南极磷虾粉替代鱼粉对圆斑星鲽幼鱼生长性能、血清和肝脏生化指标及血清非特异性免疫指标的影响
2016-12-01严俊丽陈四清王贞杰卢刘长琳胡建成
严俊丽 陈四清 常 青* 王贞杰卢 斌 刘长琳 胡建成
(1.中国水产科学研究院黄海水产研究所,青岛266071;2.上海海洋大学水产与生命学院,上海201306)
南极磷虾粉替代鱼粉对圆斑星鲽幼鱼生长性能、血清和肝脏生化指标及血清非特异性免疫指标的影响
严俊丽1,2陈四清1常 青1*王贞杰1,2卢 斌1,2刘长琳1胡建成1
(1.中国水产科学研究院黄海水产研究所,青岛266071;2.上海海洋大学水产与生命学院,上海201306)
本试验旨在探究南极磷虾粉替代鱼粉对圆斑星鲽幼鱼生长性能、血清和肝脏生化指标及血清非特异性免疫指标的影响。以鱼粉和玉米蛋白粉为蛋白质源,高筋粉为糖源,鱼油、豆油和磷脂为脂肪源配制基础饲料。以南极磷虾粉分别替代基础饲料中0(对照)、10%、20%、30%、40%、50%的鱼粉,配制6种等氮等脂的试验饲料(分别记为R0、R10、R20、R30、R40和R50),饲喂初始体重为(38.16±0.11) g的圆斑星鲽幼鱼50 d,每种饲料设3个重复,每个重复投喂30尾鱼。结果表明:1)R30、R40组圆斑星鲽幼鱼的增重率和饲料效率较高,显著高于其他各组(P<0.05);R50组的死亡率显著高于其他各组(P<0.05)。南极磷虾粉替代鱼粉水平对圆斑星鲽幼鱼的肝体比和脏体比没有产生显著影响(P>0.05)。2)R10和R30组的血清总蛋白含量显著高于其他各组(P<0.05);与对照组相比,南极磷虾粉替代10%~30%的鱼粉对圆斑星鲽幼鱼血清中谷草转氨酶和谷丙转氨酶以及肝脏中谷氨酸脱氢酶活性无显著影响(P>0.05)。R40、R50组血清中谷草转氨酶和谷丙转氨酶活性与对照组相比显著升高(P<0.05),R40组肝脏中谷草转氨酶活性较之对照组显著降低(P<0.05),且R50组肝脏中谷丙转氨酶活性显著低于其他各组(P<0.05)。3)与对照组相比,南极磷虾粉替代10%~30%的鱼粉可显著提高圆斑星鲽幼鱼血清中溶菌酶、碱性磷酸酶的活性(P<0.05)。综合来看,南极磷虾粉替代10%~30%的鱼粉可以提高圆斑星鲽幼鱼的生长性能和非特异性免疫力,并对鱼体的肝脏功能和蛋白质代谢无不利影响。
圆斑星鲽;南极磷虾粉;生长性能;生化指标;非特异性免疫力
1 材料与方法
1.1 试验饲料
以鱼粉和玉米蛋白粉为蛋白质源,高筋粉为糖源,鱼油、豆油和磷脂为脂肪源配制基础饲料。以南极磷虾粉分别替代基础饲料中0(对照)、10%、20%、30%、40%、50%的鱼粉,配制6种等氮等脂的试验饲料,并分别记为R0、R10、R20、R30、R40和R50。以南极磷虾粉替代鱼粉后,南极磷虾粉中的油脂含量高于鱼粉,通过鱼油和豆油将6种试验饲料的粗脂肪含量调至8%左右,因此随着替代水平的逐渐升高,饲料配方中鱼油和豆油的比例逐渐下降。试验饲料组成及营养水平见表1。对所有原料进行营养成分测定后,粉碎机粉碎过100目筛,按配方比例混合,最后将磷脂溶于鱼油和豆油中与其他原料混合,加35%的水搅拌,然后用制粒机制成直径为3 mm的颗粒饲料,鼓风烘干12~14 h,将制得的饲料保存在-20 ℃的冷库中。
1.2 饲养管理
试验用圆斑星鲽幼鱼取自山东省烟台市天源水产有限公司,挑选体格健壮、规格一致的同一批鱼种,初始体重为(38.16±0.11) g,随机分配到18个53 cm×72 cm×60 cm的塑料桶中,每种饲料投喂3个桶(重复),每桶30尾。试验开始前使用对照组饲料暂养1周,使之适应配合饲料。养殖期间,水温控制在14 ℃左右,盐度为35左右,溶解氧浓度在5.5 mg/L左右。试验持续50 d,每天08:30换水1次,14:00饱食投喂1次,投喂0.5 h后使用虹吸管吸取残饵,烘干称重,每天记录摄食量。
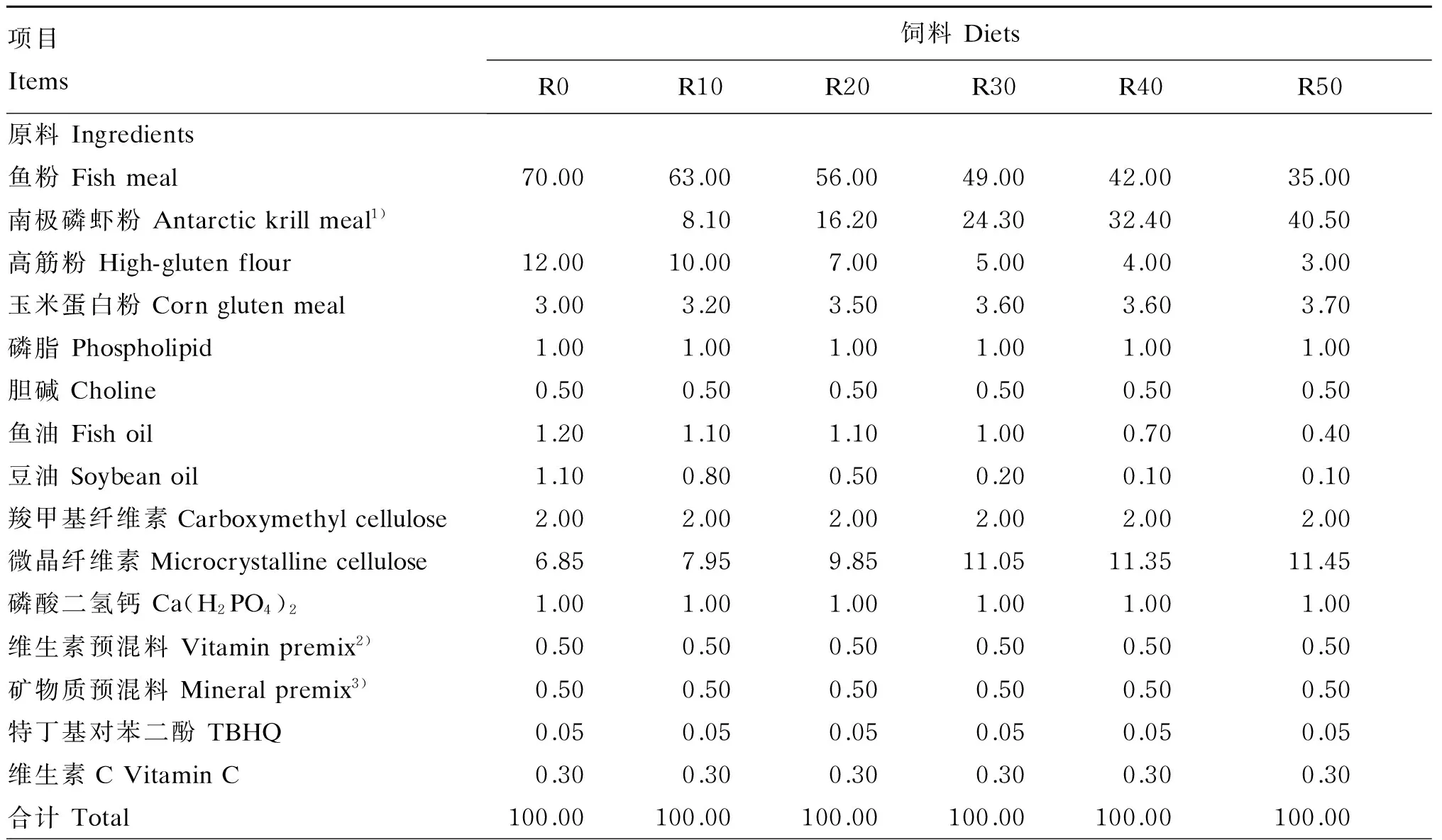
表1 试验饲料组成及营养水平(干物质基础)
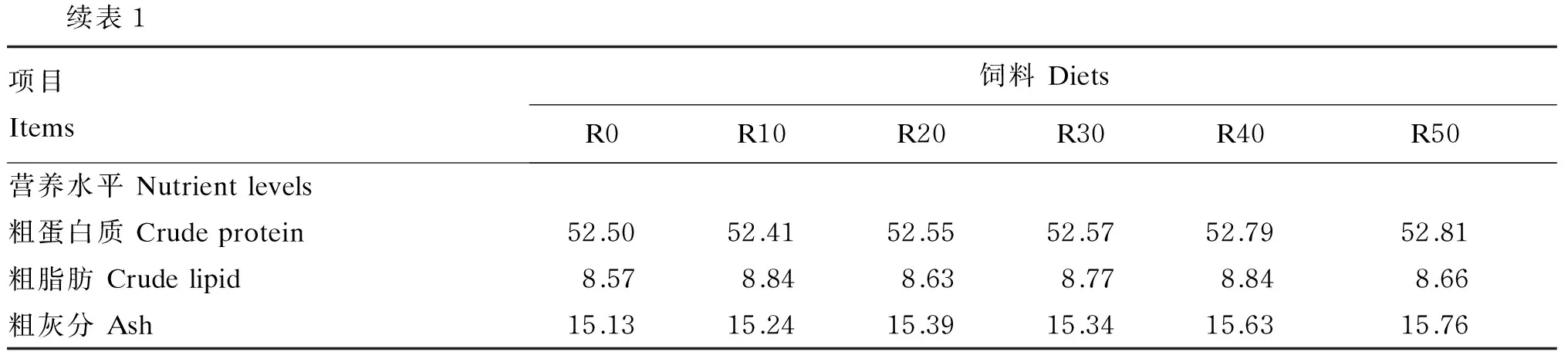
续表1项目Items饲料DietsR0R10R20R30R40R50营养水平Nutrientlevels粗蛋白质Crudeprotein52.5052.4152.5552.5752.7952.81粗脂肪Crudelipid8.578.848.638.778.848.66粗灰分Ash15.1315.2415.3915.3415.6315.76
1)南极磷虾粉粗蛋白质含量为61.45%,粗脂肪含量为12.09%,氟含量为(1 642.80±31.78) mg/kg。The contents of crude protein, crude lipid and fluoride in Antarctic krill meal were 61.45%, 12.09% and (1 642.80±31.78) mg/kg, respectively.
2)维生素预混料为每千克饲料提供Vitamin premix provided the following per kg of diets:稻壳粉 rice hull powder 1 838.8 mg,维生素A乙酸酯 VA acetate 18.0 mg,硫铵素 thiamin 25.5 mg,核黄素 riboflavin 50.0 mg,烟酰胺 nicotinamide 1 515.0 mg,泛酸钙 calcium pantothenate 510.0 mg,盐酸吡多醇 pyridoxine hydrochloride 20.2 mg,氯钴胺 chlorocobalamin 10.0 mg,VD35.0 mg,生物素 biotin 60.0 mg,维生素E乙酸酯 VE acetate 200.0 mg,VK312.0 mg,叶酸 folic acid 20.5 mg,肌醇 inositol 715.0 mg。
3)矿物质预混料为每千克饲料提供Mineral premix provided the following per kg of diets:沸石粉 zeolite powder 3 165 mg,MgSO4·H2O 1 500.0 mg,KIO380.0 mg,CoCl250.0 mg,CuSO4·5H2O 10.0 mg,FeSO4·H2O 80.0 mg,ZnSO4·H2O 50.0 mg,Na2SeO320.0 mg,MnSO4·H2O 45.0 mg。
1.3 样品收集与分析
试验结束后,采样前将试验鱼饥饿24 h,对每桶鱼进行计数、称重。每桶随机取5尾鱼,尾静脉取血3 mL左右,用体积分数为1%的肝素钠抗凝,低温放置4 h后4 000 r/min离心10 min,然后解剖分离内脏团和肝脏并称重,用于计算肝体比和脏体比。分离的血清用于总蛋白(total protein,TP)含量及谷草转氨酶(glutamic-oxaloacetic transaminase,GOT)、谷丙转氨酶(glutamic-pyruvic transaminase,GPT)、溶菌酶(lysozyme,LZM)、酸性磷酸酶(acid phosphatase,ACP)和碱性磷酸酶(alkaline phosphatase,AKP)活性的测定;分离的肝脏用于谷氨酸脱氢酶(glutamate dehydrogenase,GDH)、GOT和GPT活性的测定。
饲料样品在105 ℃烘干后采用凯氏定氮法测得粗蛋白质含量(VELP,UDK-142 Automatic Distillation Unit,意大利);采用索氏抽提法(石油醚为抽提液)测得饲料样品的粗脂肪含量(SOXTEC-2050 FOSS脂肪测定仪,瑞典);将饲料样品在马弗炉(550 ℃)中灼烧6 h测得粗灰分含量。血清中总蛋白含量及GOT、GPT、GDH、LZM、ACP和AKP活性以及肝脏中GDH、GOT和GPT活性采用南京建成生物工程研究所提供的试剂盒进行测定。
1.4 计算公式
增重率(weight gain rate,WGR,%)=100×
(末平均体重-初平均体重)/初平均体重;
死亡率(mortality rate,MR,%)=100×
(试验初鱼体总数-试验末鱼体总数)/
试验初鱼体总数;
饲料效率(feed efficiency ratio,FER,%)=
100×总增重/投饲总量;
肝体比=(heaptosomatic index,HSI,%)=
100×肝脏重/试验末鱼体重;
脏体比=(viscerosomatic index,VSI,%)=
100×内脏重/试验末鱼体重。
1.5 数据统计
试验数据采用Excel 2010和SPSS 17.0软件进行统计分析,差异显著时采用Duncan氏法进行多重比较,显著水平为P<0.05,数据以平均值±标准误(mean±SE)表示。
2 结 果
2.1 南极磷虾粉替代鱼粉对圆斑星鲽幼鱼生长性能的影响
南极磷虾粉替代鱼粉对圆斑星鲽幼鱼生长性能的影响如表2所示。随着南极磷虾粉替代水平的升高,增重率呈现先升高后降低的趋势,R30组的增重率最高,显著高于除R40组外的其他各组(P<0.05);饲料效率也呈现先升高后降低的趋势,其中对照组和R20、R50组的饲料效率没有显著差异(P>0.05),其他各组均显著高于对照组和R50组(P<0.05)。南极磷虾粉替代鱼粉会提高圆斑星鲽幼鱼的死亡率,其中R50组的死亡率最高,为13.67%,显著高于其他各组(P<0.05),其他组间无显著差异(P>0.05)。南极磷虾粉替代鱼粉水平对圆斑星鲽幼鱼的肝体比和脏体比没有产生显著影响(P>0.05)。
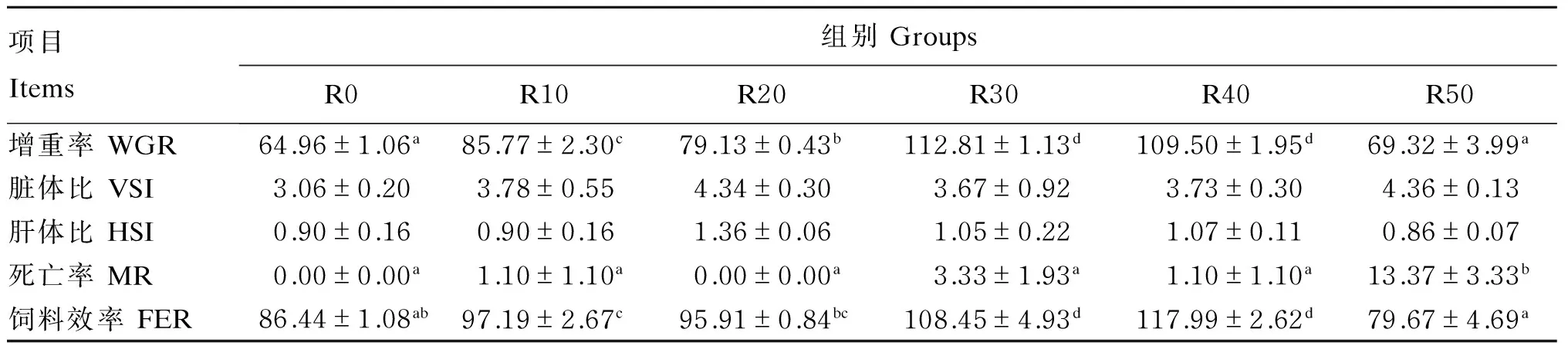
表2 南极磷虾粉替代鱼粉对圆斑星鲽幼鱼生长性能的影响
同行数据肩标相同字母或无字母表示差异不显著(P>0.05),不同字母表示差异显著(P<0.05)。下表同。
In the same row, values with the same letter or no letter superscripts indicated no significant difference (P>0.05), while with different letter superscripts indicated significant difference (P<0.05). The same as below.
2.2 南极磷虾粉替代鱼粉对圆斑星鲽幼鱼血清和肝脏生化指标的影响
南极磷虾粉替代鱼粉对圆斑星鲽幼鱼血清和肝脏生化指标的影响如表3所示。R10和R30组的血清总蛋白含量显著高于对照组(P<0.05),其余各组显著低于对照组(P<0.05)。与对照组相比,南极磷虾粉替代鱼粉水平低于或等于30%时对血清中GOT、GPT活性无显著影响(P>0.05),南极磷虾粉替代鱼粉水平为40%和50%时血清中GOT、GPT活性显著降低(P<0.05)。肝脏中GPT活性随着南极磷虾粉替代水平的升高有降低的趋势,除R50组外,其余各组均显著高于对照组(P<0.05)。肝脏中GOT活性R30组与对照组无显著差异(P>0.05),其余各组均显著低于对照组(P<0.05)。R30组肝脏中GDH活性最高,显著高于除对照组外的其余各组(P<0.05)。

表3 南极磷虾粉替代鱼粉对圆斑星鲽幼鱼血清和肝脏生化指标的影响
2.3 南极磷虾粉替代鱼粉对圆斑星鲽幼鱼血清非特异性免疫指标的影响
南极磷虾粉替代鱼粉对圆斑星鲽幼鱼血清非特异性免疫指标的影响如表4所示。各替代组血清中LZM和ACP的活性均显著高于对照组(P<0.05);此外,除R10组外,其余各组血清中AKP活性均显著高于对照组(P<0.05)。
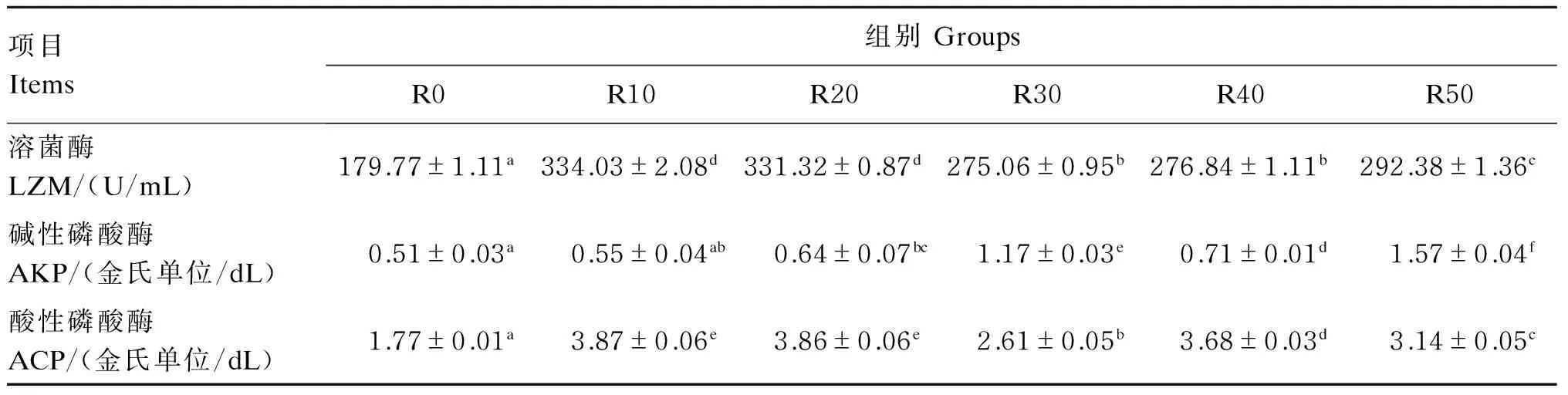
表4 南极磷虾粉替代鱼粉对圆斑星鲽幼鱼血清非特异性免疫指标的影响
3 讨 论
3.1 南极磷虾粉替代鱼粉对圆斑星鲽幼鱼生长性能的影响
本试验中,南极磷虾粉替代10%~40%的鱼粉可以提高圆斑星鲽幼鱼的增重率和饲料效率。关于磷虾粉在不同鱼类饲料中的应用已经有大量报道,部分研究结果表明,磷虾粉替代高水平的或全部的鱼粉可能会导致鱼体的生长性能和饲料效率下降[8,17],磷虾粉替代中等水平的鱼粉可能对水产动物的生长无不利影响或有一定的促进作用,例如,有报道指出以南极磷虾粉替代大西洋鲑饲料中40%的鱼粉及以南极磷虾粉替代大西洋庸鲽饲料中60%的鱼粉能够显著提高鱼体的特定生长率[18];此外,研究认为4%的磷虾粉对点带石斑鱼有一定的促生长和促摄食作用[14];孔凡华[15]也指出以磷虾粉替代20%~60%的鱼粉对大菱鲆的生长性能无不利影响。本试验中,随着南极磷虾粉替代水平的升高,圆斑星鲽幼鱼的增重率和饲料效率均出现先升高后降低的现象,饲喂南极磷虾粉替代30%鱼粉饲料的圆斑星鲽幼鱼有最好的生长性能,但是饲喂南极磷虾粉替代50%鱼粉饲料的圆斑星鲽幼鱼的死亡率显著升高,这和魏佳丽[13]得出的磷虾粉替代鱼粉的水平超过30%时星斑川鲽的生长性能下降的研究结果一致。Yoshitomi等[19]也发现磷虾粉完全替代鱼粉对黄尾鲺(Seriolaquinqueradiata)的生长会产生不利的影响。而Hansen等[8]报道,以磷虾粉完全替代鱼粉会导致大西洋鲑的生长率显著下降,但是去壳的低氟磷虾粉可以显著提高鱼体的生长性能。本试验中,南极磷虾粉中的氟含量较高,可达(1 642.80±31.78) mg/kg,随着南极磷虾粉的替代水平的升高,饲料中氟含量也会升高,替代水平为50%的组的生长性能和饲料效率下降、死亡率升高可能是由于高含量的氟造成的,因为过量的氟对鱼体有毒害作用。此外,磷虾粉中还含有大量的几丁质,在某种程度上几丁质几乎不能被鱼体吸收利用,还可降低鱼体的消化率[20-21]。鱼体的肝体比和脏体比不受饲料中南极磷虾粉替代水平的影响,这与魏佳丽[13]的研究结果一致。关于磷虾粉在不同鱼类饲料中的替代水平不一和效果各异,可能与试验鱼的种类、食性和生活环境等有关,具体的原因还需进一步的研究。
3.2 南极磷虾粉替代鱼粉对圆斑星鲽幼鱼血清和肝脏生化指标的影响
血清总蛋白的主要功能是维持渗透压平衡,同时是蛋白质代谢水平的重要指标,可反映机体蛋白质和氨基酸的消化吸收程度[22],血清中总蛋白含量增多,能够增强动物体内的代谢,对合成蛋白质和氮沉积有利,对免疫能力也有促进作用[23-24]。R10和R30组的血清总蛋白含量显著高于对照组,说明饲料中适宜水平的南极磷虾粉对圆斑星鲽幼鱼的蛋白质利用有一定的促进作用。GOT和GPT主要存在于肝脏中,正常情况下血清中这2种酶的活性很低,当组织细胞受损细胞膜通透性发生改变时才会进入血液,进而活性升高,因此血清中GOT和GPT的活性可反映肝脏的损伤情况[25]。R10、R20和R30组圆斑星鲽幼鱼血清中GPT和GOT活性与对照组没有显著差异,说明南极磷虾粉替代10%~30%的鱼粉没有对圆斑星鲽幼鱼的肝脏造成损伤和代谢负担。
GDH是非必需氨基酸合成的关键酶,其活性与蛋白质的合成与分解有密切关系[26]。饲料氨基酸主要通过转氨基作用和脱氨基作用在体内代谢转化,鱼类则主要通过联合脱氨基作用满足机体需要,GPT和GOT是氨基酸代谢中的2个关键酶,它们的活性大小不仅反映了氨基酸代谢程度的强弱,而且反映肝脏功能的正常与否[27]。本试验中,各替代组圆斑星鲽幼鱼肝脏中GDH活性与对照组没有显著差异,说明南极磷虾粉对鱼体的蛋白质代谢无不利的影响,而R30组圆斑星鲽幼鱼肝脏中GOT和GDH活性均在最高的水平,说明R30组的圆斑星鲽幼鱼蛋白质代谢最快,而且肝脏无损伤;而R40组肝脏中GOT活性较之对照组显著降低,且R50组肝脏中GPT活性显著低于其他各组,说明过高的替代水平可能会降低鱼体的蛋白质代谢水平,并且影响肝脏功能。
3.3 南极磷虾粉替代鱼粉对圆斑星鲽幼鱼血清非特异性免疫指标的影响
水生动物的特异性免疫机制不具备抗体生成的二次反应,非特异性免疫在水生动物的免疫防御中扮演着最为重要的角色。LZM能够对细菌的细胞壁造成损害,其活性大小可在一定程度上反映机体抵御细菌侵袭的能力[28]。ACP作为溶酶体的标志酶,主要参与磷酸酯的代谢调节、信号传导以及能量转化等[29],而AKP可直接参与生物体磷酸集团的转移和代谢[30-31]。本研究中,各替代组血清中LZM的活性均显著高于对照组,这与孔凡华[15]研究得出的半滑舌鳎血清中的LZM活性随着磷虾粉水平的升高而显著升高的结果相一致。同时,各替代组血清中ACP和AKP的活性也有升高的趋势,说明南极磷虾粉对圆斑星鲽幼鱼血清中的ACP和AKP的活性有提高作用,进而提高机体的非特异性免疫力,这可能与南极磷虾粉富含胡萝卜素和虾青素[3]有关,虾青素可以提高机体的免疫力和抗氧化能力[4,32],已有的研究曾报道饲料中的虾青素可以显著提高大黄鱼的免疫力和抗氧化能力[33],并可以改善虹鳟(Oncorhynchusmykiss)血清防御素含量和LZM活性等[3]。
4 结 论
① 南极磷虾粉替代鱼粉可以提高圆斑星鲽幼鱼的增重率和饲料效率,替代水平为30%时生长性能和饲料效率较好,但替代水平大于40%时会降低鱼体的存活率。
② 南极磷虾粉替代10%~30%的鱼粉对圆斑星鲽幼鱼的肝脏功能和蛋白质代谢无不利影响,同时可以提高圆斑星鲽幼鱼的非特异性免疫力。
[1] 叶建生,王兴强,马甡,等.圆斑星鲽的生物学特性及其研究进展[J].渔业经济研究,2006(6):5-7.
[2] 常青,秦帮勇,孔繁华,等.南极磷虾在水产饲料中的应用[J].动物营养学报,2013,25(2):256-262.
[3] AMAR E C,KIRON V,SATOH S,et al.Influence of various dietary synthetic carotenoids on bio-defence mechanisms in rainbow trout,Oncorhynchusmykiss(Walbaum)[J].Aquaculture Research,2001,32(Suppl.1):162-173.
[4] KALINOWSKI C T,ROBAINA L E,IZQUIERDO M S.Effect of dietary astaxanthin on the growth performance,lipid composition and post-mortem skin colouration of red porgyPagruspagrus[J].Aquaculture International,2011,19(5):811-823.
[5] FLORETO E A T,BROWN P B,BAYER R C.The effects of krill hydrolysate-supplemented soya-bean based diets on the growth,colouration,amino and fatty acid profiles of juvenile American lobster,Homarusamericanus[J].Aquaculture Nutrition,2001,7(1):33-43.
[6] YI X W,LI J,XU W,et al.Shrimp shell meal in diets for large yellow croakerLarimichthyscroceus:effects on growth,body composition,skin coloration and anti-oxidative capacity[J].Aquaculture,2015,441:45-50.
[7] RUNGRUANGSAK-TORRISSEN K.Digestive efficiency,growth and qualities of muscle and oocyte in atlantic salmon (SalmosalarL.) fed on diets with krill meal as an alternative protein source[J].Journal of Food Biochemistry,2006,31(4):509-540.
[8] HANSEN J Ø,PENN M,ØVERLAND M,et al.High inclusion of partially deshelled and whole krill meals in diets for Atlantic salmon (Salmosalar)[J].Aquaculture,2010,310(1/2):164-172.
[9] HANSEN J Ø,SHEARER K D,ØVERLAND M,et al.Replacement of LT fish meal with a mixture of partially deshelled krill meal and pea protein concentrates in diets for Atlantic salmon (Salmosalar)[J].Aquaculture,2011,315(3/4):275-282.
[10] OLSEN R E,SUONTAMA J S,LANGMYHR E,et al.The replacement of fish meal with Antarctic krill,Euphausiasuperbain diets for Atlantic salmon,Salmo salar[J].Aquaculture Nutrition,2006,12(4):280-290.
[11] KARLSEN Ø,SUONTAMA J,OLSEN R E.Effect of Antarctic krill meal on quality of farmed Atlantic cod (GadusmorhuaL.)[J].Aquaculture Research,2006,37(16):1676-1684
[12] TIBBETTS S M,OLSEN R E,LALL S P.Effects of partial or total replacement of fish meal with freeze-dried krill (Euphausiasuperba) on growth and nutrient utilization of juvenile Atlantic cod (Gadusmorhua) and Atlantic halibut (Hippoglossushippoglossus) fed the same practical diets[J].Aquaculture Nutrition,2011,17(3):287-303.
[13] 魏佳丽.磷虾粉在星斑川鲽和珍珠龙胆石斑鱼幼鱼饲料中的应用研究[D].硕士学位论文.上海:上海海洋大学,2015.
[14] 黄艳青,高露姣,陆建学,等.饲料中添加南极大磷虾粉对点带石斑鱼幼鱼生长与肌肉营养成分的影响[J].海洋渔业,2010,32(4):440-446.
[15] 孔凡华.南极磷虾粉对大菱鲆和半滑舌鳎生长、非特异性免疫及肌肉品质的影响[D].硕士学位论文.大连:大连海洋大学,2011.
[16] 严俊丽,陈四清,常青,等.南极磷虾粉作为鲽形目鱼饲料蛋白源的营养价值评价[J].渔业科学进展,2016,37(5):74-82.
[17] YOSHITOMI B,AOKI M,OSHIMA S,et al.Evaluation of krill (Euphausiasuperba) meal as a partial replacement for fish meal in rainbow trout (Oncorhynchusmykiss) diets[J].Aquaculture,2006,261(1):440-446.
[18] SUONTAMA J,KARLSEN Ø,MOREN M,et al.Growth,feed conversion and chemical composition of Atlantic salmon (SalmosalarL.) and Atlantic halibut (HippoglossushippoglossusL.) fed diets supplemented with krill or amphipods[J].Aquaculture Nutrition,2007,13(4):241-255.
[19] YOSHITOMI B,NAGANO I.Effect of dietary fluoride derived from Antarctic krill (Euphausiasuperba) meal on growth of yellowtail (Seriolaquinqueradiata)[J].Chemosphere,2012,86(9):891-897.
[20] RØDDE R H,EINBU A,VÅRUM K M,et al.A seasonal study of the chemical composition and chitin quality of shrimp shells obtained from northern shrimp (Pandalusborealis)[J].Carbohydrate Polymers,2008,71(3):388-393.
[21] FERRER J,PAEZ G,MARMOL Z,et al.Acid hydrolysis of shrimp-shell wastes and the production of single cell protein from the hydrolysate[J].Bioresource Technology,1996,57(1):55-60.
[22] 丁立云,张利民,王际英,等.饲料蛋白水平对星斑川鲽幼鱼生长、体组成及血浆生化指标的影响[J].中国水产科学,2010,17(6):1285-1292.
[23] KANJANAPRUTHIPONG J.Supplementation of milk replacers containing soy protein with threonine,methionine,and lysine in the diets of calves[J].Journal of Dairy Science,1998,81(11):2912-2915.
[24] COMA J,CARRION D,ZIMMERMAN D R.Use of plasma urea nitrogen as a rapid response criterion to determine the lysine requirement of pigs[J].Journal of Animal Science,1995,73(2):472-481.
[25] NYBLOM H,BERGGREN U,BALLDIN J,et al.High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking[J].Alcohol and Alcoholism,2004,39(4):336-339.
[26] SRERE P A.Complexes of sequential metabolic enzymes[J].Annual Review of Biochemistry,1978,56:89-124.
[27] 王香丽,麦康森,徐玮,等.蛋氨酸对瓦氏黄颡鱼幼鱼肝脏及血浆中谷草转氨酶和谷丙转氨酶活力的影响[J].中国海洋大学学报:自然科学版,2015,46(9):49-53.
[28] 黄峰,张丽,周艳萍,等.复合酶制剂对异育银鲫生长、SOD和溶菌酶活性的影响[J].华中农业大学学报,2008,27(1):96-100.
[29] 江琰,刘克武,雷远成,等.意蜂工蜂酸性磷酸酶的纯化及其酶学特性[J].昆虫学报,2004,47(3):310-315.
[30] 杜启艳,王萍,王友利,等.长期饥饿和再投喂对泥鳅不同组织糖原、酸性磷酸酶和碱性磷酸酶的影响[J].江西师范大学学报:自然科学版,2008,32(4):488-493.
[31] 廖金花,陈巧,林丽蓉,等.鲍鱼碱性磷酸酶的分离纯化和性质研究[J].厦门大学学报:自然科学版,2005,44(2):272-275.
[32] RODRIGUES E,MARIUTTI L R B,MERCADANTE A Z.Scavenging capacity of marine carotenoids against reactive oxygen and nitrogen species in a membrane-mimicking system[J].Marine Drugs,2012,10(8):1784-1798.
[33] LI M,WU W J,ZHOU P P,et al.Comparison effect of dietary astaxanthin andHaematococcuspluvialison growth performance,antioxidant status and immune response of large yellow croakerPseudosciaenacrocea[J].Aquaculture,2014,434:227-232.
*Corresponding author, professor, E-mail: changqing@ysfri.ac.cn
(责任编辑 菅景颖)
Effects of Antarctic Krill Meal Replacing Fish Meal on Growth Performance, Serum and Liver Biochemical Indices and Serum Non-Specific Immune Indices of Juvenile Spotted Halibut (Veraspervariegatus)
YAN Junli1,2CHEN Siqing1CHANG Qing1*WANG Zhenjie1,2LU Bin1,2LIU Changlin1HU Jiancheng1
(1. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China; 2. College of Fishers and Life Sciences, Shanghai Ocean University, Shanghai 201306, China)
This trial was conducted to evaluate the effects of Antarctic krill meal (AKM) replacing fish meal on growth performance, serum and liver biochemical indices and serum non-specific immune indices of juvenile spotted halibut (Veraspervariegatus). The basal diet was formulated using fish meal and corn gluten meal as protein sources, high-gluten flour as carbohydrate sources, and fish oil, soybean oil and phospholipid as lipid sources. Six isonitrogenous and isolipidic experimental diets were formulated by replacing 0 (control), 10%, 20%, 30%, 40% and 50% fish meal with AKM on the basis of the basal diet, and they were named as R0, R10, R20, R30, R40 and R50, respectively. Each diet was fed to three replicates of 30 juvenile spotted halibut with an initial body weight of (38.16±0.11) g for 50 d. The results showed as follows: 1) the weight gain rate (WGR) and feed efficiency ratio (FER) in R30 and R40 groups had the higher values, which were significantly higher than those in other groups (P<0.05). In addition, the mortality rate (MR) in R50 group was significantly higher than that in other groups (P<0.05). Replacement level of fish meal with AKM had no significant effects on viscerosomatic index (VSI) and hepatosomatic index (HSI) of juvenile spotted halibut (P>0.05). 2) Serum total protein content in R10 and R30 groups was significantly higher than that in other groups (P<0.05). Compared with the control group, AKM replacing 10% to 30% fish meal had no significant effects on the activities of serum glutamicoxalacetic transaminase (GOT) and glutamicpyruvic transaminase (GPT) and liver glutamate dehydrogenase (GDH) (P>0.05). Compared with control group, serum GOT and GPT activities in R40 and R50 groups were significantly increased (P<0.05), but the liver GOT activity in R40 group was significantly decreased (P<0.05). In addition, the liver GPT activity in R50 group was significantly lower than that in other groups (P<0.05). 3) Compared with the control group, AKM replacing 10% to 50% fish meal could significantly improve the activities of lysozyme (LZM) and alkaline phosphatase (AKP) in serum of juvenile spotted halibut (P<0.05). Above results show that AKM replacing 10% to 30% fish meal can improve the growth performance and non-specific immunity of juvenile spotted halibut, and have no adverse effect on the function of liver and protein metabolism.[ChineseJournalofAnimalNutrition, 2016, 28(11):3503-3510]
spotted halibut; Antarctic krill meal; growth performance; biochemical indices; non-specific immunity
2016-04-30
农业部“南极海洋生物资源开发利用”项目;山东省自主创新成果转化专项(2013ZHZX2A0803);鳌山科技创新计划(2015ASKJ02-03);中国水产科学研究院黄海水产研究所基本科研业务(20603022016005)
严俊丽(1990—),女,河南周口人,硕士研究生,从事鱼类营养与饲料研究。E-mail: 643497252@qq.com
*通信作者:常 青,研究员,硕士生导师,E-mail: changqing@ysfri.ac.cn
10.3969/j.issn.1006-267x.2016.11.017
S963
A
1006-267X(2016)11-3503-08
