Low-power laser therapy for carpal tunnel syndrome: effective optical power
2016-12-02YanChenChengqiangZhaoGangYeCandongLiuWendongXu
Yan Chen , Cheng-qiang Zhao, Gang Ye, Can-dong Liu, Wen-dong Xu
1 Department of Neurology, Tongji Hospital, Tongji University School of Medicine, Shanghai, China
2 Shanghai Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, Shanghai, China
3 Department of Rehabilitation Medicine, Tongji Hospital, Tongji University School of Medicine, Shanghai, China
Low-power laser therapy for carpal tunnel syndrome: effective optical power
Yan Chen1,*, Cheng-qiang Zhao2, Gang Ye3, Can-dong Liu2, Wen-dong Xu2
1 Department of Neurology, Tongji Hospital, Tongji University School of Medicine, Shanghai, China
2 Shanghai Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, Shanghai, China
3 Department of Rehabilitation Medicine, Tongji Hospital, Tongji University School of Medicine, Shanghai, China
Graphical Abstract
*Correspondence to: Yan Chen, yanchenl@sina.com.
orcid: 0000-0002-0999-0819 (Yan Chen)
Low-power laser therapy has been used for the non-surgical treatment of mild to moderate carpal tunnel syndrome, although its efficacy has been a long-standing controversy. The laser parameters in low-power laser therapy are closely related to the laser effect on human tissue. To evaluate the efficacy of low-power laser therapy, laser parameters should be accurately measured and controlled, which has been ignored in previous clinical trials. Here, we report the measurement of the effective optical power of low-power laser therapy for carpal tunnel syndrome. By monitoring the backside reflection and scattering laser power from human skin at the wrist, the effective laser power can be inferred. Using clinical measurements from 30 cases, we found that the effective laser power differed significantly among cases, with the measured laser reflection coefficient ranging from 1.8% to 54%. The reflection coefficient for 36.7% of these 30 cases was in the range of 10—20%, but for 16.7% of cases, it was higher than 40%. Consequently, monitoring the effective optical power during laser irradiation is necessary for the laser therapy of carpal tunnel syndrome.
nerve regeneration; peripheral nerve regeneration; carpal tunnel syndrome; laser therapy; median nerve; diode laser; skin absorption; skin reflection; skin scattering; laser power measurement; laser therapy; neural regeneration
Introduction
Laser beams can be used to induce biological stimulation, promote cell regeneration, improve blood circulation, diminish inflammation, relieve pain, and regulate immune functions (Zhu, 2003; Hashmi et al., 2010). Short wavelength laser beams (e.g., 650 nm) can be used to induce pathological changes in superficial tissues, while infrared laser beams (e.g., 810 nm) have a better penetration into the skin, fat and muscle tissues as well as bone tissues, and therefore can be used for the treatment of deep tissue lesions. The overall effect of different laser wavelength combinations can be used to obtain therapeutic efficacy in tissues of different depths.
Low-power laser therapy has been widely used for the physical treatment of orthopedics, wound surgery and pain diseases such as osseous pain, muscle pain, soft tissue pain, neuralgia, and trauma pain. However, there is a long-standing dispute regarding the efficacy of low-power laser therapy for some diseases. For example, in the conservative treatment of mild to moderate carpal tunnel syndrome (CTS), a common entrapment neuropathy involving the median nerve at the wrist (Phalen, 1966; Chen, 2008), using low-power laser beams to irradiate the wrist of patients was reported to be a simple and non-invasive physical therapy (Weintraub, 1997; Padua et al., 1998; Naeser et al., 2002; Bakhtiary and Rashidy-Pour, 2004; Naesera, 2006; Evcik et al., 2007; Piazzini et al., 2007; Rochkind et al., 2007a; Chang et al., 2008; Dincer et al., 2009; Bales and Meals, 2010;Tascioglu et al., 2012; Fusakul et al., 2014). However, the effects of laser treatment reported by different studies were different and often contradictory (Enwemeka et al., 2004; Irvine et al., 2004; Naesera, 2006; Piazzini et al., 2007; Rochkind et al., 2007b; Shooshtari et al., 2008; Yagci, 2009; Wu and Yu, 2010; Casale et al., 2013). Piazzini et al. (2007) systematically reviewed all published results (based on MEDLINE database) regarding the laser therapy of CTS (confirmed by clinical symptoms and electrophysiological tests) between January 1985 and May 2006 and reported that the conclusions regarding the efficacy of laser therapy of CTS were highly inconsistent.
Laser parameters in low-power laser irradiation therapy, including the laser power onto the action point, irradiation area, time duration of laser action, time interval between each laser action, and especially the optical power and power density at therapeutic targets, are closely related to the laser effect on human tissues (van Breugel and Bar, 1992; Bjordal et al., 2003; Zhu, 2003). To evaluate the efficacy of low-power laser therapy, laser parameters should be accurately measured and controlled, which were ignored in most previous clinical trials (Bjordal et al., 2003).
Naesera (2006) reviewed seven CTS cases undergoing laser therapy and found that the total laser irradiation doses (9 J, 12—30 J, 32 J/cm2, and 225 J/cm2) in five cases where the positive effect of laser treatment was concluded were significantly higher than in those of two cases where a negative effect was concluded (1.8 J or 6 J/cm2). However, in the five cases showing a positive effect, there were large variations from case to case regarding the laser parameters. For example, the laser wavelength in these cases was single or a combination of 830-nm, 632.8-nm, 904-nm, and 670-nm laser beams. The laser energy density, time duration of each laser action, and the time interval between laser actions also differed for different cases.
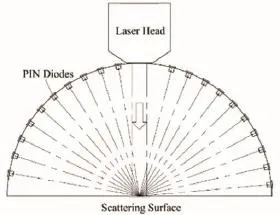
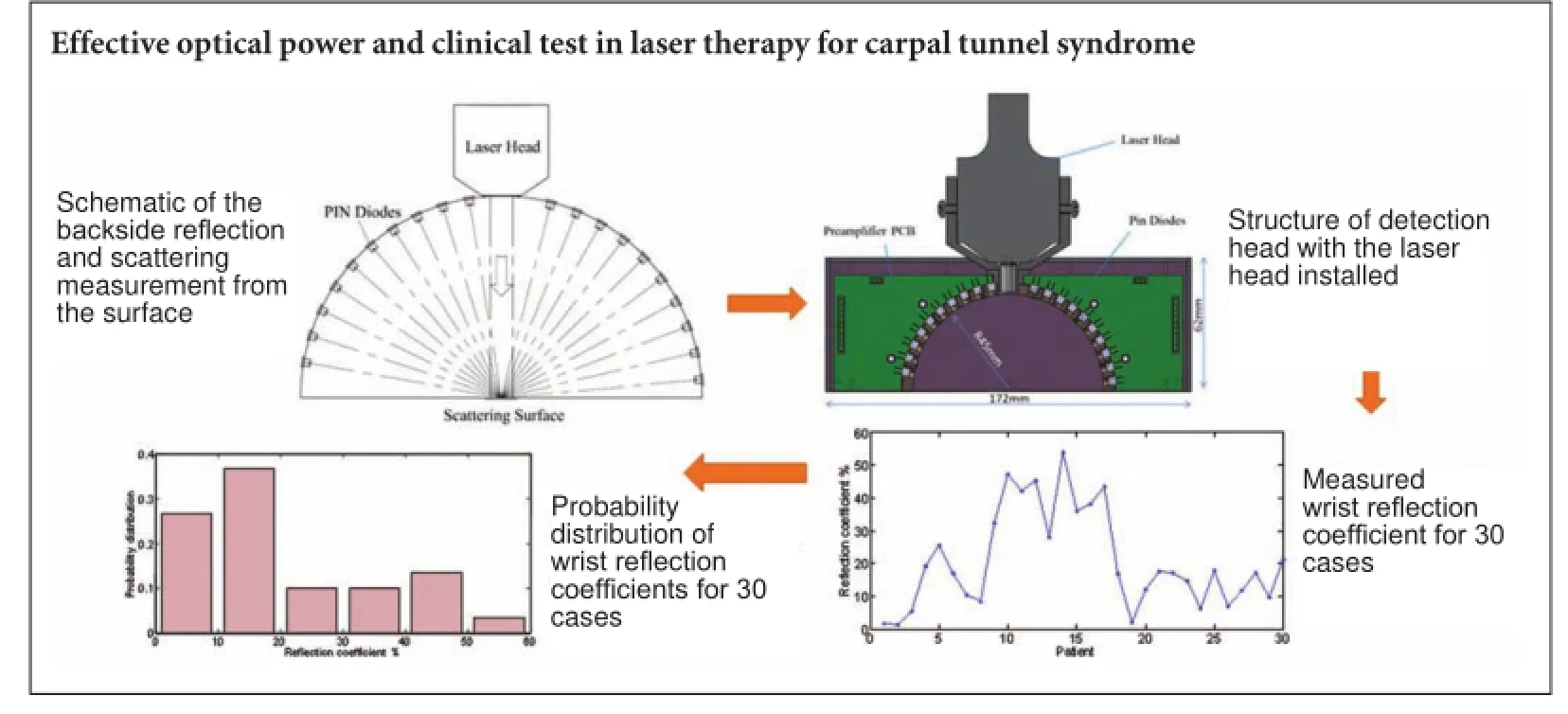
Figure 1 Schematic of the backside reflection and scattering measurement from the surface of wrist skin.
Does a higher laser power or a higher laser energy density always lead to a positive result of laser therapy for CTS? The answer is no. In 2007, Bjordal (2007) reported that high laser energy and energy density in laser therapy for CTS may lead to unfavorable factors.
In recent studies using a combination of 1,064 nm + 830-nm double wavelength laser beams (Casale et al., 2013), the authors claimed that an obvious curative effect was achieved because the long-wavelength laser beam penetrated deeper and scattered less. However, the laser power used for the treatment was as high as 25 W, which is two orders of magnitude higher than that of the hundreds of mW generally used for low-power laser therapy. Consequently, it is unclear whether the high-laser power or the long wavelength mediated the positive effect of the laser treatment.
What is the best laser parameter for CTS therapy? Are there thresholds for laser power, energy density, and total irradiation laser energy? In a clinical trial of lower-power laser therapy, usually only the output laser power from the laser head is recorded or adjusted. Based on standard formulas, some studies took into account the scattering loss inside human tissues before arriving at the action target; however, no individual difference was considered. To the best of our knowledge, previous clinical trials of CTS therapy never monitored the actual laser parameters at the skin of the wrist.
When a laser beam irradiates human skin, significant reflection and scattering occur (Xie et al., 2003; Michel et al., 2013), which leads to a lower effective laser power compared with that from the laser head. There were no measurements of the effective laser power at the wrist of patients in previous studies of laser therapy for CTS. In addition, clinical studies have shown that the laser reflection and scattering of human skin show significant individual differences (Xie et al., 2003). Therefore, it is necessary to measure the effective laser power for every patient receiving low-power laser therapy to compare and evaluate the efficacy of laser therapy.
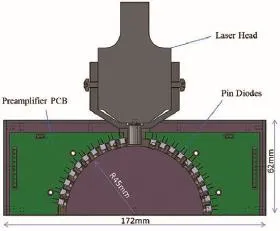
Figure 2 Structure of the detection head with the laser head installed.
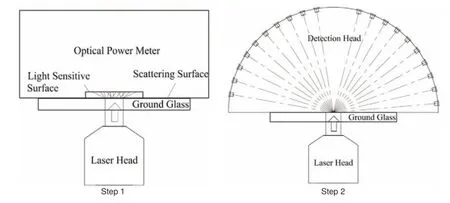
Figure 3 Relationship between the signal from the detection head and the absolute value of reflecting and scattering laser power.
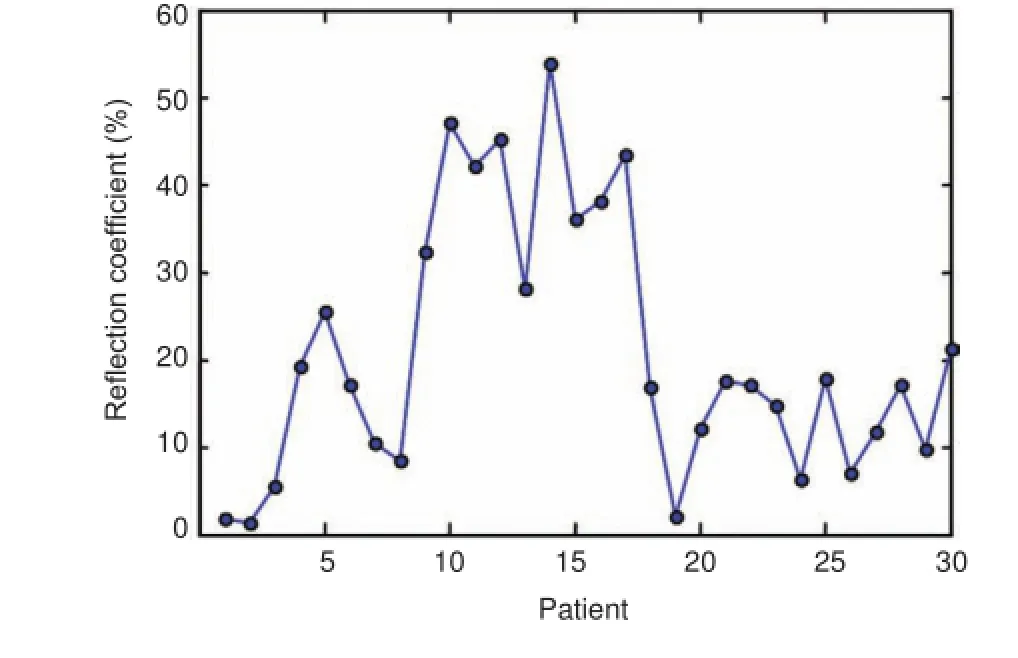
Figure 4 Measured wrist reflection coefficient for 30 carpal tunnel syndrome patients.
To solve these existing problems in the low-power laser therapy for CTS, we proposed and developed a device to monitor quantitatively the effective laser power into the wrist skin of CTS patients to control quantitatively the laser parameters in clinical laser therapy. By monitoring the laser backside reflection and scattering signal, we obtained the effective laser power at the skin surface of a patient's wrist. This device is expected to become useful auxiliary equipment for monitoring effective laser parameters in many clinical applications using low-power laser therapy.
Subjects and Methods
Subjects
By using the detection head coupled with a commercial laser therapeutic apparatus (Diode Laser System for Pain MDC-500-I, nominal output laser power: 0—500 mW, laser wavelength: 810 ± 20 nm; Shanghai Mandisen Optoelectronics Co., Ltd., Shanghai, China), we investigated the effective laser power for 30 CTS patients during laser therapy. The enrolled patients with mild to moderate CTS were treated in the Department of Neurology at Shanghai Tongji Hospital, Shanghai, China from January 2013 to May 2014. The 30 (26 females) enrolled patients had a mean age of 54.2 years (range 35—70 years).

Figure 5 Probability distribution of the measured wrist reflection coefficients for 30 carpal tunnel syndrome patients.
Inclusion criteria: (1) mild to moderate CTS diagnosed according to the literature (Gu, 2005, 2011); (2) no tumor or infectious diseases; (3) no photosensitivity reaction; (4) no serious heart, liver or kidney diseases; and (5) no other peripheral neuropathy.
Exclusion criteria: (1) acute wrist trauma and surgery; (2) treated with other methods; and (3) pregnancy.
This clinical research was approved by the Ethics Committee of Shanghai Tongji Hospital and all patients received laser therapy after providing informed consent.
Schematic of effective laser power measurement
The schematic for measuring the backside reflection and scattering of the laser beam in the full hemisphere is shown in Figure 1. The backside reflection and scattering from the surface (skin) were assumed to be axial symmetric; therefore, the measurement can be achieved in the plane of incidence, which is then integrated to obtain data for the full hemisphere. Sixteen photodiodes (S2386-18K Pin diode, Hamamatsu Co., Ltd., Hamamatsu, Japan) were uniformly arranged around a circle on the two sides of the laser head. Four more were used for calibration. In the calibration, filtering, fitting, and weighted operations were performed to ensure the measurement accurately accounted for the backside reflection and scattering in the full hemisphere.
Device for effective laser power measurement
We developed a device composed of three main parts: a detection head, a data collector, and a computer with control software. The structure of the detection head is shown in Figure 2. The detection head is directly fixed to the existing laser head of the commercial laser therapy apparatus and the Hamamatsu S2386-18k Pin diode is used as the detector. The signal current from the Pin diode is converted to a 0 to 10-V signal in the pre-amplifier circuit to facilitate subsequent data collection and analysis. The detection head size is 172 × 62 × 40 mm3, which fits well with the laser head of the commercial laser therapy apparatus (Diode Laser System for Pain MDC-500-I, Shanghai Mandisen Optoelectronics Co., Ltd.).
Data acquisition and procession
The data collector includes a USB data acquisition card (NI USB-6210) and its power supply. The USB acquisition card converts the analog signals collected by the 16 Pin Diodes into digital signals, and transmits data to the computer through the USB port, where data processing is performed.
Software interface
To enhance the practical experience and make operation easy during clinical applications, a good user interface is an indispensable part of the device. We developed the software for this device by adopting LabVIEW programming software, with the help of a graphical programming environment and intuitive icons and wires. The software provides functions including data collection, data processing, data storage, and reading modules. The data storage and reading modules help to catalog different kinds of information (including the reflection and scattering power at different angles in the incidence plane shown in Figure 1), which is convenient for the follow-up of patients and historical data processing.
Device calibration
First, the device should be fully calibrated to acquire the correct backside reflecting and scattering laser power values. All the Pin detectors should be calibrated to the same sensitivity and magnification factor. According to the existing conditions, we put all the detectors on the same status and exposed them to sunlight, which can be considered a uniform parallel light source. The voltage signals from all 16 Pin detectors were then adjusted to the same value to complete the first calibration step. Then, the final data value through integral processing represents the relative value of backside reflecting and scattering laser power. To obtain the absolute value, the next calibration process is divided into two steps, as shown in Figure 3. First, the laser head of the therapy apparatus is used to irradiate a piece of ground glass, and behind the ground glass, we placed an optical power meter to record the total power of the transmitted light. Second, the optical power meter is replaced by the detection head. Thus, the corresponding relationship between the signal from the detection head and the absolute value of the reflecting and scattering laser power was obtained.
Results
We observed a significant inter-individual difference between CTS patients. We defined the ratio between the measured reflection and scattering laser power in the backside hemisphere to the output power from the laser head as the reflection coefficient. The measured laser reflection coefficient from CTS patient wrists varied from 1.8% to 54%, as shown in Figure 4. We calculated the patient numbers in different ranges of the measured reflection coefficients (<10%, 10—20%, 20—30%, 30—40%, 40—50%, and 50—60%), and obtained the probability distribution of wrist reflection coefficients using the ratios between patient numbers in different ranges and the total patient number of 30. Figure 5 shows the probability distribution of wrist reflection coefficients for 30 cases. The reflection coefficient for 36.7% of the 30 cases was in the range of 10—20%, while that of 16.7% cases was higher than 40%.
Discussion
The clinical measurements obtained in this study clearly indicate the inter-individual differences between CTS patients, but also that the effective laser power at the wrist skin is significantly lower than at the laser head. Additional optical power loss during the propagation of laser beams from the skin to the median nerve at the wrist should be taken into account to determine the laser power at therapeutic targets. Analysis of the optical penetration depth and scattering in human tissues has been reported (Xie et al., 2003; Michel et al., 2013). Further measurement and modeling should be conducted to understand the individual differences found in this study.
This clinical trial shows that the proposed and developed device for monitoring the effective laser power for CTS therapy is necessary and feasible. Further investigation should be performed to determine the relationship between effective laser power and efficacy of laser therapy for CTS. Once the actual laser power at the wrist skin can be measured, it will be possible to determine the appropriate laser parameter range for individual CTS therapy and therefore to clarify the discrepancy in the literature.
In conclusion, we report the first measurement of effective optical power during laser therapy of CTS by monitoring the backside reflection and scattering laser power from the skin of human wrists. Using clinical measurements from 30 CTS cases, we found that the effective laser power differed significantly between patients, with a measured laser reflection coefficient ranging from 1.8% to 54%. Consequently, monitoring of the effective optical power during laser therapy will help clarify the dispute on the efficacy of laser therapy for CTS. Furthermore, this developed method and device might be useful for other lower-power laser therapies.
Author contributions: YC and WDX designed the study and wrote the paper. YC, CQZ, GY, CDL, and WDX conducted experiments. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Bakhtiary AH, Rashidy-Pour A (2004) Ultrasound and laser therapy in the treatment of carpal tunnel syndrome. Aust J Physiother 50:147-151.
Bales JG, Meals RA (2010) Low-level laser treatment. Evid Based Med 35:469-471.
Bjordal J (2007) Inadequate statistical analysis hides significant effect of low level laser therapy in carpel tunnel syndrome. Photomed Laser Surg 25:530-531.
Bjordal JM, Couppe C, Chow RT, Tuner J, Ljunggren EA (2003) A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust J Physiother 49:107-116.
Casale R, Damiani C, Maestri R, Wells CD (2013) Pain and electrophysiological parameters are improved by combined 830-1064 high-intensity LASER in symptomatic carpal tunnel syndrome versus transcutaneous electrical nerve stimulation. Eur J Phys Rehabil Med 49:205-211.
Chang WD, Wu JH, Jiang JA, Yeh CY, Tsai CT (2008) Carpal tunnel syndrome treated with a diode laser: a controlled treatment of the transverse carpal ligament. Photomed Laser Surg 26:551-557.
Chen Y, Chen H, Chen SR, Gu Q, Zhan Q, Hou HG (2008) The clinical feature and neuro-electrophysiological changes of carpal tunnel syndrome. J Clin Neurol 21:191.
DincerU, Cakar E, Kiralp MZ, Kilac H, Dursun H (2009) The effectiveness of conservative treatments of carpal tunnel syndrome: splingting, ultrasound and low level laser therapies. Photomed Laser Surg 27:119-125.
Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Woodruff LD (2004) The efficacy of low-power laser in tissue repair and pain control: a meta-analysis study. Photomed Laser Surg 22:323-329.
Evcik D, Kavuncu V, Cakir T, Subasi V, Yaman M (2007) Laser therapy in the treatment of carpal tunnel syndrome: a randomized controlled trial. Photomed Laser Surg 25:34-39.
Fusakul Y, Aranyavalai T, Saensri P, Thiengwittayaporn S (2014) Low-level laser therapy with a wrist splint to treat carpal tunnel syndrome: a double-blinded randomized controlled trial. Lasers Med Sci 29:1279-1287.
Gu YD (2005) Pay more attentions to the diagnosis and treatment of carpal tunnel syndrome. Orthop J Chin 13:325-326.
Gu YD (2011) Clinical classification for carpal tunnel syndrome and Cubital tunnel syndrome: status and suggestion. Chin J Orthop 31:818-819.
Hashmi JT, Huang YY, Osmani BZ (2010) Role of low-level laser therapy in neurorehabilitation. PM R 2:S292-305.
Irvine J, Chong SL, Amirjani N, Chan KM (2004) Double-blind randomized controlled trial of low-level laser therapy in carpal tunnel syndrome. Muscle Nerve 30:182-187.
Michel AP, Liakat S, Bors K, Gmachl CF (2013) In vivo measurement of mid-infrared light scattering from human skin. Biomed Opt Exp 4:520.
Naeser MA, Hahn KA, Lieberman BE, Branco KF (2002) Carpal tunnel syndrome pain treated with low-level laser and microamperes transcutaneous electric nerve stimulation: a controlled study. Arch Phys Med Rehabil 83:978-987.
Naesera M (2006) Photobiomodulation of pain in carpal tunnel syndrome: review of seven laser therapy studies. Photomed Laser Surg 24:101-110.
Padua L, Padua R, Aprile I, Tonali P (1998) Noninvasive laser neurolysis in carpal tunnel syndrome. Muscle Nerve 21:1232-1233
Phalen GS (1966) The carpal tunnel syndrome: seventeen years' experience in diagnosis and treatment of six hundred fifty-four hands. J Bone Joint Surg Am 48:211-228.
Piazzini D, Aprile I, Ferrara PE, Bertolini C, Tonali P, Maggi L, Rabini A, Piantelli S, Padua L (2007) A systematic review of conservative treatment of carpal tunnel syndrome. Clin Rehabil 21:299-314.
Rochkind S, Drory VM, Nissan M, Ouaknine G (2007a) Laser phototherapy (780 nm), a new modality in treatment of long-term incomplete peripheral nerve injury: a randomized double-blind placebo-controlled study. Photomed Laser Surg 25:436-442.
Rochkind S, Leider-Trejo LE, Nissan M, Shamir M, Kharenko O, Alon M (2007b) Efficacy of 780-nm laser phototherapy on peripheral nerve regeneration after neurotube reconstruction procedure (double-blind randomized study). Photomed Laser Surg 25:137-143.
Shooshtari SM, Badiee V, Taghizadeh SH, Nematollahi AH, Amanollahi AH, Grami MT (2008) The effects of low level laser in clinical outcome and neurophysiological results of carpal tunnel syndrome. Electromyogr Clin Neurophysiol 48:229-231.
Tascioglu F, Degirmenci NA, Ozkan S, Mehmetoglu O (2012) Low-level laser in the treatment of carpal tunnel syndrome: clinical, electrophysiological, and ultrasonographical evaluation. Rheumatol Int 32:409-415.
van Breugel HH, Bar PR (1992) Power density and exposure time of He-Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro. Lasers Surg Med 12:528-537.
Weintraub MI (1997) Noninvasive laser neurolysis in carpal tunnel syndrome. Muscle Nerve 20:1029-1031.
Wu P, Yu C (2010) Progress on the conservative treatments of mild to moderate carpal tunnel syndrome. Int J Orthop 31:26.
Xie S, Li H, Li B (2003) Measurement of optical penetration depth and refractive index of human tissue. Chin Opt Lett 1:44.
Yagci I, Elmas O, Akcan E, Ustun I, Gunduz OH, Guven Z (2009) Comparison of splinting and splinting plus low-power laser therapy in idiopathic carpal tunnel syndrome. Clin Rheumatol 28:1059-1065.
Zhu J (2003) Laser Medicine. Shanghai: Shanghai Science and Technology Press.
Copyedited by Croxford L, Norman C, Wang J, Qiu Y, Li CH, Song LP, Zhao M
How to cite this article: Chen Y, Zhao CQ, Ye G, Liu CD, Xu WD (2016) Low-power laser therapy for carpal tunnel syndrome∶ effective optical power. Neural Regen Res 11(7)∶1180-1184.
Funding: This study was supported in part by the National Natural Science Foundation of China, No. 61108077.
10.4103/1673-5374.187063
2016-05-22
RESEARCH ARTICLE
杂志排行
中国神经再生研究(英文版)的其它文章
- Recovery of an injured corticospinal tract by subcortical peri-lesional reorganization in a patient with intracerebral hemorrhage
- Dose response and time course of manganeseenhanced magnetic resonance imaging for visual pathway tracing in vivo
- Extracellular matrix from human umbilical cordderived mesenchymal stem cells as a scaffold for peripheral nerve regeneration
- Differential temporal expression of matrix metalloproteinases following sciatic nerve crush
- Protective effects of ginsenoside Rg1 against hydrogen peroxide-induced injury in human neuroblastoma cells
- Genistein suppresses the mitochondrial apoptotic pathway in hippocampal neurons in rats with Alzheimer’s disease