Central CB2receptors in inflammation-driven neurodegeneration: dysregulation and therapeutic potential
2016-12-01RuthM.Concannon,EilísDowd
Central CB2receptors in inflammation-driven neurodegeneration: dysregulation and therapeutic potential
In recent times there has been an intensification of interest in the pathological role of neuroinflammation in neurodegenerative disease. Neuroprotective strategies to slow, halt or reverse neurodegeneration have not proven fruitful clinically, and the notion of a multi-hit hypothesis in the progression of neurodegenerative disease has steered focus towards other contributory pathological factors, particularly neuroinflammation. Neuroinflammation is believed to sustain the neurodegenerative pathology, forming a cyclical and self-sustaining pathological process, with dying neurons activating microglia, which, once activated, can release several factors that kill further neurons (reviewed in Blandini, 2013). Indeed, evidence of neuroinflammation has been observed around sites of neuropathological lesions in many neurodegenerative conditions including Parkinson’s disease (Gómez-Gálvez et al., 2015). Furthermore, epidemiological evidence suggests that chronic administration of drugs with anti-inflammatory action, including non-steroidal anti-inflammatory drugs (NSAIDs), may reduce the risk of developing neurodegenerative conditions. With this in mind, the concept of anti-inflammatory therapy for disease-modification in neurodegenerative disease has emerged, with a view to identifying specific, novel targets to reduce inflammation in neurodegeneration. One such anti-inflammatory target that has garnered particular attention in the context of Parkinson’s disease is the anti-inflammatory and immunosuppressant cannabinoid type-2 (CB2) receptor. The endocannabinoid system has been the focus of much interest in Parkinson’s disease research, initially due to the role of the CB1receptor in the basal ganglia neurocircuitry. Indeed, in Parkinson’s disease patients, elevated levels of the endogenous cannabinoid, anandamide, and dysregulation of the central CB1receptor have been reported, and preclinically, targeting the CB1receptor has shown therapeutic benefit in treating both symptomatic and drug-induced motoric features (reviewed in Concannon et al., 2015a). In contrast, preclinical studies targeting the CB1receptor have been less successful as a neuroprotective strategy, and this has led to increased interest in the CB2receptor for disease-modification. The CB2receptor has emerged as an exciting target for modulation of neuroinflammation because it is expressed on microglial cells, and when activated, has profound anti-inflammatory and immunosuppressant effects on these neuroimmune cells (Mecha et al., 2016). This perspective will draw together some of the recent evidence indicating that CB2receptor expression is profoundly increased at sites of injury in the brain, thus supporting the concept that specific targeting of this receptor may have the potential to break the pathological cycle of neuroinflammation and neurodegeneration in neurodegenerative disease.
The response of the central cannabinoid CB2receptor to injury, and its potential as an anti-inflammatory, neuroprotective target in Parkinson's disease: The first evidence that the CB2receptor expression is altered in the diseased human brain came in 2003 when Cristina Benito, Julián Romero and colleagues (Benito et al., 2003) showed that the CB2receptor was selectively expressed in microglia and astrocytes surrounding neuritic plaques in the post mortem brains of patients with Alzheimer’s disease. This was the first evidence that expression of the CB2receptor, which was considered the“peripheral” cannabinoid receptor, was altered in the human brain in a neurodegenerative disease. Since then, upregulation of microglial CB2receptor expression has been reported to be associated with the degenerative pathology of many human diseases including in the degenerating substantia nigra of patients with Parkinson’s disease, the degenerating striatum of patients with Huntington’s disease, the degenerating cerebellum of patients with spinocerebellar ataxia, and in the demyelinated cortex of patients with multiple sclerosis (Concannon et al., 2015b, Gómez-Gálvez et al., 2015, Mecha et al., 2016).
Although these studies are crucially important in that they have shown that the microglial CB2receptor undergoes upregulation in the diseased human brain, the key question is the role they playing in the pathophysiological cycle of neurodegeneration and neuroinflammation — is CB2receptor upregulation contributing to inflammation-driven neurodegeneration or is it a compensatory attempt to keep it in check? The probable answer to this question comes from a multitude of in vitro studies which have provided overwhelming evidence to support a role for the CB2receptor in attenuating the microglial neuroinflammatory response to injury and neurodegeneration (extensively reviewed in Mecha et al., 2016). These studies have shown that activation of CB2receptors produces a less active microglial phenotype with supressed phagocytic and antigen presentation functionality, reduced release of pro-inflammatory cytokines and other neurotoxic factors, and enhanced release of anti-inflammatory cytokines. If these in vitro observations are reflective of the functions of microglial CB2receptors in the diseased human brain, then it follows that pharmacological targeting of CB2receptors should reduce neuronal degeneration in conditions where this is associated with neuroinflammation.
In vivo preclinical studies provide the interface between such in vitro studies and the human findings, and provide a means for probing dynamic changes in CB2receptor expression in response to brain injury, as well as for assessing the functionality of any such changes. Moreover, valid animal models in which microglial CB2receptor upregulation is associated with neurodegenerative lesions (such as occurs in the human brain), will also facilitate the development of novel therapeutic strategies targeting this receptor. To this end, we have recently embarked on a series of studies investigating the change in expression of the brain’s CB2receptor in response to neurotoxic, environmental and inflammatory stimuli in the rat (Concannon et al., 2015b, 2016). We specifically investigated CB2receptor expression in response to direct intrastriatal infusion of the catecholaminergic neurotoxin, 6-hydroxydopamine, the organic pesticide, rotenone, the bacterial inflammagen, liposaccharide (LPS), and the viral inflammagen, polyinosinic:polycytidylic acid (Poly I:C). Although chosen because of their relevance to the etiology and pathogenesis of Parkinson’s disease, the inflammagens, in particular, are relevant to a wider range of neurodegenerative diseases in which neuroinflammation plays a pathological role. The toxins were also chosen because they initiate neuroinflammation and neurodegeneration in different ways: both 6-hydroxydopmaine and rotenone are direct neurotoxins causing neuronal death from within the cell through oxidative stress, while LPS and Poly I:C cause inflammation-driven neurodegeneration secondary to microglial activation. Thus selecting neurotoxins with different inductive/degenerative mechanisms allowed for assessment of CB2receptor expression where inflammation plays a greater or lesser role in the neurodegenerative process. We found that all neurotoxins, whether direct or indirect, were associated with pronounced upregulation in CB2receptor expression in the rat striatum which peaked two weeks after the initial insult (Figure 1). However, intriguingly, the upregulation in CB2receptor expression was substantially more pronounced in response to the bacterial and viral inflammagens, LPS and Poly I:C, respectively. These data show that, similar to the human degenerating brain, the central CB2receptor undergoes dynamic changes in response to brain injury, and is profoundly upregulated when the brain’s innate immune system is engaged in the neurodegenerative process (Figure 1).
Other in vivo preclinical evidence that supports the potential of targeting the CB2receptor for anti-inflammatory and neuroprotective disease modification in Parkinson’s disease comes from pharmacological and genetic studies. Several pharmacological studies haveshown that non-selective cannabinoid drugs have anti-inflammatory and/or neuroprotective efficacy in animal models of Parkinson’s disease, and these effects are mediated by CB2receptor activation. The first such study, reported that the non-selective cannabinoid agonist, WIN55, 212-2, reduced MPTP-induced microglial marker expression in the mouse ventral midbrain, and this was blocked by the CB2antagonist, JTE-907 (Price et al., 2009). Likewise, more recent studies using inflammation-driven mouse models of Parkinson’s disease, have shown that pharmacological activation of CB2receptors can reduce LPS-induced microglial activation and protect the nigrostriatal pathway (García et al., 2011; Gómez-Gálvez et al., 2015). Several genetic studies have also revealed the functional neuroprotective capacity of the CB2receptor with deletion of CB2causing increased vulnerability to LPS-induced nigrostriatal damage (García et al., 2011), and overexpression of CB2receptor causing less susceptibility to 6-hydroxydopamine (Ternianov et al., 2012).
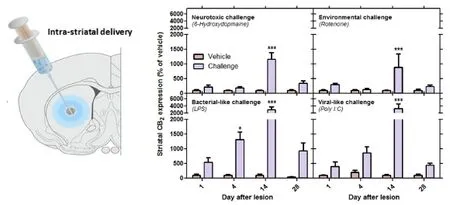
Figure 1 The response of the central CB2receptor to neurotoxic, environmental and inflammatory neurodegenerative challenges.
Thus, the emerging preclinical in vivo studies, taken together with the evidence from human post mortem reports and substantive in vitro indications, strongly suggest that the CB2receptor should be pursued as a therapeutic target to break the pathological cycle of neuroinflammation and neurodegeneration in neurodegenerative disease. Moreover, our recent studies revealing dynamic changes in CB2receptor expression in response to neurotoxic, environmental and inflammatory stimuli support the use of these models for validation of emerging cannabinoid therapies targeting the receptor. In particular, these studies have shown that the CB2receptor may play an important role in attenuating inflammation-driven neurodegeneration, and may be of particular relevance to neurodegenerative disease in which the brain’s innate immune system is engaged in the neurodegenerative process. Although reduction of neuroinflammation will probably not be sufficient to solely halt neurodegenerative disease progression, it may serve to slow progression, and by ameliorating the inflammatory milieu it may enhance the effectiveness of current and emerging restorative treatments.
Our research in this field is supported by the Irish Health Research Board (HRA_POR/2012/12).
Ruth M. Concannon#, Eilís Dowd*,#
Pharmacology & Terapeutics and Galway Neuroscience Centre, National University of Ireland, Galway, Ireland
*Correspondence to: Eilís Dowd, Ph.D., eilis.dowd@nuigalway.ie.
#Both authors contributed equally to this article.
Accepted: 2016-08-25
orcid: 0000-0001-9619-2657 (Ruth M. Concannon)
0000-0002-2668-539X (Eilís Dowd)
How to cite this article: Concannon RM, Dowd E (2016) Central CB2receptors in inflammation-driven neurodegeneration: dysregulation and therapeutic potential. Neural Regen Res 11(9):1409-1410.
References
Benito C, Núñez E, Tolón RM, Carrier EJ, Rábano A, Hillard CJ, Romero J (2003) Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci 23:11136-11141.
Blandini F (2013) Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J Neuroimmune Pharmacol. 8:189-201.
Concannon R, Finn DP, Dowd E (2015a) Cannabinoids in Parkinson’s disease. In: Cannabinoids in Neurological and Mental Disease (Fattore L, ed.), pp 35-59. London, UK: Elsevier.
Concannon RM, Okine BN, Finn DP, Dowd E (2015b) Differential upregulation of the cannabinoid CB 2 receptor in neurotoxic and inflammation-driven rat models of Parkinson’s disease. Exp Neurol 269:133-141.
Concannon RM, Okine BN, Finn DP, Dowd E (2016) Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson’s disease. Exp Neurol 283:204-212.
García C, Palomo-Garo C, García-Arencibia M, Ramos JA, Pertwee RG, Fernández-Ruiz J (2011) Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ9-THCV in animal models of Parkinson’s disease. Br J Pharmacol 163:1495-1506.
Gómez-Gálvez Y, Palomo-Garo C, Fernández-Ruiz J, García C (2015) Potential of the cannabinoid CB2 receptor as a pharmacological target against inflammation in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 64:200-208.
Mecha M, Carrillo-Salinas FJ, Feliú A, Mestre L, Guaza C (2016) Microglia activation states and cannabinoid system: Therapeutic implications. Pharmacol Ther doi:10.1016/j.pharmthera.2016.06.011.
Price DA, Martinez AA, Seillier A, Koek W, Acosta Y, Fernandez E, Strong JR, Lutz B, Marsicano G, Roberts JL (2009) WIN55, 212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the MPTP mouse model of Parkinson’s disease. Eur J Neurosci 29:2177.
Ternianov A, Pérez-Ortiz JM, Solesio ME, García-Gutiérrez MS, Ortega-Álvaro A, Navarrete F, Leiva C, Galindo MF, Manzanares J (2012) Overexpression of CB2 cannabinoid receptors results in neuroprotection against behavioral and neurochemical alterations induced by intracaudate administration of 6-hydroxydopamine. Neurobiol Aging 33:421.e1-16.
10.4103/1673-5374.191208
杂志排行
中国神经再生研究(英文版)的其它文章
- Recovery of corticospinal tract injured by traumatic axonal injury at the subcortical white matter: a case report
- Galanin and its receptor system promote the repair of injured sciatic nerves in diabetic rats
- Stem Cell Ophthalmology Treatment Study (SCOTS):improvement in serpiginous choroidopathy following autologous bone marrow derived stem cell treatment
- Changes in microtubule-associated protein tau during peripheral nerve injury and regeneration
- Does crossover innervation really affect the clinical outcome? A comparison of outcome between unilateral and bilateral digital nerve repair
- Effects of triptolide on hippocampal microglial cells and astrocytes in the APP/PS1 double transgenic mouse model of Alzheimer's disease