the careful-ly-trimmed donor nerve slightly exceed the length of the ablated segment to avoid stretching the host or donor nerves when micro-suturing proximal and distal cut ends.
Unanswered questions on PEG-fusion: While we know some of the cellular/molecular mechanisms by which PEG-fusion restores axonal continuity to open, vesicle free, axonal ends apposed by micro-sutures, we recognize that these fusion events cannot selectively reconnect distal and proximal portions of severed motor or sensory axons, much less individually identifiable axons. Hence, we do not yet know the cellular/molecular mechanisms that prevent such “hybrid sensory-motor axons” from undergoing Wallerian degeneration. These mechanisms are surely identifiable by using immunological stains selective for motor or sensory axons distal and proximal to sites of PEG-fusion or examining axonal transport of tracer substances in motor or sensory axons at various post-operative times (Bittner et al, 2015b).
We continue to investigate the mechanisms by which successful PEG-fusion greatly enhances behavioral recovery at the systems level. For donor allografts, there are no axons with appropriate connections to PEG-fuse. Possible mechanisms include the respecification of spinal connections made by the proximal side in response to changes in retrograde signals, or segmental reassignment of allograft axons by the proximal and distal inputs. Alternatively, collateral outgrowths from surviving distal and motor axons could quickly remake new peripheral connections or synapses. Central pattern generators in spinal cords or higher CNS brain centers could be very plastic and quickly alter sensory and motor outputs. These are also unanswered questions when considering natural regeneration by outgrowth from severed proximal ends. These mechanisms should be identifiable by various procedures such as examining changes in dendritic arborization of PEG-fused motor axons, collateral sprouting of motor and sensory axons, and sprouting of motor axons within distal muscles. We are also investigating why successfully PEG-fused allografts are not quickly rejected in contrast to negative control or unsuccessfully PEG-fused allografts that are often rejected within a few days by assessing acute immune response in the spleen, lymphocyte and macrophage infiltration of the surgical wound site, and cytokine production in plasma.
Prospectus: While the specific mechanisms responsible for retarding Wallerian degeneration and rapidly restoring behaviors lost after complete axonal severance of peripheral nerves are not yet known, PEG-fusion may translate well to clinical procedures since all drugs are benign, readily available, and are already FDA-approved for human use. Successful allograft repair suggests that tissue banks might consider the inclusion of donor nerve tissue alongside donor corneas, livers, hearts, etc. This PEG-fusion technology developed to restore many behaviors lost after peripheral nerve severance or ablation has also recently been reported to restore many behaviors lost after spinal contusion injuries (Bittner et al., 2015a).
This study was supported by grants from the Lone Star Paralysis Foundation and an NIH grant R01 NS081063 to GDB.
George D. Bittner*, Michelle Mikesh, Cameron L. Ghergherehchi Department of Neuroscience, University of Texas at Austin, Austin, TX, USA
*Correspondence to: George D. Bittner, Ph.D., bittner@austin.utexas.edu.
Accepted: 2016-02-15
Bittner GD, Rokkappanavar KK, Peduzzi JD (2015a) Application and implications of PEG-fusion as a novel technology to repair injured spinal cords. Neural Regener Res. In Press.
Bittner GD, Sengelaub DR, Trevino RC, Peduzzi JD, Mikesh M, Ghergherehchi CL, Schallert T, Thayer WP (2015b) The curious ability of PEG-fusion technologies to restore lost behaviors after nerve severance. J Neurosci Res doi: 10.1002/jnr.23685.
Bittner GD, Keating CP, Kane JR, Britt JM, Spaeth CS, Fan JD, Zuzek A, Wilcott RW, Thayer WP, Winograd JM, Gonzalez-Lima F, Schallert T (2012) Rapid, effective, and long-lasting behavioral recovery produced by microsutures, methylene blue, and polyethylene glycol after completely cutting rat sciatic nerves. J Neurosci Res 90:9679-9680.
Ghergherehchi CL, Bittner GD, Hastings RL, Mikesh M, Riley DC, Trevino RC, Schallert T, Thayer WP, Sunkesula SR, Ha TA, Munoz N, Pyarali M, Bansal A, Poon AD, Mazal AT, Smith TA, Wong NS, Dunne PJ (2015) Effects of extracellular calcium and surgical techniques on restoration of axonal continuity by PEG-fusion following complete cut- or crush-severance of rat sciatic nerves. J Neurosci Res doi: 10.1002/jnr.23704.
Green DP, Wolfe SW (2011) Green’s Operative Hand Surgery. 6th ed Philadelphia: Elsevier/ Churchill Livingstone.
Jin X (2014) Membrane resealing as a promising strategy for early treatment of neurotrauma. Neural Regen Res 9:1876-1877.
Lore AB, Hubbell JA, Bobb DSJ, Ballinger ML, Loftin KL, Smith JW (1999) Rapid induction of functional and morphological continuity between severed ends of mammalian or earthworm myelinated axons. J Neurosci 19:2442-2454.
Riley DC, Bittner GD, Mikesh M, Cardwell NL, Pollins AC, Ghergherehchi CL, Bhupanapadu Sunkesula SR, Ha TN, Hall BT, Poon AD, Pyarali M, Boyer RB, Mazal AT, Munoz N, Trevino RC, Schallert T, Thayer WP (2015) Polyethylene glycol-fused allografts produce rapid behavioral recovery after ablation of sciatic nerve segments. J Neurosci Res 93:572-583.
Sexton KW, Pollins AC, Cardwell NL, Del Corral GA, Bittner GD, Shack RB, Nanney LB, Thayer WP (2012) Hydrophilic polymers enhance early functional outcomes after nerve autografting. J Surg Res 177:392-400.
Spaeth CS, Robison T, Fan JD, Bittner GD (2012) Cellular mechanisms of plamalemmal sealing and axonal repair by polyethylene glycol and methylene blue. J Neursosci Res 90:955-966.
10.4103/1673-5374.177716 http://www.nrronline.org/
How to cite this article: Bittner GD, Mikesh M, Ghergherehchi CL (2016) Polyethylene glycol-fusion retards Wallerian degeneration and rapidly restores behaviors lost after nerve severance. Neural Regen Res 11(2):217-219.
杂志排行
中国神经再生研究(英文版)的其它文章
- Tissue-type plasminogen activator is a modulator of the synaptic vesicle cycle
- Impaired consciousness caused by injury of the lower ascending reticular activating system: evaluation by diffusion tensor tractography
- Considering calcium-binding proteins in invertebrates: multi-functional proteins that shape neuronal growth
- Cardiovascular dysfunction following spinal cord injury
- Practical application of the neuroregenerative properties of ketamine: real world treatment experience
- Exergames: neuroplastic hypothesis about cognitive improvement and biological effects on physical function of institutionalized older persons
