Protective effects of ginsenoside Rg1 against hydrogen peroxide-induced injury in human neuroblastoma cells
2016-12-02ZhigaoSunLipingChenFaweiWangChengyongXuMiaoGeng
Zhi-gao Sun, Li-ping Chen , Fa-wei Wang Cheng-yong Xu, Miao Geng
1 Department of Traditional Chinese Medicine, Hainan Branch Hospital of Chinese PLA General Hospital, Sanya, Hainan Province, China
2 Department of Traditional Chinese Medicine, Chinese PLA General Hospital, Beijing, China
3 Institute of Gerontology, Chinese PLA General Hospital, Beijing, China
Protective effects of ginsenoside Rg1 against hydrogen peroxide-induced injury in human neuroblastoma cells
Zhi-gao Sun1, Li-ping Chen2,*, Fa-wei Wang2, Cheng-yong Xu1, Miao Geng3
1 Department of Traditional Chinese Medicine, Hainan Branch Hospital of Chinese PLA General Hospital, Sanya, Hainan Province, China
2 Department of Traditional Chinese Medicine, Chinese PLA General Hospital, Beijing, China
3 Institute of Gerontology, Chinese PLA General Hospital, Beijing, China
Graphical Abstract

*Correspondence to: Li-ping Chen, M.D., lipingschen@aliyun.com.
orcid: 0000-0002-5568-9293 (Li-ping Chen)
The active ingredient of ginseng, ginsenosides Rg1, has been shown to scavenge free radicals and improve antioxidant capacity. This study hypothesized that ginsenosides Rg1 has a protective role in human neuroblastoma cells injured by H2O2. Ginsenosides Rg1 at different concentrations (50 and 100 μM) was used to treat H2O2(150 μM)-injured SH-SY5Y cells. Results demonstrated that ginsenoside Rg1 elevated the survival rate of SH-SY5Y cells injured by H2O2, diminished the amount of leaked lactate dehydrogenase, and increased superoxide dismutase activity. Ginsenoside Rg1 effectively suppressed caspase-3 immunoreactivity, and contributed to heat shock protein 70 gene expression, in a dose-dependent manner. These results indicate that ginsenoside Rg1 has protective effects on SH-SY5Y cells injured by H2O2and that its mechanism of action is associated with anti-oxidation and the inhibition of apoptosis.
nerve regeneration; traditional Chinese medicine monomer; ginsenoside Rg1; SH-SY5Y cells; H2O2; cerebral ischemia; cell apoptosis; lactate dehydrogenase; superoxide dismutase; caspase-3; heat shock protein 70; dose-effect relationship; neural regeneration
Introduction
The effects of ischemic cerebrovascular disease on learning and memory and the neurobiological mechanisms involved are a hot topic in neuroscience (Dong et al., 2013). Ischemic cerebrovascular disease refers to a decrease in chronic blood flow caused by various factors, which promotes pathological and biochemical alterations, disorders energy metabolism (Liang et al., 2012; Chen et al., 2014; Ji et al., 2014), and causes oxygen free radical injury (Fraser et al., 2011), changes in neurotransmitters (Shen et al., 2011), cholinergic receptor deletion (Tracey et al., 2007), white matter damage and neuronal deletion (Xiong et al., 2012). These changes form the pathophysiological basis of chronic cerebral ischemia-induced dysfunction (Inoue et al., 2012). Jian et al. (2013) suggested that free radical injury was a key factor in the injury to ischemic neurons. Ischemia and hypoxia in brain tissues induce a large amount of oxygen free radicals. Free radicals with a strong oxidative capacity attack vascular endothelial cells, destroy lipid membranes and cross-link membrane proteins to phospholipids, resulting in lipid peroxidation, increased permeability of the cell membrane to Ca2+, destruction of the blood-brain barrier, and irreversible protein deactivation (Lu et al., 2012). Free radicals also increase proapoptotic gene caspase-3 expression and suppress anti-apoptotic gene heat shock protein 70 (HSP70) activity, causing cell membrane destruction, neuronal injury, and apoptosis (Tirapelli et al., 2012).
Recently, increasing numbers of studies have focused on the effects of traditional Chinese medicine to counter oxidative stress (Wang et al., 2013). Panax ginseng C.A.Meyer is a traditional Chinese herb that has been reported to regulate immunity, promote excitability, resist oxidation and fatigue, improve brain function, and contribute to the recovery of learning and memory functions (Zheng et al., 2011). Ginsenoside Rg1, a major component of Panax ginseng C.A.Meyer, has been shown to enhance superoxide dismutase (SOD) activity, inhibit the production of malondialdehyde, scavenge accumulating free radicals, and elevate antioxidative effects (Kim et al., 2009). Another study confirmed that a Shenlong decoction containing ginsenoside Rg1 reduced nitric oxide and inducible nitric oxide synthase contents, elevated the ability of learning and memory in rats with cerebral ischemia, and strengthened vascular endothelial growth factor expression in the rat hippocampus after cerebral ischemia (Zhang et al., 2011). Studies addressing the antioxidative mechanism of ginsenoside Rg1 for treatment of ischemic brain damage have mainly focused on the inhibitory effects of ginsenoside against neuronal apoptosis and its protective effects on neuronal cells (Li et al., 2015), but have seldom focused on the antioxidative mechanism of cells in vitro (Huang et al., 2016).
SH-SY5Y cells generated from human neuroblastoma have a low level of differentiation and are pyramidal with the presence of apparent axons (Lee et al., 2010). Some physiological functions of SH-SY5Y cells are similar to those of normal neurons (Waly et al., 2016). SH-SY5Y cells are commonly used in studies of the onset of nervous system disease and the mechanisms involved in the action of drugs (Ccy et al., 2014).
The current study investigated the regulatory effects of ginsenoside Rg1 on the survival rate, amount of leaked lactate dehydrogenase (LDH), SOD activity, caspase-3 expression, and HSP70 gene activity in SH-SY5Y cells injured by H2O2to determine its protective effects and the mechanisms involved in its antioxidative and antiapoptotic effects.
Materials and Methods
Cells
Human dopaminergic neuroblastoma cell strain (SH-SY5Y) was a gift from the Sixth Institute of Academy of Military Medical Sciences, China.
Drugs
Ginsenoside Rg1 powder was purchased from Nanjing Zelang Medical Technology Co., Ltd., (Nanjing, Jiangsu Province, China, batch No. ZL201003; purity > 95%).
The experiments were approved by the Animal Ethics Committee, Chinese PLA General Hospital, China.
SH-SY5Y cell culture
SH-SY5Y cells were thawed and, digested with 0.25% trypsin and 0.02% ethylenediamine tetraacetic acid for 3 minutes, incubated with Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (Hyclone, Logan, UT, USA), 100 U/mL penicillin and 100 U/mL streptomycin in a 37°C 5% CO2incubator. The medium was replaced every 3 days. When cells reached 90% confluence, they were digested with 0.25% trypsin (Gibco, Carlsbad, CA, USA) for passage. Cells in the logarithmic phase were collected for further experiments.
Establishment of a cell model of H2O2-induced injury
SH-SY5Y cell concentrations in each group were adjusted to 1 × 106/mL. After removal of primary medium, cells in each well were incubated in complete medium containing 50, 100, 150, or 200 μM H2O2in a 5% CO2, 37°C incubator (Thermo, American) for 12 hours. The experimental cells were allocated to control, model (H2O2150 μM), 50 μM ginsenoside Rg1 (H2O2150 μM + ginsenoside Rg1 50 μM) and 100 μM ginsenoside Rg1 (H2O2150 μM + ginsenoside Rg1 100 μM) groups.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay to measure rate of cell survival
Cells from each group were adjusted to 1 × 106/mL. MTT (5 g/L; Sigma, St. Louis, MO, USA) 20 μL was added to each well of a tissue culture plate in a 5% CO2incubator at 37°C (Shellab, Cornelius, NC, USA) for 4 hours. When blue-purple crystals formed, the supernatant was removed. The samples were incubated with 150 μL dimethyl sulfoxide (Sigma) in each well, and shaken in a shaking bed for 10 minutes to dissolve the blue-purple crystals in cells completely. Optical density values were measured at 570 nm with a microplate reader (Polar star Galaxy; BMG, Offenburg, Germany). The average optical density value of cells from six wells was calculated by the following formula: survival rate = optical densityexperimentalgroup/optical densitycontrolgroup× 100%. The experiment was performed in triplicate. Cell viability was determined by MTT assay to identify the optimal H2O2concentration (150 μM in this study). Different doses of ginsenosides Rg1 (10, 50, and 100 μM) combined with 150 μM H2O2were used for 12 hours to observe the protective effects of different concentrations of cells.
Measurement of LDH leakage and SOD activity in cells The cells (method described above) were treated with 150 μM H2O2. SH-SYSY cells were additionally treated with 10, 50, or 100 μM ginsenoside Rg1 in a 5% CO2incubator at 37°C for 24 hours following H2O2(150 μM) treatment. The amount of leaked LDH and SOD activity in supernatants were examined using an LDH assay kit and SOD activity assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China).
Immunofluorescence histochemistry for caspase-3 expression in cells
The experimental procedure followed the instructions of the caspase-3 fluorescence detection kit (Beijing Boaosen Bioengineering Institute, Beijing, China). SH-SY5Y cells (1 × 106/mL) were washed three times with PBS, fixed with 4% paraformaldehyde for 20 minutes, washed three times with PBS, blocked with normal goat serum at 37°C for 20 minutes, incubated with primary antibody (rabbit anti-caspase-3 polyclonal antibody; Bioss, Woburn, MA, USA) at 4°C overnight, rewarmed for 10 minutes, and washed three times with PBS (each for 5 minutes). Subsequently, the samples were incubated with secondary antibody (goat anti-rabbit IgG, 1:20—1:100) at 37°C for 90 minutes, washed three times with PBS (each for 5 minutes), mounted with glycerol buffer, and then observed under a fluorescence (fluorescein isothiocyanate, Cy3 labeled) microscope (BX-60; Olympus, Tokyo, Japan). The Image-Pro Plus 5.1 image analytical system (Media Cybernetics, Seattle, WA, USA) was used to measure the number of caspase-3-positive cells and the fluorescence intensity.
Reverse transcription-polymerase chain reaction (RT-PCR) to measure HSP70 mRNA expression
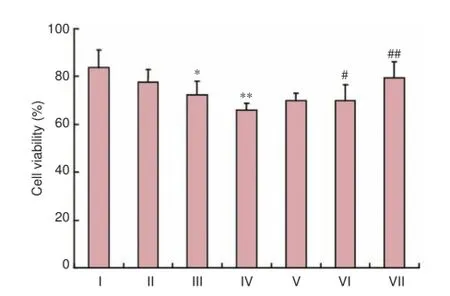
Figure 1 Effect of ginsenoside Rg1 on the viability of H2O2-treated SH-SY5Y cells.
Cells were adjusted to 1 × 106/mL, and total RNA was extracted (Liu et al., 2006). An ultraviolet spectrophotometer was utilized to measure nucleic acid concentrations. Total RNA (0.5 μg) was treated with DNaseI (EN0521, Fermentas, Canada), and reverse transcribed (K1622, Fermentas) into cDNA. cDNA (1 μL) was mixed with 8.2 μL ddH2O. HSP70 and β-actin were amplified on a quantitative PCR device. Primers were prepared as previously described (Liu et al., 2006).
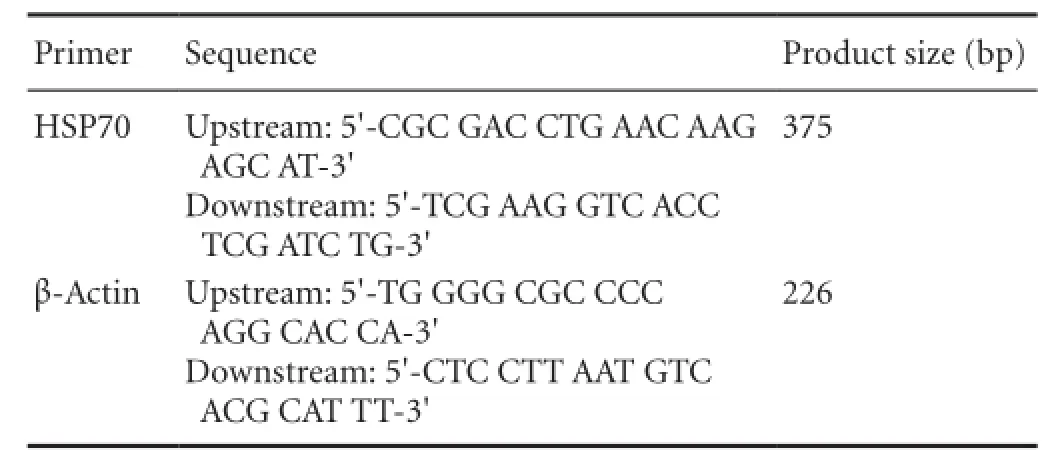
Primer sequences used in this study:
Amplification conditions were as follows: predenaturation at 94°C for 5 minutes, denaturation at 94°C for 30 seconds, annealing at 57°C for 45 seconds, extension at 72°C for 20 seconds, for 40 cycles, followed by 72°C for 10 minutes. PCR products were electrophoresed on a 2% agarose gel, and photographed using a gel imaging system (Media Cybernetics). Results were expressed as the relative optical density value (HSP70/β-actin).
Statistical analysis
Measurement data, expressed as the mean ± SD, were analyzed with SPSS 13.5 software (SPSS, Chicago, IL, USA).

Figure 2 Effect of ginsenoside Rg1 on caspase-3 expression in SH-SY5Y cells injured by H2O2 (red immunofluorescence staining, inverted fluorescence microscope, magnification × 200).

Table 1 Effect of ginsenoside Rg1 on the release of LDH and SOD activity in SH-SY5Y cells injured by H2O2
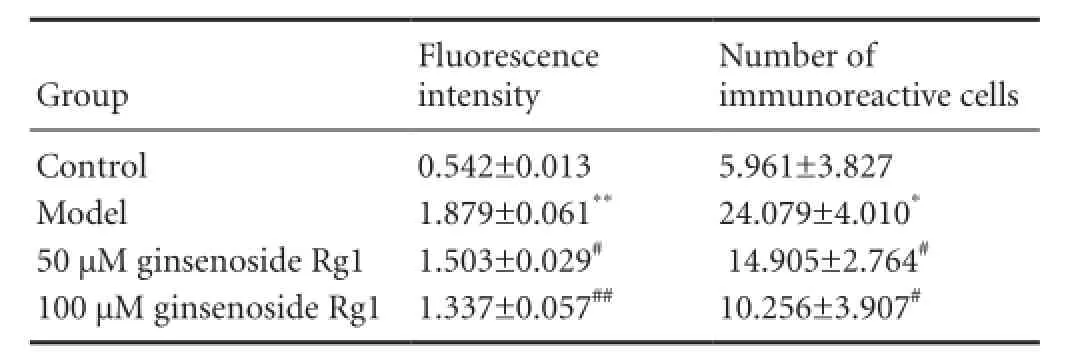
Table 2 Effects of ginsenoside Rg1 on caspase-3 immunoreactivity in H2O2-treated SH-SY5Y cells
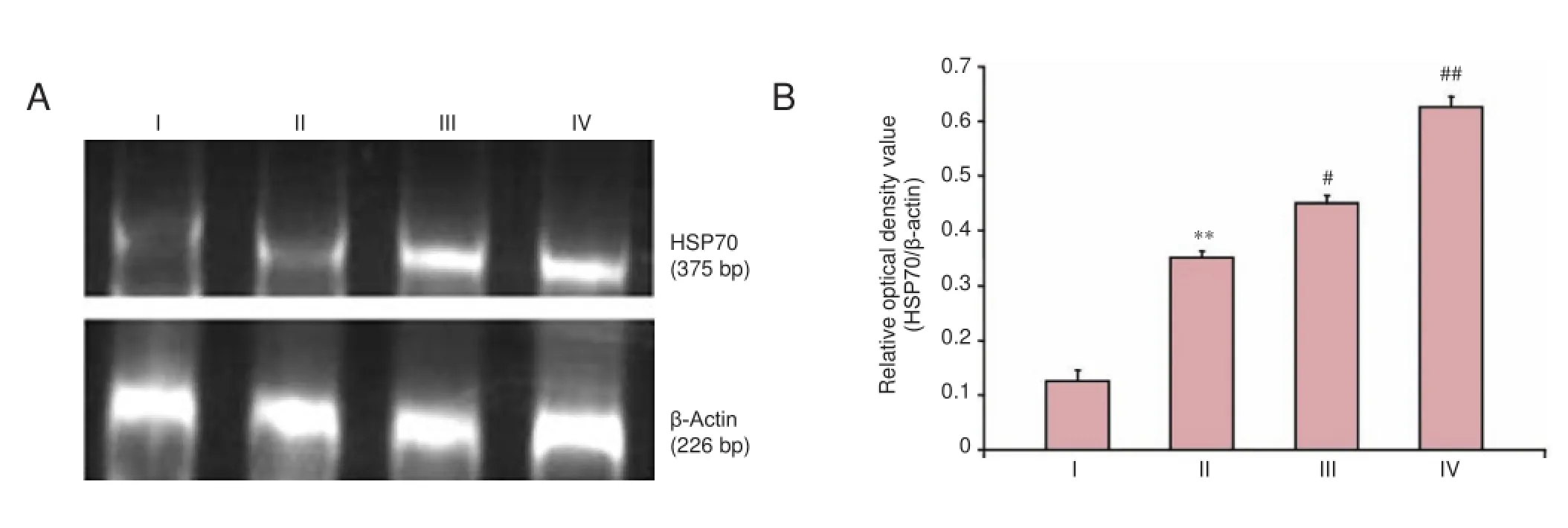
Figure 3 Effects of ginsenoside Rg1 on HSP70 mRNA expression in H2O2-treated SH-SY5Y cells.
One-way analysis of variance and post hoc least significant difference test were used at α = 0.05.
Results
Effects of ginsenoside Rg1 on the survival rate of H2O2-treated SH-SY5Y cells
As shown in Figure 1, the survival rate of SH-SY5Y cells was gradually decreased with an increasing concentration of H2O2. When SH-SY5Y cells were treated with 150 μM H2O2for 12 hours, the optical density of cells was significantly decreases compared with the control group (P < 0.01). This indicated that the ability of cells to reduce MTT decreased, and the cell survival rate was diminished. Therefore, 150 μM H2O2was used in all the following experiments. After 12 hours of H2O2treatment, 10 μM ginsenoside Rg1 had no significant effects on the cell survival rate compared with the model group (P > 0.05). However, 50 and 100 μM ginsenoside Rg1 had significant protective effects on SH-SY5Y cells injured by 150 μM H2O2compared with the model group (P< 0.05, P < 0.01, respectively). Thus, 50 and 100 μM ginsenoside Rg1 were utilized in the following experiments.
Effects of ginsenoside Rg1 on the amount of leaked LDH from H2O2-treated SH-SY5Y cells
LDH leakage was significantly greater in SH-SY5Y cells injured by 150 μM H2O2(model group) compared with the control group (P < 0.01). In addition, 50 and 100 μM ginsenoside Rg1 effectively significantly inhibited the LDH leakage in SH-SY5Y cells injured by H2O2compared with the model group (P < 0.05, P < 0.01, respectively). The inhibitory effect was significantly enhanced with an increased dose of ginsenoside Rg1. Significant differences in LDH leakage were observed between the 50 and 100 μM ginsenoside Rg1 groups (P < 0.05; Table 1).
Effects of ginsenoside Rg1 on SOD activity in SH-SY5Y cells injured by H2O2
SOD activity was significantly lower in SH-SY5Y cells injured by H2O2(model group) compared with the control group (P < 0.01). SOD activity was significantly higher in the 50 and 100 μM ginsenoside Rg1 groups in a dose-dependent manner compared with the model group (P < 0.05, P< 0.01, respectively). Nevertheless, no significant differences in SOD activity were detectable between the 50 and 100 μM ginsenoside Rg1 groups (P > 0.05; Table 1).
Effects of ginsenoside Rg1 on caspase-3 expression in SH-SY5Y cells injured by H2O2
As shown in Figure 2 and Table 2, immunofluorescence staining revealed that caspase-3 expression was significantly higher in SH-SY5Y cells injured by H2O2(model group) than in hippocampal neurons of the control group (P < 0.05, P < 0.01, respectively). In addition, 50 and especially 100 μM ginsenoside Rg1, significantly diminished the caspase-3 expression in injured cells (P < 0.05, P < 0.01, respectively).
Effects of ginsenoside Rg1 on HSP70 mRNA expression in SH-SY5Y cells injured by H2O2
Clear bands of different brightness at 377 bp RT-PCR indicated the presence of HSP70 mRNA expression in cells from each group. Compared with the control group, HSP70 was obviously activated in cells injured by H2O2for 12 hours. Furthermore, 50 and 100 μM ginsenoside Rg1 enhanced HSP70 expression in the injured cells. The optical density ratio of HSP70 to β-actin was considered a measurable indicator of the expression of HSP70 mRNA. The ratios in the control, model, 50, and 100 μM ginsenoside Rg1 groups were 0.630, 0.351, 0.457, and 0.630, respectively. Significant differences in the above ratios were detectable between the 50 and 100 μM ginsenoside Rg1 groups and the model group (P <0.05, P < 0.01, respectively; Figure 3).
Discussion
The pathogenesis of neurons injured by cerebral ischemia is complicated, and is associated with oxygen free radical injury, inflammatory factor damage, excitatory amino acid injury, and intracellular Ca2+overload (Nakase et al., 2008; Sierra et al., 2011). Of these, oxidative stress-induced oxygen free radical injury has become the focus of most attention (Allen et al., 2009). Brain tissues contain abundant unsaturated fatty acids and are therefore more susceptible to damage by free radicals (Kim et al., 2008). A recent study confirmed that oxygen free radical injury to ischemic neurons was correlated with caspase-3 and HSP70 expression in the brain (Ueda et al., 2002). When brain tissues experienced oxidative stress, such as during ischemia or hypoxia, caspase-3, a key executor of neuronal apoptosis, i.e., apoptotic effector molecule (Awasthi et al., 2013), becomes activated. Caspase-3 destroys collagen, intervenes in mRNA splicing, blocks DNA replication and repair, and induces cell apoptosis (Broughton et al., 2009). Simultaneously, caspase-3 activation was reported to exhaust intracellular nicotinamide adenine dinucleotide/adenosine triphosphate, resulting in cell loss (Jie et al., 2011). HSP70 is expressed at low levels in normal cells, but this expression is increased during stress (Dong et al., 2012). Under ischemic/hypoxic conditions, intracellular nucleoproteins are denatured, heat shock factors bind to heat shock elements, and many molecules of HSP70 are synthesized (Franklin et al., 2005). HSP70 inhibits caspase-3 activation, cleaves caspase cascade reactions, and prevents cell apoptosis. HSP70 also prevents protein aggregation or incorrect folding under stress, maintains protein homeostasis, and prevents degeneration-induced disorders of DNA (Wang et al., 2012).
LDH leakage reflects the degree of cell membrane injury (Ya et al., 2013). Increased LDH concentrations in the extracellular fluid are a marker for irreversible damage or cell necrosis (Noh et al., 2011). SOD is a scavenger enzyme of superoxide free radical anions in vivo (Park et al., 2011). During oxidative stress, increased SOD consumption led to a reduction in SOD activity (Cui et al., 2013). Another study showed that ginsenoside Rg1 elevated SOD production and scavenged oxygen free radicals (Li et al., 2010). In this study, when SH-SY5Y cells were treated with 150 μM H2O2for 12 hours, the amount of LDH leaked was markedly increased, but the SOD concentration was decreased. Ginsenoside Rg1 increased the survival rate of H2O2-injured SH-SY5Y cells, diminished the amount of leaked LDH and increased SOD activity. These results indicated that ginsenoside Rg1 strongly inhibited oxidative stress injury. Furthermore, ginsenoside Rg1 reduced caspase-3 immunoreactivity, promoted HSP70 gene expression, and reduced oxygen free radical injury in SH-SY5Y cells injured by H2O2.
Ginsenoside Rg1 had a dose-dependent mechanism involved in improving cell apoptosis because the protective effect of ginsenoside Rg1 increased with an increasing dose of ginsenoside Rg1.
In conclusion, cerebral ischemia-induced nerve cell apoptosis is a key neuropathological process. After cerebral ischemia, multiple factors and mechanisms interact, participate in the occurrence and development of nerve cell apoptosis, finally resulting in apoptosis. Ginsenoside Rg1 resists oxidative stress and free radical injury, increases the survival rate of damaged cells, reduces the amount of leaked LDH and caspase-3 activation, increases SOD activity and HSP70 expression, and finally suppresses cell apoptosis, in a dose-dependent manner.
Author contributions: LPC conceived and designed this study, and obtained funding. ZGS wrote the paper and provided data. FWW, CYX, and MG participated in the study concept and design, data analysis and paper writing. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Allen CL, Bayraktutan U (2009) Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke 4:461-470.
Awasthi YC, Sharma R, Cheng JZ, Yang Y, Sharma A, Singhal SS, Awasthi S (2003) Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Mol Aspects Med 24:219-230.
Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40:331-339.
Chen WQ, Sun YY, Liu KY, Sun XJ (2014) Autophagy: a double-edged sword for neuronal survival after cerebral ischemia. Neural Regen Res 9:1210-1216.
Dong B, Cai M, Fang Z, Wei H, Zhu F, Li G, Dong H, Xiong L (2013) Hemopexin induces neuroprotection in the rat subjected to focal cerebral ischemia. BMC Neuroscience 14:58.
Franklin TB, Krueger-Naug AM, Clarke DB, Arrigo AP, Currie RW (2005) The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system. Int J Hyperthermia 21:379-392.
Fraser PA (2011) The role of free radical generation in increasing cerebrovascular permeability. Free Radic Biol Med 51:967-977.
Huang L, Liu LF, Liu J, Dou L, Wang GY, Liu XQ, Yuan QL (2016) Ginsenoside Rg1 protects against neurodegeneration by inducing neurite outgrowth in cultured hippocampal neurons. Neural Regen Res 11:319-325.
Inoue M, Kojima Y, Mima T, Sawamoto N, Matsuhashi M, Fumuro T, Kinboshi M, Koganemaru S, Kanda M, Shibasaki H (2012) Pathophysiology of unilateral asterixis due to thalamic lesion. Clin Neurophysiol 123:1858-1864.
Ji XY, Zhang LN, Liu R, Liu YZ, Song JF, Dong H, Jia YF, Zhou ZG (2014) Potential targets for protecting against hippocampal cell apoptosis after transient cerebral ischemia-reperfusion injury in aged rats. Neural Regen Res 9:1122-1128.
Jie L, Yuqin C, Yao D, Shuai H, Yuanyuan W (2011) Occlusion of middle cerebral artery induces apoptosis of cerebellar cortex neural cells via caspase-3 in rats. Turk Neurosurg 21:567-574.
Kim JS, Yun I, Choi YB, Lee KS (2008) Ramipril protects from free radical induced white matter damage in chronic hypoperfusion in the rat. J Neurosci 15:174-178.
Kim YO, Kim HJ, Kim GS, Park HG, Lim SJ, Seong NS, Ham YW, Lee SD, Jang KH, Jung KH, Chung JH, Kang SA (2009) Panax ginseng protects against global ischemia injury in rat hippocampus. J Medicinal Food 12:71-76.
Lee DH, Lee YJ, Kwon KH (2010) Neuroprotective effects of astaxanthin in oxygen-glucose deprivation in SH-SY5Y cells and global cerebral ischemia in rat. J Clin Biochem Nutr 47:121-129.
Li M, Zhao J, Hu Y, Lu H, Guo J (2010) Oxygen free radicals regulate energy metabolism via AMPK pathway following cerebral ischemia. Neurol Res 32:779-784.
Li X, Zhang X, Yuan HF (2010) Expermiental research on effect of gensenoside Rg1 on expressions of P-Tau and caspase-3 in brain slices from AD model rats. Zhongguo Zhongyao Zazhi 35:369-372.
Li YB, Wang Y, Tang JP, Chen D, Wang SL (2015) Neuroprotective effects of ginsenoside Rg1-induced neural stem cell transplantation on hypoxic-ischemic encephalopathy. Neural Regen Res 2015;10:753-759.
Liang J, Yao J, Wang G, Wang Y, Wang B, Ge P (2012) Ischemic postconditioning protects neuronal death caused by cerebral ischemia and reperfusion via attenuating protein aggregation. Int J Med Sci 9:923-932.
Liu P, Wang JY, Li Q, Xu FY, Wang ZY, Xu HY, Liu ZP, Zhang XM (2006) Effect of baicalin on HSP70 expression of hippocampal neurons in focal brain ischemia-reperfusion injury rats. Yao Xue Xue Bao 41:619-284.
Lu ZQ, Deng YJ, Lu JX (2012) Effect of aloe polysaccharide on caspase-3 expression following cerebral ischemia and reperfusion injury in rats. Mol Med Rep 6:371-374.
Nakase T, Yamazaki T, Ogura N (2008) The impact of inflammation on the pathogenesis and prognosis of ischemic strokev. J Neurol Sci 271:104-109.
Noh SJ, Lee SH, Shin KY (2011) SP-8203 reduces oxidative stress via SOD activity and behavioral deficit in cerebral ischemia. Pharmacol Biochem Behav 98:150-154.
Park HJ, Shim HS, Kim KS, Shim I (2011) The protective effect of black ginseng against transient focal ischemia-induced neuronal damage in rats. Korean J Physiol Pharmacol 15:333-338.
Sierra C, Coca A, Schiffrin EL (2011) Vascular mechanisms in the pathogenesis of stroke. Curr Hypertens Rep 13:200-207.
Tirapelli DP, Carlotti Jr CG, Leite JP, Tirapelli LF, Colli BO (2010) Expression of HSP70 in cerebral ischemia and neuroprotetive action of hypothermia and ketoprofen. Arq Neuropsiquiatr 68:592-596.
Tracey KJ (2007) Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117:289-296.
Ueda S, Masutani H, Nakamura H (2002) Redox control of cell death. Antioxid Redox Signal 4:405-414.
Waly M, Power-Charnitsky VA, Hodgson N, Sharma A, Audhya T, Zhang Y, Deth R (2016) Alternatively spliced methionine synthase in sh-sy5y neuroblastoma cells: cobalamin and gsh dependence and inhibitory effects of neurotoxic metals and thimerosal. Oxid Med Cell Longev 2016:6143753.
Wang K, Deng G, Chen G, Liu M, Yi Y, Yang T, McMillan DR, Xiao X (2012) Heat shock protein 70 inhibits hydrogen peroxide-induced nucleolar fragmentation via suppressing cleavage and down-regulation of nucleolin. Cell Stress Chaperones 17:121-130.
Wang QY, Liu F, Wu FJ, Li JL (2013) Effects of ginsenoside Rg1 on the expressions of p-eRK1/2 and p-JNK in local cerebral ischemia/reperfusion injury rats. Zhongguo Zhong Xi Yi Jie He Za Zhi 33:229-234.
Wong CC, Meaburn EL, Ronald A, Price TS, Jeffries AR, Schalkwyk LC, Plomin R, Mill J (2014) Methylomic analysis of monozygotic twins discordant for autism spectrum disorder and related behavioural traits. Mol Psychiatry 19:495-503.
Xiong ZG, Xu TL (2012) The role of ASICS in cerebral ischemia. Wiley Interdiscip Rev Membr Transp Signal 1:655-662.
Yao J, Shen XN, Shen H, Wu M (2011) Effects of theanine on cerebral ischemia-reperfusion injury in rats. Zhonghua Yu Fang Yi Xue Za Zhi 40:684-687.
Yu CC, Wang JK (2013) Neuroprotective effect of penehyclidine hydrochloride on focal cerebral ischemiareperfusion injury. Neural Regen Res 8:622-632.
Zhang D, Fu M, Song C, Wang C, Lin X, Liu Y (2012) Expressions of apoptosis-related proteins in rats with focal cerebral ischemia after Angong Niuhuang sticker point application. Neural Regen Res 30:2347-2353.
Zhang G, Xu CY, Geng M (2011) Effect of shenlongjiannao capsule on oxidation damage and acetylcholine neurotransmitter in cortex of chronic cerebral ischemia rats. Zhongguo Zhongyiyao Xinxi Zazhi 1:41-43.
Zheng GQ, Cheng W, Wang Y, Wang XM, Zhao SZ, Zhou Y, Liu SJ, Wang XT (2011) Ginseng total saponins enhance neurogenesis after focal cerebral ischemia. J Ethnopharmacol 133:724-728.
Copyedited by Croxford L, Raye W, Wang J, Qiu Y, Li CH, Song LP, Zhao M
How to cite this article: Sun ZG, Chen LP, Wang FW, Xu CY, Geng M (2016) Protective effects of ginsenoside Rg1 against hydrogen peroxide-induced injury in human neuroblastoma cells. Neural Regen Res 11(7)∶1159-1164.
Funding: This research was supported by the Research and Development Project Fund of Science and Technology Plan Program of Science and Technology Bureau of Beijing of China, No. Z111107067311022.
10.4103/1673-5374.187057
2016-05-30
RESEARCH ARTICLE
杂志排行
中国神经再生研究(英文版)的其它文章
- Recovery of an injured corticospinal tract by subcortical peri-lesional reorganization in a patient with intracerebral hemorrhage
- Dose response and time course of manganeseenhanced magnetic resonance imaging for visual pathway tracing in vivo
- Low-power laser therapy for carpal tunnel syndrome: effective optical power
- Extracellular matrix from human umbilical cordderived mesenchymal stem cells as a scaffold for peripheral nerve regeneration
- Differential temporal expression of matrix metalloproteinases following sciatic nerve crush
- Genistein suppresses the mitochondrial apoptotic pathway in hippocampal neurons in rats with Alzheimer’s disease