皮质醇对斜带石斑鱼原代培养肝细胞糖代谢的影响
2016-12-01张春晓游文煌陈晓卉
宋 凯 骆 源 张春晓 王 玲 游文煌 陈晓卉 阳 达
(农业部东海海水健康养殖重点实验室,厦门市集美大学饲料检测与安全评价重点实验室,集美大学水产学院,厦门361021)
皮质醇对斜带石斑鱼原代培养肝细胞糖代谢的影响
宋 凯 骆 源 张春晓 王 玲 游文煌 陈晓卉 阳 达
(农业部东海海水健康养殖重点实验室,厦门市集美大学饲料检测与安全评价重点实验室,集美大学水产学院,厦门361021)
本试验旨在研究皮质醇对斜带石斑鱼原代培养肝细胞糖代谢的影响。分离斜带石斑鱼肝细胞,选取2个孵育时间(24和36 h)和3个皮质醇浓度[0(对照)、100 和1 000 nmol/L],测定培养上清液中葡萄糖含量(即肝细胞葡萄糖释放量),肝细胞糖原和丙酮酸含量以及肝细胞糖代谢关键酶[磷酸烯醇丙酮酸羧激酶(PEPCK)、葡萄糖-6-磷酸酶(G6Pase)、糖原合成酶(GSase)、苹果酸脱氢酶(MDH)、异柠檬酸脱氢酶(ICD)和丙酮酸激酶(PK)]的活性。结果表明:在孵育24和36 h时,与对照组相比,100 和1 000 nmol/L皮质醇组肝细胞葡萄糖释放量显著提高(P<0.05),而肝细胞丙酮酸含量显著降低(P<0.05);皮质醇孵育时间对肝细胞葡萄糖释放量、丙酮酸含量均无显著影响(P>0.05)。在孵育24 h时,皮质醇浓度对肝细胞糖原含量无显著影响(P>0.05),而在孵育36 h时,肝细胞糖原含量则随着皮质醇浓度的升高而显著降低(P<0.05);随着皮质醇孵育时间的延长,肝细胞糖原含量显著下降(P<0.05)。在孵育24和36 h时,100 和1 000 nmol/L皮质醇显著提高了肝细胞PEPCK和G6Pase的活性(P<0.05),显著降低了肝细胞MDH和ICD的活性(P<0.05),但对肝细胞GSase活性则无显著影响(P>0.05);在孵育24 h时,皮质醇浓度对肝细胞PK活性无显著影响(P>0.05),但在孵育36 h时,100 和1 000 nmol/L皮质醇则显著提高了肝细胞PK活性(P<0.05)。随着孵育时间的延长,肝细胞G6Pase活性显著下降(P<0.05),PK活性显著升高(P<0.05),而MDH和ICD活性均无显著变化(P>0.05)。由此可见,皮质醇能够促进斜带石斑鱼原代培养肝细胞糖异生作用,抑制葡萄糖的分解代谢,而对糖原合成无调节作用。
斜带石斑鱼;原代培养;皮质醇;糖代谢;酶活性
斜带石斑鱼(Epinepheluscoioides)是世界范围内重要的海水养殖鱼类之一,而外界不良环境因子引起的应激反应会对其生长繁殖造成不利影响,导致下丘脑-垂体-肾上腺皮质轴兴奋和一系列物质代谢的变化。皮质醇是鱼类重要的皮质类固醇类激素,广泛作用于机体的各个组织器官[1-2],是鱼类应激反应、生长、新陈代谢和免疫应答等生理过程的重要调节激素[3-4]。研究表明,当鱼类受到外界不良环境刺激之后,下丘脑-垂体-肾间腺皮质轴兴奋并释放皮质醇,导致血液中皮质醇浓度升高[2,5-6],伴随而来的是机体物质和能量代谢的剧烈变化。鱼类糖代谢的主要途径包括糖酵解、糖异生、糖原合成、肝糖分解、三羧酸循环和磷酸戊糖途径,是鱼类机体代谢的重要组成部分[7-8]。鱼类和其他脊椎动物类似,糖代谢与内分泌活动紧密相关,一些内分泌激素对糖代谢的调节发挥重要作用,如胰岛素和胰高血糖素等[9-10]。肝脏是鱼类参与糖代谢的重要器官,同时也是机体内分泌系统作用的靶器官[11-12],皮质醇作为鱼类机体新陈代谢调节的重要内分泌激素,它与肝脏物质代谢调节密切相关。研究表明,体外培养肝细胞能够表达绝大部分在体肝脏的生理功能,并且保留和维持了活体肝细胞的完整形态及代谢活性,能够很好地反映体内的代谢状态[13]。肝细胞培养模型已成为研究激素对糖代谢途径调节作用的重要工具。然而,大部分有关皮质醇对肝脏物质代谢的研究以整体动物为模型,由于动物机体中多种激素的交互作用,为确定某一种激素独立的调节作用带来很大困难,而且,皮质醇对鱼类肝脏糖代谢的研究主要是探讨皮质醇与糖代谢途径中关键酶活性的关系[8]。利用体外分离培养肝细胞模型可以克服在体难以确定某一激素对糖代谢调节作用的不足。皮质醇对鱼类体外分离培养肝细胞糖代谢的研究鲜有报道,而其对石斑鱼原代培养肝细胞物质代谢机制方面的研究尚未见报道。因此,本研究利用建立的斜带石斑鱼原代培养肝细胞模型,探讨皮质醇对斜带石斑鱼糖代谢的作用机制。
1 材料与方法
1.1 试验动物及饲养管理
健康的斜带石斑鱼(体重约为50 g)购于厦门当地一家水产公司。将斜带石斑鱼放于600 L循环桶中暂养。每天08:30和18:30饱食投喂商业饲料,投喂0.5 h后吸去残饵和粪便,吸完残饵和粪便后换水,换水量为循环桶的1/3~1/2。养殖期间,采用自然光照,海水盐度为30‰,水温为(27±2)℃,溶解氧浓度>6.5 mg/L,氨氮浓度<0.02 mg/L,pH 8.0~8.2。
1.2 主要试剂
皮质醇(含皮质醇50 μmol/L的盐溶液,适用于细胞培养),购于Sigma公司。0.25%胰蛋白酶、L-15培养基、青链霉素(10 000 IU/mL、10 000 μg/mL)、两性霉素B、胎牛血清(FBS),购于Gibco公司;台盼蓝、二甲基亚砜(DMSO),购于上海捷瑞生物工程有限公司;葡萄糖测定试剂盒、肝糖原测定试剂盒、丙酮酸测定试剂盒、磷酸烯醇式丙酮酸激酶(PEPCK)测定试剂盒、葡萄糖-6-磷酸酶(G6Pase)测定试剂盒、糖原合成酶(GSase)测定试剂盒、苹果酸脱氢酶(MDH)测定试剂盒、异柠檬酸脱氢酶(ICD)测定试剂盒、丙酮酸激酶(PK)测定试剂盒,购于南京建成生物工程研究所。
1.3 肝细胞原代培养与皮质醇孵育试验
在Segner等[14]和Qin等[15]方法的基础上进行改良,具体操作如下:采用胰蛋白酶消化法分离肝细胞,新鲜分离的肝细胞在含20%胎牛血清的L-15培养液中培养48 h后,弃去培养上清液,并用L-15培养基(无胎牛血清)清洗3遍,以去除其他激素。然后再加入含有不同浓度皮质醇的新鲜L-15培养基,皮质醇浓度分别为0(作为对照,为了使培养体系中L-15培养液中加入的处理药物体积一致,故加入与后2个处理中等体积的磷酸盐缓冲液)、100和1 000 nmol/L,分别在孵育24和36 h后收集培养上清液和肝细胞,用于后续试验指标测定。每个处理设4个重复,新鲜分离的肝细胞以2×105个/mL的浓度按照不同处理接种于12孔细胞培养板进行培养,剩下的刚分离的细胞按照不同处理接种于25 cm2培养瓶培养,以用于后续试验指标(生化分析)的测定。
1.4 生化分析
肝细胞培养上清液中葡萄糖含量(即肝细胞葡萄糖释放量)以及肝细胞内糖原和甘油三酯含量、糖代谢相关酶活性的测定均采用在25 cm2培养瓶培养的细胞进行测定。孵育试验结束后,细胞采用胰蛋白酶消化法进行收集,并用磷酸盐缓冲液清洗2次。收集的细胞采用台盼蓝拒染法鉴定细胞活力,试验中的活细胞用于酶活性和代谢物含量的测定。
肝细胞培养上清液中葡萄糖含量采用Kikuchi等[16]的操作方法进行测定;为测定肝细胞内糖原和甘油三酯的含量,肝细胞收集后加入适量磷酸盐缓冲液进行超声破碎,肝细胞糖原含量采用de Frutos等[17]描述的方法进行测定,肝细胞糖原含量表示为每毫克细胞蛋白质所含的肝糖原毫克数。
肝细胞GSase活性采用Vijayan等[18]描述的方法测定,MDH和ICD活性采用Sunny等[19]描述的方法测定,G6Pase活性采用Chan等[20]描述的方法测定。
肝细胞加入缓冲液(100 mmol Tris-HCl,pH 7.5,10 ℃)后匀浆,采用Sullivan等[21]所描述的方法测定PK活性。
肝细胞PEPCK活性采用Polakof等[22]描述的方法测定,操作如下:取预先收集好的肝细胞,在4 ℃下加入10倍缓冲液(缓冲液配制参照Polakof等[22])匀浆,匀浆液于10 000 r/min离心30 min后取上清,在30 ℃、340 nm波长下用酶标仪测定吸光度值。
细胞内蛋白质浓度以牛血清蛋白(BSA)作为标准品采用考马斯亮蓝法[23]定量测定。所有酶活性均采用特定活性(U/mg prot)表示。
1.5 数据处理
试验数据用平均值±标准误(mean±SE)表示。数据采用SPSS 19.0软件进行统计和分析,先对数据进行方差齐性检验,如满足方差齐性条件则对数据进行单因素方差分析(one-way ANOVA)和独立样本t检验,若组间存在显著性差异,则再采用Duncan氏法进行多重比较,以P<0.05表示差异显著。
2 结 果
2.1 皮质醇对斜带石斑鱼原代培养肝细胞葡萄糖释放量及糖原和丙酮酸含量的影响
皮质醇对斜带石斑鱼原代培养肝细胞葡萄糖释放量及糖原和丙酮酸含量的影响结果见图1。皮质醇孵育肝细胞24或36 h时,肝细胞葡萄糖含量均随着皮质醇浓度的升高而逐渐增加,与对照组相比,100和1 000 nmol/L皮质醇组的肝细胞葡萄糖含量均显著增加(P<0.05),且以1 000 nmol/L皮质醇组为最高,显著高于其他组(P<0.05)。皮质醇孵育肝细胞24 h时,肝细胞糖原含量各组之间无显著差异(P>0.05),而在孵育36 h时,肝细胞糖原含量则随着皮质醇浓度的升高而显著降低(P<0.05),且以1 000 nmol/L皮质醇组为最低。皮质醇孵育36 h的肝细胞糖原含量显著低于皮质醇孵育24 h的肝细胞糖原含量(P<0.05)。皮质醇孵育肝细胞24或36 h后,100和1 000 nmol/L皮质醇组肝细胞丙酮酸含量均显著低于对照组(P<0.05),但100和1 000 nmol/L皮质醇组之间无显著差异(P>0.05)。皮质醇孵育时间对肝细胞葡萄糖和丙酮酸含量均没有显著影响(P>0.05)。
2.2 皮质醇对斜带石斑鱼原代培养肝细胞糖代谢相关酶活性的影响
皮质醇对斜带石斑鱼原代培养肝细胞糖代谢相关酶活性的影响见图2。皮质醇孵育肝细胞24或36 h时,随着皮质醇浓度的升高,肝细胞PEPCK活性呈现逐渐升高的趋势,且各组之间差异显著(P<0.05)。在皮质醇浓度为100 nmol/L时,随着孵育时间的延长,肝细胞PEPCK活性显著升高(P<0.05)。皮质醇孵育肝细胞24或36 h时,与对照组相比,100和1 000 nmol/L皮质醇组肝细胞G6Pase活性均显著升高(P<0.05),但100和1 000 nmol/L皮质醇组之间无显著差异(P>0.05)。在皮质醇浓度为100和1 000 nmol/L时,肝细胞G6Pase活性随着孵育时间的延长显著下降(P<0.05)。皮质醇孵育肝细胞24或36 h时,肝细胞MDH和ICD活性均随着皮质醇浓度的升高而显著下降(P<0.05),且在1 000 nmol/L皮质醇组达到最小值。孵育时间对同一浓度皮质醇组的肝细胞MDH和ICD活性均无显著影响(P>0.05)。皮质醇孵育肝细胞24或36 h时,肝细胞G6Pase活性在各组之间均无显著差异(P>0.05)。皮质醇孵育肝细胞24 h时,肝细胞PK活性各组之间无显著差异(P>0.05);在孵育36 h时,肝细胞PK活性随着皮质醇浓度的升高而增加,100和1 000 nmol/L皮质醇组显著低于对照组(P<0.05),但100和1 000 nmol/L皮质醇组之间无显著差异(P>0.05)。随着孵育时间的延长,100和1 000 nmol/L皮质醇组的肝细胞PK活性显著升高(P<0.05)。但皮质醇浓度及孵育时间对肝细胞GSase活性均未产生显著影响(P>0.05)。
3 讨 论
在鱼类中,皮质醇对物质代谢的调节作用已有广泛研究[24-27],主要是研究外源性皮质醇对鱼类在血浆和肝脏组织代谢水平以及肝脏组织中代谢相关酶活性变化规律的影响。由于鱼体内存在各种激素的扰动,很难对单个激素如皮质醇在鱼体糖代谢中的独特调节作用进行研究。本试验以斜带石斑鱼原代培养肝细胞为模型,用不同浓度皮质醇对原代培养的肝细胞进行处理,探讨皮质醇对鱼类糖代谢的调节作用。

数据柱标注不同小写字母表示在同一孵育时间下不同皮质醇浓度之间存在显著差异(P<0.05),不同大写字母表示在同一皮质醇浓度下不同孵育时间之间存在显著差异(P<0.05)。下图同。
Date columns with different small letters indicate significant difference among different cortisol concentrations at the same exposure time (P<0.05), and with different capital letters indicate significant difference between different exposure time at the same cortisol concentration (P<0.05). The same as below.
图1 皮质醇孵育时间和浓度对斜带石斑鱼原代培养肝细胞葡萄糖释放量及糖原、丙酮酸含量的影响
Fig.1 Effects of cortisol incubation time and concentration on glucose production, glycogen and pyruvate contents in primary cultured hepatocytes fromEpinepheluscoioides
本试验中,皮质醇孵育离体培养肝细胞后,其物质代谢水平发生了显著变化。皮质醇可以显著提高肝细胞葡萄糖的释放量,并且葡萄糖释放量与皮质醇浓度存在剂量依赖性关系。皮质醇的孵育时间长短并不会影响肝细胞葡萄糖释放量,但皮质醇的孵育时间长短影响了肝细胞糖原含量,孵育24 h时,皮质醇浓度对肝细胞糖原含量不产生显著影响,而在孵育36 h时皮质醇浓度越高肝细胞糖原含量则越低。这表明,在皮质醇孵育24 h时,肝细胞葡萄糖释放量的增加不是由肝糖原分解产生的,可能是肝细胞利用培养液中的氨基酸通过糖异生途径合成的葡萄糖。而孵育36 h时,葡萄糖释放量的增加不仅由肝糖原分解得到,而且可能还由其他糖代谢途径合成葡萄糖。已有研究表明,皮质醇能够刺激鱼类肝脏氨基酸和甘油三酯用于糖异生[28-29]。本研究结果与Vijayan等[30]对美洲杜父鱼(Hemitripterusamericanus)和Mommsen等[31]对大马哈鱼(Oncorhynchusketa)有关皮质醇对糖代谢的研究结果一致。
糖酵解、糖异生与三羧酸循环是在动物体内糖代谢的重要途径,在维持血糖平衡中发挥重要作用[32]。本试验中,皮质醇显著提高了斜带石斑鱼原代培养肝细胞PEPCK和G6Pase活性,但延长皮质醇孵育时间提高了PEPCK的活性却抑制了G6Pase的活性。这说明PEPCK和G6Pase活性与皮质醇存在剂量和时间依赖性关系。PEPCK和G6Pase均为糖异生作用的关键酶,其活性的升高说明皮质醇提高了肝细胞糖异生能力,促进了肝细胞葡萄糖的生成,这是肝细胞葡萄糖释放量增加的重要原因。本试验结果也证明了皮质醇能够刺激鱼类肝脏糖异生作用以维持应激对葡萄糖的需要量这一结论[33-34]。Jones等[35]研究表明,地塞米松能够刺激小鼠原代培养肝细胞利用丙酮酸作为基质物的生糖作用。本试验中,斜带石斑鱼原代培养肝细胞MDH和ICD活性均随着皮质醇浓度的升高而呈显著降低,MDH和ICD是三羧酸循环中的限速酶,其活性降低说明皮质醇抑制了肝细胞糖的有氧分解,促使肝细胞释放更多的葡萄糖供给机体其他组织对能量的需求。Sunny等[19]研究表明,皮质醇能够降低罗非鱼分离培养肝细胞MDH和ICD活性,与本试验结果一致。本试验结果表明,斜带石斑鱼原代培养肝细胞PK活性随着皮质醇孵育时间的延长而显著增加,PK是糖酵解过程中催化磷酸烯醇式丙酮酸转化成丙酮酸的调节酶,其活性增加表明皮质醇能够刺激糖酵解中丙酮酸的生成,而本研究中添加皮质醇后丙酮酸的含量非但没有增加,还较对照组显著下降,这说明皮质醇抑制了肝细胞的糖分解作用。研究表明,在鱼类中皮质醇对肝细胞糖原代谢的调节发挥重要作用[2]。本试验中,作者利用肝细胞原代培养模型探讨皮质醇对肝细胞糖原合成过程中GSase的直接调节作用,但结果发现,无论是皮质醇浓度还是孵育时间都没有对斜带石斑鱼原代培养肝细胞GSase的活性产生显著影响,说明原代培养肝细胞糖原合成能力不受皮质醇的调节,与Vijayan等[18]对大马哈鱼的研究结果一致。相反,有研究表明,腹腔注射皮质醇能够刺激金头鲷肝脏糖原的合成[6]。还有学者指出,皮质醇通过上调海鲷离体肝细胞中GSase的转录水平来提高肝细胞的糖原合成能力[8]。这说明,皮质醇对肝细胞糖原合成的调节作用不仅与鱼的种类有关,而且与皮质醇的作用浓度和孵育时间有关。
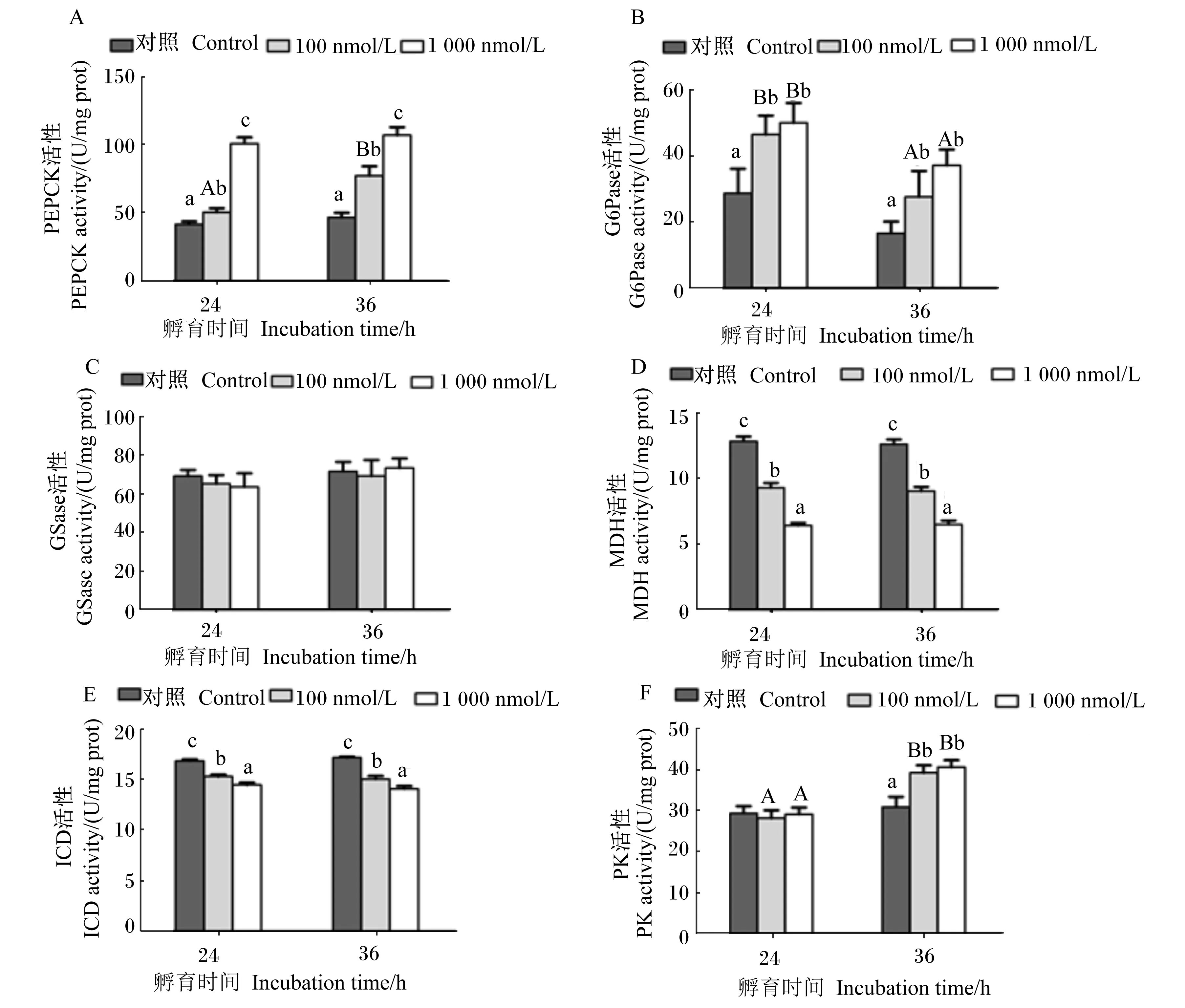
图2 皮质醇孵育时间和浓度对斜带石斑鱼原代培养肝细胞磷酸烯醇丙酮酸羧激酶、葡萄糖-6-磷酸酶、糖原合成酶、苹果酸脱氢酶、异柠檬酸脱氢酶和丙酮酸激酶活性的影响
4 结 论
综上所述,皮质醇提高了斜带石斑鱼原代培养肝细胞的糖异生能力,促进了葡萄糖的生成,抑制了葡萄糖的分解,而对糖原合成无调节作用。
[1] IWAMA G K,VIJAYAN M M,FORSYTH R B,et al.Heat shock proteins and physiological stress in fish[J].American Zoologist,1999,39(6):901-909.
[2] MOMMSEN T P,VIJAYAN M M,MOON T W.Cortisol in teleosts:dynamics,mechanisms of action,and metabolic regulation[J].Reviews in Fish Biology and Fisheries,1999,9(3):211-268.
[3] GAAB J,ROHLEDER N,NATER U M,et al.Psychological determinants of the cortisol stress response:the role of anticipatory cognitive appraisal[J].Psychoneuroendocrinology,2005,30(6):599-610.
[4] TORT L.Stress and immune modulation in fish[J].Developmental & Comparative Immunology,2011,35(12):1366-1375.
[5] BARTON B A,IWAMA G K.Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids[J].Annual Review of Fish Diseases,1991,1:3-26.
[6] WENDELAAR B S E.The stress response in fish[J].Physiological Reviews,1997,77(3):591-625.
[7] WILSON R P.Utilization of dietary carbohydrate by fish[J].Aquaculture,1994,124(1/2/3/4):67-80.
[8] LEUNG L Y,WOO N Y S.Effects of growth hormone,insulin-like growth factor I,triiodothyronine,thyroxine,and cortisol on gene expression of carbohydrate metabolic enzymes in sea bream hepatocytes[J].Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,2010,157(3):272-282.
[9] PUVIANI A C,OTIOLENGHI C,GAVIOLI M E,et al.Action of glucagon and glucagon-like peptide on glycogen metabolism of trout isolated hepatocytes[J].Comparative Biochemistry and Physiology Part B:Comparative Biochemistry,1990,96(2):387-391.
[10] MOMMSEN T P,PLISETSKAYA E M.Insulin in fishes and agnathans:history,structure,and metabolic regulation[J].Reviews in Aquatic Sciences,1991,4(2/3):225-259.
[11] REINECKE M.Influences of the environment on the endocrine and paracrine fish growth hormone-insulin-like growth factor-Ⅰ system [J].Journal of Fish Biology,2010,76(6):1233-1254.
[12] WOOD A W,DUAN C M,BERN H A.Insulin-like growth factor signaling in fish[J].International Review of Cytology,2005,243:215-285.
[13] BATTLE T,STACEY G.Cell culture models for hepatotoxicology[J].Cell Biology and Toxicology,2001,17(4/5):287-299.
[14] SEGNER H,BÖHM R,KLOAS W.Binding and bioactivity of insulin in primary cultures of carp (Cyprinuscarpio) hepatocytes[J].Fish Physiology and Biochemistry,1993,11(1/2/3/4/5/6):411-420.
[15] QIN Q W,WU T H,JIA T L,et al.Development and characterization of a new tropical marine fish cell line from grouper,Epinepheluscoioidessusceptible to iridovirus and nodavirus[J].Journal of Virological Methods,2006,131(1):58-64.
[16] KIKUCHI K,FURUTA T,HONDA H.Utilization of soybean meal as a protein source in the diet of juvenile japanese flounder,Paralichthysolivaceus[J].Aquaculture Science,1994,42(4):601-604.
[17] DE FRUTOS P G,BONAMUSA L,FERNANDEZ F,et al.Fructose 2,6-bisphosphate in liver ofSparusaurata:influence of nutritional state[J].Comparative Biochemistry and Physiology Part B:Comparative Biochemistry,1990,96(1):63-65.
[18] VIJAYAN M M,MAULE A G,SCHRECK C B,et al.Hormonal control of hepatic glycogen metabolism in food-deprived,continuously swimming coho salmon (Oncorhynchuskisutch)[J].Canadian Journal of Fisheries and Aquatic Sciences,1993,50(8):1676-1682.
[19] SUNNY F,LAKSHMY P S,OOMMEN O V.Rapid action of cortisol and testosterone on lipogenic enzymes in a fresh water fishOreochromismossambicus:short-terminvivoandinvitrostudy[J].Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology,2002,131(3):297-304.
[20] CHAN D K,WOO N Y S.Effect of hypophysectomy on the chemical composition and intermediary metabolism of the Japanese eel,Anguillajaponica[J].General and Comparative Endocrinology,1978,35(2):169-178.
[21] SULLIVAN K M,SOMERO G N.Enzyme activities of fish skeletal muscle and brain as influenced by depth of occurrence and habits of feeding and locomotion [J].Marine Biology,1980,60(2/3):91-99.
[22] POLAKOF S,MGUEZ J M,SOENGAS J L.A hepatic protein modulates glucokinase activity in fish and avian liver:a comparative study[J].Journal of Comparative Physiology B:Biochemical,Systemic,and Environmental Physiology,2009,179(5):643-652.
[23] BRADFORD M M.A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J].Analytical Biochemistry,1976,72(1/2):248-254.
[24] CHAN D K O,WOO N Y S.Effect of glucagon on the metabolism of the eal,Anguillajaponica[J].General and Comparative Endocrinology,1978,35(3):216-225.
[25] FOSTER G D,MOON T W.Cortisol and liver metabolism of immature American eels,Anguillarostrata(LeSueur)[J].Fish Physiology and Biochemistry,1986,1(2):113-124.
[26] VIJAYAN M M,BALLANTYNE J S,LEATHERLAND J F.Cortisol-induced changes in some aspects of the intermediary metabolism ofSalvelinusfontinalis[J].General and Comparative Endocrinology,1991,82(3):476-486.
[27] VIJAYAN M M,LEATHERLAND J F.Invivoeffects of the steroid analogue RU486 on some aspects of intermediary and thyroid metabolism of brook charr,Salvelinusfontinalis[J].Journal of Experimental Zoology,1992,263(3):265-271.
[28] DAVE G,JOHANSSON-SJÖBECK M L,LARSSON Å,et al.Effects of insulin on the fatty acid composition of the total blood plasma lipids in the european EEL,AnguillaanguillaL[J].Comparative Biochemistry and Physiology Part A:Physiology,1979,62(3):649-653.
[29] SHERIDAN M A.Effects of thyroxin,cortisol,growth hormone,and prolactin on lipid metabolism of coho salmon,Oncorhynchuskisutch,during smoltification[J].General and Comparative Endocrinology,1986,64(2):220-238.
[30] VIJAYAN M M,FOSTER G D,MOON T W.Effects of cortisol on hepatic carbohydrate metabolism and responsiveness to hormones in the sea raven,Hemitripterusamericanus[J].Fish Physiology and Biochemistry,1993,12(4):327-335.
[31] MOMMSEN T P,MOON T W.Metabolic response of teleost hepatocytes to glucagon-like peptide and glucagon[J].Journal of Endocrinology,1990,126(1):109-118.
[32] RADZIUK J,PYE S.Hepatic glucose uptake,gluconeogenesis and the regulation of glycogen synthesis[J].Diabetes/Metabolism Research and Reviews,2001,17(4):250-272.
[33] VIJAYAN M M,MOON T W.The stress response and the plasma disappearance of corticosteroid and glucose in a marine teleost,the sea raven[J].Canadian Journal of Zoology,2011,72(3):379-386.
[34] VIJAYAN M M,REDDY P K,LEATHERLAND J F,et al.The effects of cortisol on hepatocyte metabolism in rainbow trout:a study using the steroid analogue RU486[J].General and Comparative Endocrinology,1994,96(1):75-84.
[35] JONES C G,HOTHI S K,TITHERADGE M A.Effect of dexamethasone on gluconeogenesis,pyruvate kinase,pyruvate carboxylase and pyruvate dehydrogenase flux in isolated hepatocytes[J].The Biochemical Journal,1993,289(Pt 3):821-828.
Author, SONG Kai, associate professor, E-mail: songkai@jmu.edu.cn
(责任编辑 菅景颖)
Effects of Cortisol on Glycometabolism in Primary Cultured Hepatocytes formEpinepheluscoioides
SONG Kai LUO Yuan ZHANG Chunxiao WANG Ling YOU Wenhuang CHEN Xiaohui YANG Da
(Key Laboratory of Healthy Mariculture for the East China Sea of Ministry of Agriculture, Key Laboratory for Feed Quality Testing and Safety Evaluation, Fish College, Jimei University, Xiamen 361021, China)
This experiment was conducted to study the effects of cortisol on glycometabolism in primary cultured hepatocytes formEpinepheluscoioides. Freshly hepatocytes were isolated fromEpinepheluscoioides, and cultured at different cortisol concentrations [0 (control), 100 and 1 000 nmol/L] and different incubation time (24 and 36 h). Medium glucose content (hepatocyte glucose production), hepatocyte glycogen and pyruvate contents, and the activities of glycometabolism related enzymes including phosphoenolpyruvate carboxykinase (PECK), glucose-6-phosphatase (G6Pase), glycogen synthase (GSase), malate dehydrogenase (MDH), isocitrate dehydrogenase (ICD) and pyruvate kinase (PK) were assessed at 24 and 36 h of incubation, respectively. The results showed as follows: compared with the control group, the hepatocyte glucose production in 100 and 1 000 nmol/L cortisol groups was significantly increased (P<0.05), but the hepatocyte pyruvate content was significantly decreased (P<0.05), both at 24 and 36 h of incubation. The time of incubate to cortisol showed no significant effect on hepatocyte glucose production and pyruvate content (P>0.05). At 24 h of incubation, cortisol concentration showed no significant effect on hepatocyte glycogen content (P>0.05), however, at 36 h of incubation, hepatocyte glycogen content was significantly decreased with cortisol concentration increasing (P<0.05). With the cortisol incubation time extending, hepatocyte glycogen content was significantly decreased (P<0.05). At 24 and 36 h of incubation, 100 and 1 000 nmol/L cortisol significantly increased the activities of hepatocyte PEPCK and G6Pase (P<0.05), and significantly decreased the activities of hepatocyte MDH and ICD (P<0.05), however, there was no significant difference in the activity of hepatocyte GSase (P>0.05). At 24 h of incubation, cortisol concentration showed no significant effect on the activity of hepatocyte PK (P>0.05), but at 36 h of incubation, 100 and 1 000 nmol/L cortisol significantly increased the activity of hepatocyte PK (P<0.05). With the cortisol incubation time extending, the activity of hepatocyte G6Pase was significantly decreased (P<0.05), the activity of hepatocyte PK was significantly increased (P<0.05), however, there were no significant differences in the activities of hepatocyte MDH and ICD (P>0.05). The present study demonstrates that cortisol can enhance the gluconeogenesis, and has no regulating effect on synthesis of hepatic glycogen, but inhibits the catabolism of glucose of the primary cultured hepatocytes fromEpinepheluscoioides.[ChineseJournalofAnimalNutrition, 2016, 28(11):3520-3527]
Epinepheluscoioides; primary cultured; cortisol; glycometabolism; enzyme activities
2016-05-11
国家自然科学基金青年科学基金项目(31302198);国家公益性行业(农业)专项(201303053);集美大学大学生创新性试验计划项目(201610390103)
宋 凯(1978—),男,黑龙江哈尔滨人,副教授,博士,从事动物营养与饲料学研究。E-mail: songkai@jmu.edu.cn
10.3969/j.issn.1006-267x.2016.11.019
S963
A
1006-267X(2016)11-3520-08
