Galanin and its receptor system promote the repair of injured sciatic nerves in diabetic rats
2016-12-01XiaofengXuDandanZhangJinchiLiaoLiXiaoQingWangWeiQiuTheThirdAffiliatedHospitalofSunYatsenUniversityGuangzhouGuangdongProvinceChinaSouthChinaBotanicalGardenChineseAcademyofSciencesGuangzhouGuangdongProvinceChina
Xiao-feng Xu, Dan-dan Zhang, Jin-chi Liao Li Xiao Qing Wang Wei Qiu The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong Province, China South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, Guangdong Province, China
Galanin and its receptor system promote the repair of injured sciatic nerves in diabetic rats
Xiao-feng Xu1,*, Dan-dan Zhang2, Jin-chi Liao1, Li Xiao1, Qing Wang1, Wei Qiu1
1 The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong Province, China
2 South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, Guangdong Province, China
How to cite this article: Xu XF, Zhang DD, Liao JC, Xiao L, Wang Q, Qiu W (2016) Galanin and its receptor system promote the repair of injured sciatic nerves in diabetic rats. Neural Regen Res 11(9):1517-1526.
Funding: This work was supported by the National Natural Science Foundation of China, No. 31440047; the Natural Science Foundation of Guangdong Province in China, No. 2015A030310152.
Xiao-feng Xu, M.D., Ph.D.,
xuxf5@mail.sysu.edu.cn.
orcid:
0000-0002-8413-3278
(Xiao-feng Xu)
Accepted: 2016-08-15
Graphical Abstract
Various studies have reported that galanin can promote axonal regeneration of dorsal root ganglion neurons in vitro and inhibit neuropathic pain. However, little is known about its effects on diabetic peripheral neuropathy, and in vivo experimental data are lacking. We hypothesized that repeated applications of exogenous galanin over an extended time frame may also repair nerve damage in diabetic peripheral neuropathy, and relieve pain in vivo. We found that neuropathic pain occurred in streptozotocin-induced diabetic rats and was more severe after sciatic nerve pinch injury at 14 and 28 days than in diabetic sham-operated rats. Treatment with exogenous galanin alleviated the neuropathic pain and promoted sciatic nerve regeneration more effectively in diabetic rats than in non-diabetic rats after sciatic nerve pinch injury. This was accompanied by changes in the levels of endogenous galanin, and its receptors galanin receptor 1 and galanin receptor 2 in the dorsal root ganglia and the spinal dorsal horn when compared with nerve pinch normal rats. Our results show that application of exogenous galanin daily for 28 days can promote the regeneration of injured sciatic nerves, and alleviate neuropathic pain in diabetic rats.
nerve regeneration; peripheral nerve injury; diabetes; sciatic nerve; galanin; galanin receptor 1; galanin receptor 2; neuropathic pain; dorsal root ganglion; spinal dorsal horn; neural regeneration
Introduction
Galanin (Gal) is a neuropeptide expressed in less than 5% of dorsal root ganglion (DRG) neurons and interneurons in the spinal dorsal horn (SDH). After peripheral nerve injury, Gal expression increases rapidly and markedly in DRG and SDH neurons (Hokfelt et al., 1987; Villar et al., 1989), implying that Gal may be associated with pain modulation and nerve regeneration. Many studies have shown that Gal plays an important role in modulating nociception and protecting neurons from damage in the central and peripheral nervous systems (Suarez et al., 2006; Hulse et al., 2011; Cordero-Llana et al., 2014). Galanin receptors 1 and 2 (GalR1 and GalR2) are highly expressed in DRG and SDH neurons (Brumovsky et al., 2006; Shi et al., 2006) and mediate the important biological roles of Gal (Landry et al., 2005; Pope et al., 2010).
Diabetic neuropathy is the most common clinical peripheralneuropathy and DRG neurons injury may be a causative factor (Shi et al., 2013; Menichella et al., 2014). SDH neurons are important mediators of nociceptive signals, and the hyperactivity of these neurons is critical in diabetic neuropathy (Wang et al., 2011). However, the mechanisms underlying nerve dysfunction and neuropathic pain in diabetic neuropathy have not been delineated.
Experimental models of diabetic neuropathy can spur the development of novel therapies by providing an understanding of how diabetes alters sensory processing. Sciatic nerve pinch injury is a reliable animal model for evaluating pain behavior and nerve regeneration (Hirose et al., 2010). Previous studies, such as ours (Xu et al., 2012a), have focused on pain regulation by Gal over short time frames and neglected the potential effect of Gal as a neurotrophic factor. Gal has been confirmed to promote the axonal regeneration of DRG neurons in vitro (Mahoney et al., 2003), but not in vivo, experiments especially in diabetic neuropathy. Therefore, whether extended exogenous Gal treatment can improve the regeneration of peripheral nerves and regulate diabetic pain in vivo, needs investigation. The present study explored the effect of Gal on neuropathic pain and nerve regeneration after sciatic nerve pinch injury in streptozotocin (STZ)-induced diabetic rats. The expression of Gal and its receptors (GalR1, GalR2) in the DRG and SDH was also investigated.
Materials and Methods
Establishment of animal models of diabetic neuropathic pain and group assignment
We used 96 healthy specific-pathogen-free adult male Wistar rats (200—250 g) taken from a breeding colony maintained at the Experimental Animal Center at Sun Yat-sen University of China (license No. SCXT (Yue) 2011-0029). All procedures were reviewed by, and had the prior approval of, the Ethical Committee for Animal Experimentation of the Sun Yat-sen University. All surgery was performed under anesthesia, and all efforts were made to minimize suffering.
Half of the 96 rats were used to establish diabetic peripheral neuropathy models. Each was administered one intravenous injection of STZ (Sigma, St. Louis, MO, USA; 55 mg/kg freshly dissolved in 0.1 M citric acid buffer, pH 4.5) (Freeman et al., 2016). The blood sugar levels, body weights, and behaviors of these rats were monitored. The diabetic rats included in this study had to meet the two following criteria: blood sugar ≥ 20 mM after STZ injection and maintained until the end of the experiment, and significant neuropathic pain at 12 weeks after STZ injection (tactile threshold ≤ 5 g, thermal threshold ≤ 10 seconds, see Section Evaluation of tactile allodynia and thermal hyperalgesia).
Twelve weeks after injection of STZ, 48 diabetic rats were divided randomly into three different groups as follows: diabetic pinch + Gal (DP + Gal) (n = 16), diabetic rats with sciatic nerve pinch injury (see section Establishment of rat models of sciatic nerve pinch injury pain) and treated with Gal (3 μg/d, intrathecal injection) (Tocris Bioscience, Bristol, UK); DP (n = 16), diabetic rats with sciatic nerve pinch injury and intrathecally treated with vehicle solution; and diabetic sham (DS) (n = 16), sham-operated diabetic rats intrathecally treated with vehicle solution.
Forty-eight normal rats of the same age were also divided randomly into three different groups as follows: normal pinch + Gal (NP + Gal) (n = 16), non-diabetic rats with sciatic nerve pinch injury and treated with Gal (3 μg/d, intrathecally); NP (n = 16), non-diabetic rats with sciatic nerve pinch injury intrathecally treated with vehicle solution; and controls (n = 16), sham-operated normal rats intrathecally treated with vehicle solution.
At 14 and 28 days after left sciatic nerve pinch injury, eight rats were sacrificed and the corresponding DRG, SDH, and sciatic nerve tissue was collected.
Catheter implantation and intrathecal drug administration
Prior to intrathecal injection of Gal, all rats were anesthetized with 10% chloral hydrate (300 mg/kg) intraperitoneally. A sterile polyethylene catheter (PE-10, 15 cm length) (Instech Laboratories Incorporation, Plymouth Meeting, PA, USA) was inserted into the subarachnoid space through an incision in the gap between the sixth lumbar (L6) and first sacral (S1) vertebrae. The tip of the catheter was implanted between the L4and L5DRGs (Wu et al., 2004).
Gal was dissolved in artificial cerebrospinal fluid at 0.3 μg/min. The composition of artificial cerebrospinal fluid (pH 7.4) was as follows (mM): NaCl, 138.6; KCl, 3.35; CaCl2·2H2O, 1.26; MgCl2·6H2O, 1.16; NaH2PO4·2H2O, 0.58; NaHCO3, 21.0; and glucose, 10.0.
Establishment of rat models of sciatic nerve pinch injury pain
Sixty-four rats (32 diabetic rats and 32 normal rats) were divided into DP + Gal, DP, NP + Gal, and NP groups and used to create a sciatic nerve injury model. Sciatic nerve pinch injury was performed similarly to a previously described method (Hirose et al., 2010). Briefly, the rats were anesthetized with 10% chloral hydrate after baseline pain behavior tests were completed. The left sciatic nerve was exposed and pinched for 3 seconds with a microsurgical clamp (0.3-mm tip) at the point where the nerve crosses the adductor brevis muscle. The other 32 rats (16 diabetic rats and 16 normal rats) were divided into DS and control groups, and were used to create sham-operated models. The surgical procedure was identical except that the nerve pinch injury was not performed.
Real-time polymerase chain reaction (PCR) analysis of Gal, GalR1, and GalR2 mRNA expression
Anesthetized rats were sacrificed by decapitation (14 or 28 days after sciatic nerve pinch injury or sham operation) and tissue (DRG and SDH tissue) collected. The mRNA levels of Gal, GalR1, and GalR2 in L4—5DRGs and the corresponding SDH on the left side were analyzed by real time-PCR. Total RNA was isolated using TRIzol (Invitrogen, Grand Island, NY, USA) and cDNA was synthesized using a RevertAid First Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)mRNA expression was also determined as an internal control. The synthetic oligonucleotide primer sequences are shown in Table 1.
Real-time PCR was performed using Maxima SYBR Green qPCR Master Mix (2×) (Fermentas, Vilnius, Lithuania) and a Realplex PCR system (Eppendorf, Hamburg, Germany). The PCR cycle conditions were as follows: activation at 95°C for 10 minutes, followed by 40 cycles of amplification and quantification at 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. A comparative cycle of threshold fluorescence (Ct) method was used, and the relative transcript amount of the target gene was normalized to that of GAPDH using the 2—ΔΔCtmethod (Livak and Schmittgen, 2001).
Western blot assay of Gal, GalR1, and GalR2
The levels of Gal, GalR1, and GalR2 in L4—5DRG and the corresponding SDH on the left side were analyzed by western blot assay (14 and 28 days after sciatic nerve pinch injury or sham operation). The tissue was homogenized in 10 mM Tris homogenization buffer (pH 7.4) with protease inhibitors. After protein concentrations of the samples were measured, 50 mg protein of each sample was electrophoresed using a 10% sodium dodecyl sulphate gel. Proteins were transferred to a nitrocellulose membrane for immunoblotting. Following blocking in 5% nonfat milk blocking buffer for 2 hours at room temperature, the membranes were incubated overnight at 4°C with the primary antibody goat anti-Gal polyclonal antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), goat anti-GalR1 polyclonal antibody (1:500; Santa Cruz Biotechnology), goat anti-GalR2 polyclonal antibody (1:500; Santa Cruz Biotechnology), or mouse anti-β-actin monoclonal antibody (1:1,000; Santa Cruz Biotechnology). The membranes were then washed with a commercial washing solution (Beyotime Biotechnology, Nantong, Jiangsu Province, China), and incubated with the secondary antibody rabbit anti-goat IgG-horseradish peroxidase (1:4,000; Santa Cruz Biotechnology) or goat anti-mouse IgG-horseradish peroxidase (1:4,000; Santa Cruz Biotechnology). The immunoreactive bands were visualized on light-sensitive film using an enhanced chemiluminescence western blotting detection kit (Millipore, Billerica, MA, USA). The films were scanned and grayscale values of the bands were analyzed quantitatively using ImagJ 1.39u image analysis software (National Institutes of Health, Bethesda, MD, USA). The levels of Gal, GalR1, and GalR2 were normalized to β-actin.
Evaluation of tactile allodynia and thermal hyperalgesia
This experiment had two periods of behavioral tests: after STZ injury and before sciatic nerve pinch injury operation or sham operation (for confirming the successful model of diabetic peripheral neuropathy, data not shown) and after sciatic nerve pinch injury operation or sham operation until the end of this experiment (for assessing the effect of exogenous Gal). The left hind paw threshold responses to tactile stimuli were measured using von Frey filaments (BME-403; Chinese Academy of Medical Sciences Institute of Biomedical Engineering, China). The threshold responses to thermal stimuli were measured using a plantar analgesia tester (BME-400C; Chinese Academy of Medical Sciences Institute of Biomedical Engineering). The procedures and calculations were performed as detailed previously (Zhang et al., 2012).
Briefly, tactile allodynia was determined using von Frey filaments applied to the plantar region of the hind paw. Rats were acclimated in clear plastic chambers with a stainless steel wire grid floor individually for at least 15 minutes. Rapid withdrawal or flinching was recorded as a positive response by applying a series of filaments (0.1, 0.5, 1.0, 3.0, 3.8, 5.0, 8.2, and 14.6 g) to the plantar region of the hind paw until the filament bent slightly. Filaments were applied in ascending order for 3 seconds, with an interval of 5 seconds between each. Left hind paws were tested twice to determine an average score. Scoring was cut off at a force of 14.6 g to prevent hind paw injury. The median 50% withdrawal threshold was interpolated according to the sequence of positive and negative scores.
Thermal hyperalgesia was determined as the left paw latency to withdrawal from a heat source radiating a 10-W light beam. Rats were placed individually in transparent plastic chambers. The bottom of the chamber was a thin glass platform that conducted heat from a source below the cage to stimulate the plantar region of the hind paw. The room temperature was maintained at 25°C. The beam source was set with a cut-off time of 20 seconds to avoid tissue injury. Six measurements were taken at intervals of at least 1 minute. Four values were used to calculate the mean paw withdrawal latency after excluding the minimal and maximal values.
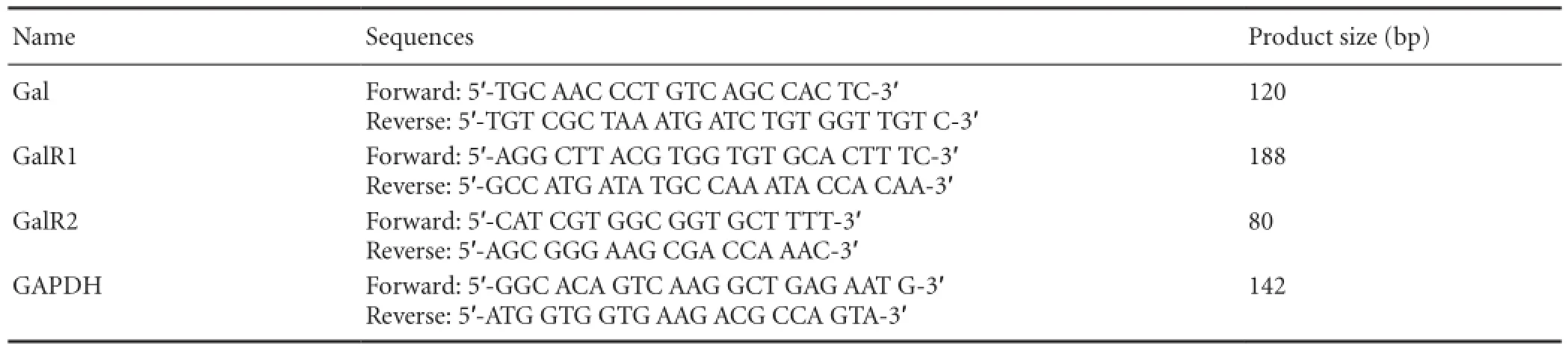
Table 1 Synthetic oligonucleotide primer sequences
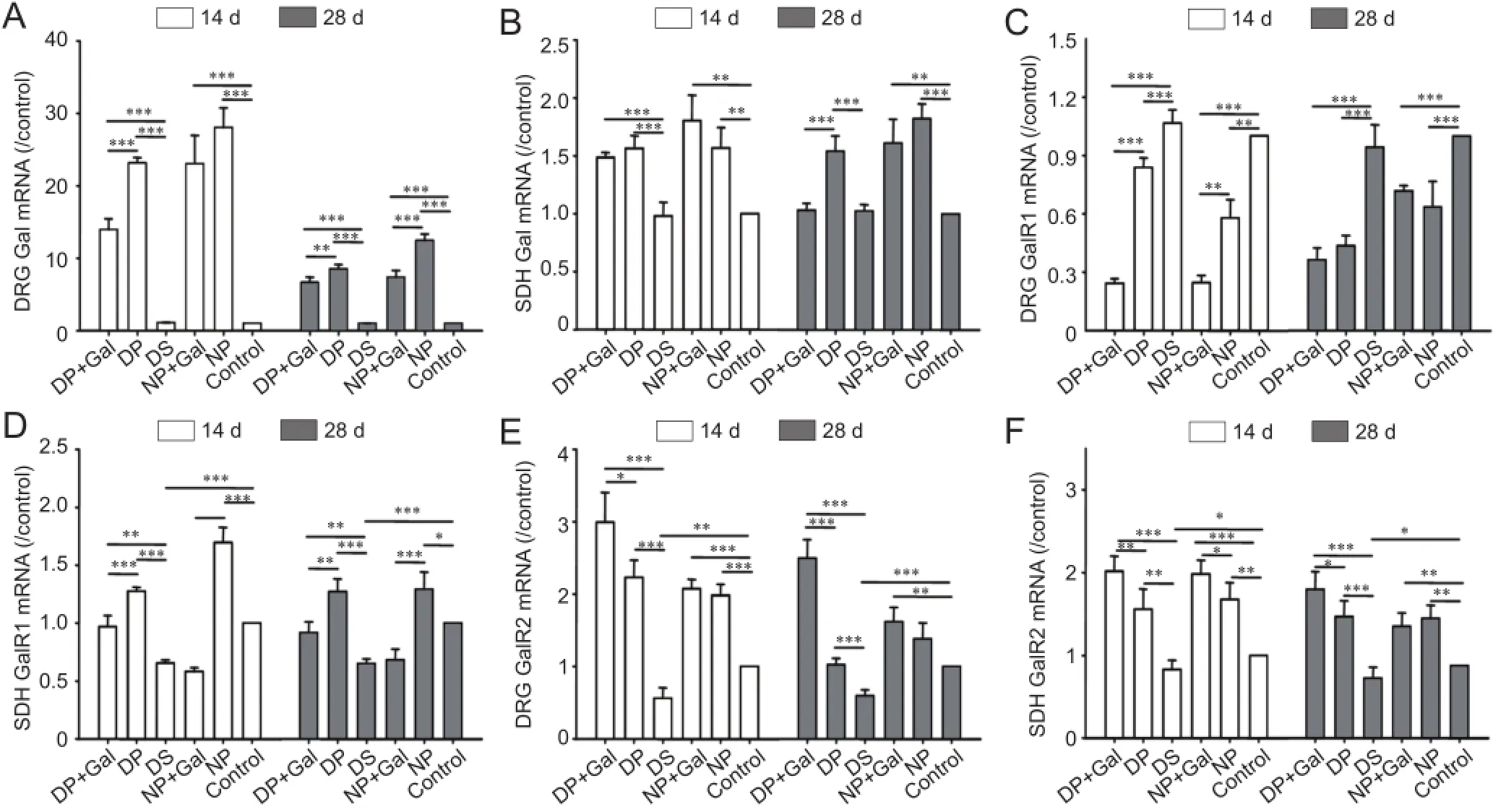
Figure 1 Gal, GalR1, and GalR2 mRNA levels in the DRG and SDH under different experimental conditions on days 14 and 28 after sciatic nerve pinch injury.
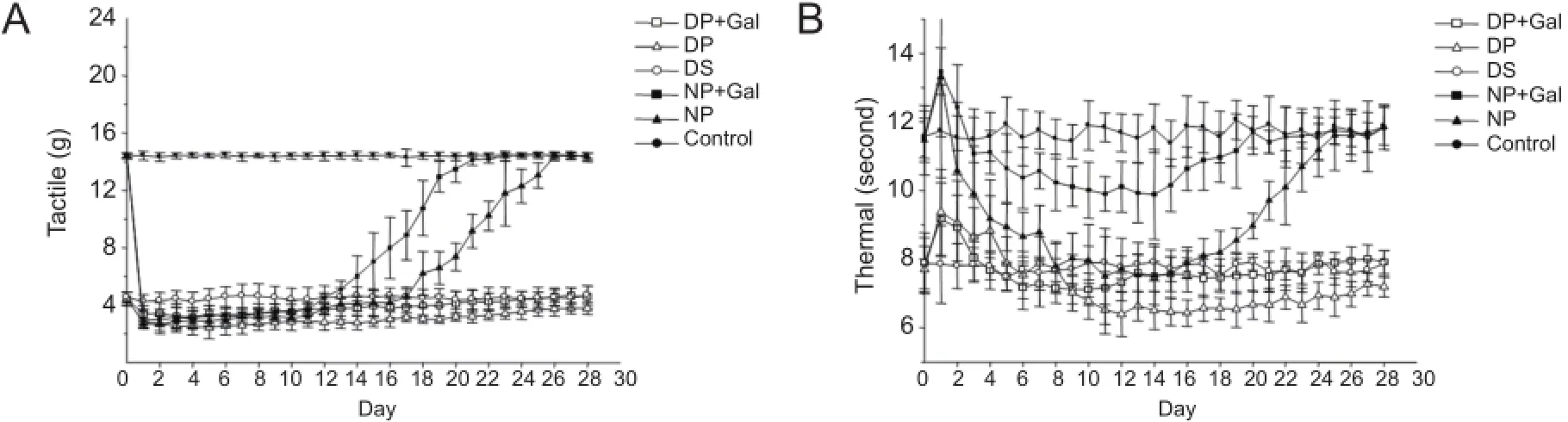
Figure 3 Tactile allodynia (A) and thermal hyperalgesia (B) in rats under different treatment conditions after sciatic nerve pinch injury or sham operation from day 0 (the basal threshold before pinch injury or sham operation) to day 28.
Determination of nerve conduction velocity (NCV)
Both motor nerve conduction velocity (MNCV) and sensory nerve conduction velocity (SNCV) of the sciatic nerves were determined using a Keypoint electromyography/evoked potentials system (Dantec, Copenhagen, Denmark) at 14 and 28 days after sciatic nerve pinch injury or sham operation. Sciatic nerve conduction was assessed by stimulating proximally at the sciatic notch, which is almost 0.7 cm above the pinch point, and distally at the Achilles tendon with bipolar electrodes as described previously (Xu et al., 2012b). Briefly, the latencies of M-wave (compound muscle action potential) and H-reflex, representing the motor and sensory conduction time respectively, were recorded. Nerve conduction velocity (recorded as meters per second) was derived from the distance between the two stimulation points divided by the difference in the latency.
Morphological observation
When the experimental procedures were complete, sciatic nerve explants (2 mm distal to the pinch injury point) were fixed in a 4% paraformaldehyde/3% glutaraldehyde solution for 30 minutes at 4°C. The samples were post-fixed in 1% osmium tetroxide in 0.1 M phosphate buffer for 2 hours at 4°C and embedded in Epon for 72 hours at 60°C. Transverse semi-thin sections (1 μm) of sciatic nerve were stained with 0.5% toluidine blue and observed under a light microscope (Nikon Eclipse 80i, Japan) to detect morphological changes. To enable ultrastructural observations to be made, ultrathin sections (70 nm) of sciatic nerve were processed for electron microscopy, negatively stained with uranyl acetate and lead citrate, and examined using a JEM-1400 transmission electron microscope (JEOL, Akishima, Tokyo, Japan).
Statistical analysis
The data were analyzed using SPSS 19.0 software (IBM, Armonk, NY, USA) and expressed as the mean ± SD. One-way analysis of variance followed by the Student-Newman-Keuls test for significance was used to compare the differences between various groups. P < 0.05 was considered statistically significant.
Results
Blood glucose levels, body weights, and neuropathic pain in diabetic models
Rats treated with STZ (55 mg/kg, intravenously) developed hyperglycemia within 1 week, and their serum glucose concentrations were consistently higher than the vehicle-treated rats (12 weeks after STZ injection, diabetes vs. control: 25.03 ± 2.06 mM vs. 6.07 ± 1.25 mM, P < 0.05). The weights of diabetic rats were significantly lower than those of vehicle-treated rats during the 12 weeks of observation (12 weeks after STZ injection, diabetes vs. control: 325.15 ± 18.10 g vs. 475.67 ± 19.44 g, P < 0.05). The diabetic rats had a significant decrease in nociceptive threshold. Paw withdrawal from tactile stimulation threshold and thermal stimulation significantly decreased 4 weeks after STZ injection and persisted for the entire study (12 weeks after STZ injection, tactile diabetes vs. control: 4.53 ± 0.68 g vs. 14.36 ± 0.23 g, P < 0.05; thermal diabetes vs. control: 7.79 ± 0.40 seconds vs. 11.60 ± 0.86 seconds, P < 0.05).
Gal, GalR1, and GalR2 mRNA expression in the DRG and SDH
The Gal mRNA levels in the DRG and SDH of normal or diabetic rats under different experimental conditions on days 14 and 28 after sciatic nerve pinch injury are shown in Figure 1A, B. Gal mRNA expression was dramatically upregulated after sciatic nerve pinch injury in the DRG and SDH of diabetic and normal rats (P < 0.001). However, this effect was smaller in diabetic rats than in normal rats (P < 0.05). Exogenous Gal treatment reduced endogenous Gal mRNA expression in the DRG after sciatic nerve pinch injury (P < 0.01). This phenomenon was not apparent in the SDH, and was only observed in diabetic rats on day 28.
The GalR1 mRNA levels in the DRG and SDH of normal or diabetic rats under different experimental conditions on days 14 and 28 after sciatic nerve pinch injury are shown in Figure 1C, D. GalR1 mRNA expression was downregulated in the SDH of diabetic rats (P < 0.01). After sciatic nerve pinch injury, GalR1 mRNA expression was downregulated in the DRG and upregulated in the SDH in both normal and diabetic rats (P < 0.01). Exogenous Gal treatment reduced GalR1 mRNA expression in both the DRG and SDH (P < 0.01).
GalR2 mRNA levels in the DRG and SDH of normal or diabetic rats under different experimental conditions on days 14 and 28 after sciatic nerve pinch injury are shown in Figure 1E, F. GalR2 mRNA expression was downregulated in both the DRG and SDH of diabetic rats (P < 0.05). After sciatic nerve pinch injury, GalR2 mRNA expression was upregulated in both the DRG and SDH of normal and diabetic rats (P < 0.05). Exogenous Gal treatment increased GalR2 mRNA expression in both the DRG and SDH of diabetic rats (P < 0.05).
Gal, GalR1, and GalR2 expression in the DRG and SDH
Gal, GalR1, and GalR2 protein levels in the DRG and SDH of normal or diabetic rats under different experimental conditions injury on days 14 and 28 after sciatic nerve pinch are shown in Figure 2. The standard deviation (SD) of the western blot assay was larger than that of real-time PCR, but the trend of protein expression was consistent with the mRNA findings.
Effects of exogenous Gal expression on pain responses after sciatic nerve pinch injury
Sciatic nerve pinch injury caused severe tactile allodynia and thermal hyperalgesia in both normal and diabetic rats. The tactile threshold responses to the von Frey test in rats under different treatments are shown in Figure 3A. The tactile threshold decreased significantly from day 1 to day 25 after sciatic nerve pinch injury in normal rats (P < 0.05). Gal treatment increased the tactile threshold significantly between days 12 and 25 after sciatic nerve pinch injury in normal rats (P < 0.05). The tactile threshold decreased significantly in sham-operated diabetic rats compared with sham-operated normal rats (P < 0.05). Sciatic nerve pinch injury caused more severe decreases in the tactile threshold in diabetic rats (P < 0.05). Gal treatment increased the tactile threshold significantly between days 4 and 28 after sciatic nerve pinch injury in diabetic rats (P < 0.05).
The thermal threshold responses in rats under different treatments are shown in Figure 3B. The thermal threshold decreased significantly between days 3 and 23 after sciatic nerve pinch injury in normal rats (P < 0.05). Gal treatment increased the thermal threshold significantly between days 4 and 23 after sciatic nerve pinch injury in normal rats (P < 0.05). The thermal threshold decreased significantly in sham-operated diabetic rats compared with sham-operated normal rats (P < 0.05). Sciatic nerve pinch injury induced more severe decreases in the thermal threshold in diabetic rats (P < 0.05). Gal treatment increased the thermal threshold significantly between days 12 and 28 after sciatic nerve pinch injury in diabetic rats (P < 0.05).
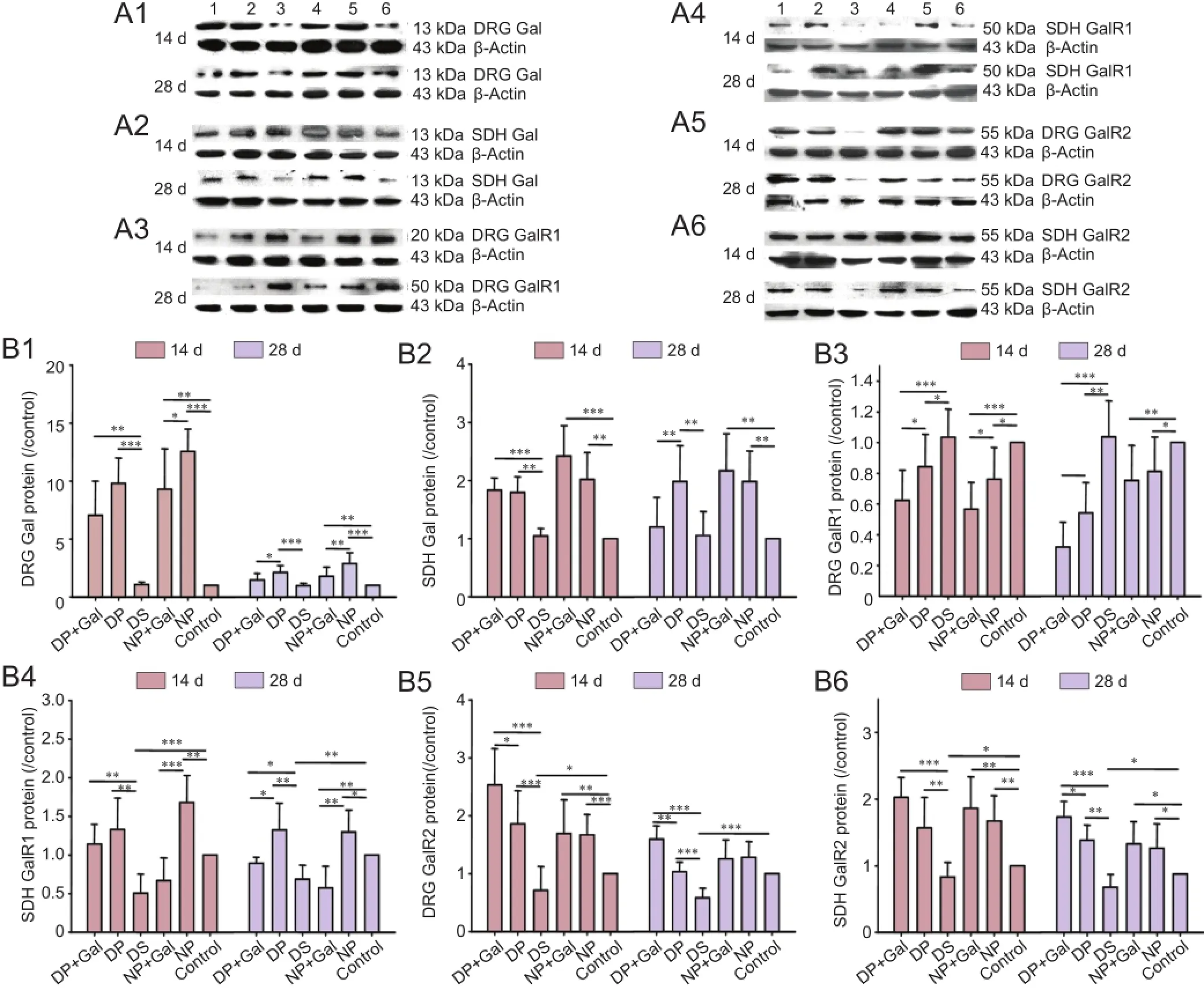
Figure 2 Gal, GalR1, and GalR2 protein expression levels in the DRG and SDH under different experimental conditions on days 14 and 28 after sciatic nerve pinch injury.
Effects of exogenous Gal on NCV after sciatic nerve pinch injury
MNCV and SNCV decreased significantly after sciatic nerve pinch injury on days 14 and 28 in normal rats compared with sham-operated control normal animals (P < 0.001). Gal administration improved MNCV and SNCV significantly after sciatic nerve pinch injury on days 14 and 28 in normal rats compared with sciatic nerve pinch normal rats without Gal treatment (P < 0.05) (Figure 4).
MNCV and SNCV decreased significantly in diabetic rats as compared with sham-operated control normal animals (P < 0.05). Sciatic nerve pinch injury in diabetic rats severely decreased MNCV and SNCV on days 14 and 28 compared with sham-operated diabetic rats (P < 0.01). Gal treatment increased both MNCV and SNCV at days 14 and 28 in sciatic nerve pinch diabetic rats compared with sciatic nerve pinch diabetic rats without Gal treatment (P < 0.05; Figure 4).
Sciatic nerve fiber alterations with toluidine blue staining On day 14 following sciatic nerve pinch injury, most of the fibers in normal rats were undergoing some degree of dystrophy and the internal structure of the nerve was severely disorganized. The fibers recovered by day 28 after sciatic nerve pinch injury. The dystrophy decreased on day 14 after sciatic nerve pinch injury in normal rats with Gal treatment. Most of the fibers recovered to almost normal status on day 28 after sciatic nerve pinch injury in normal rats with Gal treatment. Axons with regular shape and arrangement were seen in normalcontrol rats following sham operations (Figure 5).
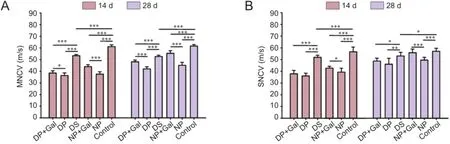
Figure 4 MNCV (A) and SNCV (B) of rat sciatic nerves under the different treatment conditions.
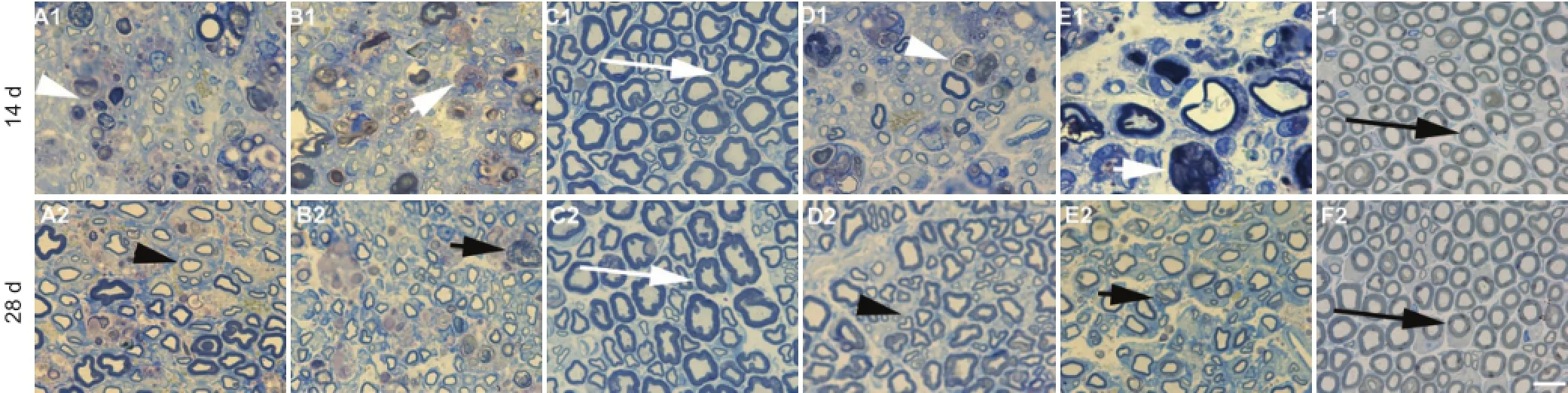
Figure 5 Semi-thin cross sections of sciatic nerves under different treatment conditions (toluidine blue staining, optical microscope).
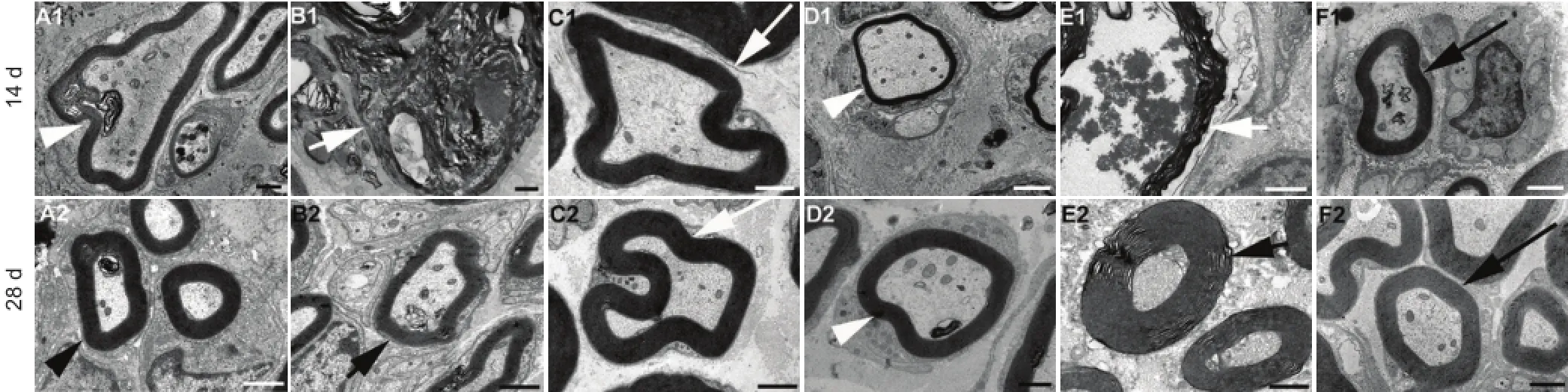
Figure 6 Transmission electron microscope micrographs of sciatic nerves under different treatment conditions (osmium negative staining, transmission electron microscopy) on days 14 and 28 following sciatic nerve pinch injury.
The shape of myelin became irregular in diabetic rats. The dystrophy was more severe after sciatic nerve pinch injury on days 14 and 28 in diabetic rats compared with normal rats. The dystrophy was less severe after sciatic nerve pinch injury on day 14 in diabetic rats with Gal treatment compared with sciatic nerve pinch diabetic rats. Most of the fibers recovered partially, with irregular shape, on day 28 following sciatic nerve pinch injury in Gal treated diabetic rats (Figure 5).
Sciatic nerve ultrastructural alterations
In normal rats myelinated axons degenerated to myelin debris and most of the axoplasm was lost at day 14 after sciatic nerve pinch injury. The myelin and axoplasm were partially recovered on day 28. There was no obvious axoplasm loss and myelin breakdown on day 14 after sciatic nerve pinch injury in Gal treated normal rats , and the myelin and axoplasm recovered at day 28. Axons with normal myelin and axoplasm were observed in sham operated normal control rats (Figure 6).
Irregularly shaped myelin was seen in diabetic rats. The myelin had completely broken down to myelin debris and the axoplasm disappeared completely on day 14 after sciatic nerve pinch injury in diabetic rats. The myelinated axons with irregular shape were regenerating on day 28 after sciatic nerve pinch injury in diabetic rats, and on day 14 in Gal treated diabetic rats. Myelinated axons with regular shape and normal myelin were seen on day 28 after sciatic nerve pinch injury in Gal treated diabetic rats (Figure 6).
Discussion
Diabetic neuropathy is characterized by a slowing of conduction velocity and axonal atrophy with neuropathic pain (Zochodne et al., 2004). In this study, we found that neuropathic pain was more severe in sciatic nerve pinch diabetic rats that were in a stable pain hypersensitiveness stage 12 weeks after the induction of diabetes. Significant improvements in hyperalgesia and nerve regeneration were observed in sciatic nerve pinch diabetic rats treated with exogenous Gal. These phenomena were accompanied by alterations in the expression of endogenous Gal and its receptors (GalR1 and GalR2) in the DRG and SDH. This plasticity may be involved in diabetic neuropathy.
Gal is only expressed at low levels in a small group of neurons in the DRG and SDH under normal conditions but expression significantly increases following nerve injury (Hokfelt et al., 1987; Villar et al., 1989). In this study, Gal expression was not obviously changed in STZ-induced diabetic rats, suggesting that complex mechanisms exist to adjust endogenous Gal expression after the induction of diabetes. Low levels of endogenous Gal expression were observed in diabetic rats after sciatic nerve pinch injury, suggesting that insufficient endogenous Gal might correspond with more severe and longer neuropathic pain and delayed nerve regeneration in diabetic rats. The exact molecular mechanisms of this phenomenon need to be elucidated, but the current data clearly shows that DRG and SDH Gal levels are suppressed in diabetic neuropathy, regardless of any additional injury. In this study, Gal was up-regulated in the DRG and SDH on day 28 after nerve injury in diabetic rats, and this upregulation was decreased by exogenous Gal administration. The decrease in endogenous Gal expression induced by intrathecal injection of exogenous Gal may be due to two possible reasons: degenerative feedback, which is widespread in gene expression regulation, and better neuronal recovery due to the neurotrophic effect of exogenous Gal (Hobson et al., 2010).
It has been demonstrated that sciatic nerve pinch injury induces an upregulation of a neuropeptide pain marker calcitonin gene-related peptide in the DRG of rats (Hirose et al., 2010). The release of neuropeptide pain markers in the spinal cord can be prevented by intrathecal injection of Gal (Hua et al., 2005). Gal has a moderate inhibitory effect on spinal nociception under normal conditions, whereas Gal expression changes following peripheral nerve injury, and plays a critical regulatory role in nociception in neuropathic states both in the DRG and SDH (Xu et al., 2010). This study showed that exogenous Gal can attenuate tactile and thermal responses both in normal and diabetic nerve pinch rats. These effects of Gal may partially correlate with reduced spinal hyperexcitability through a postsynaptic blockade of the excitatory effects of substance P and calcitonin gene-related peptide released from active afferent nerve terminal fibers.
Many studies have shown that nerve regeneration after injury is impaired in diabetic animals and patients as a result of delayed Wallerian degeneration, and deficiencies of axonal sprouting, elongation and maturation (King et al., 1989; Said et al., 1992; Thomas et al., 1996; Chen et al., 2010; Ali et al., 2014). Although several mechanisms may target peripheral neurons, they produce a degenerative pattern of damage that begins in distal terminals. Moreover, sensory neurons are involved earlier than motor neurons (Zochodne et al., 2008). It is important to enhance nerve regeneration as well as to prevent nerve degeneration in the treatment of diabetic neuropathy (Yasuda et al., 2003). Experimental diabetic peripheral neuropathy is marked by impaired NCV in STZ-injected diabetic rats (Srinivasan et al., 2000; Stevens et al., 2000). In this study, the neurotrophic effect of Gal was confirmed by NCV evaluation and morphological observation. Exogenous Gal administration improved NCV and the morphology of the sciatic nerve after pinch injury in both diabetic and normal animals. Gal plays an important role in nerve regeneration, and thereby may be implicated in the generation of pain sensation. The neurotrophic effects of Gal, particularly the long term effects, are important and could lessen nerve damage in diabetic rats, and thus reduce neuropathic pain.
In addition to altered Gal expression under different experimental conditions, changes in the expression of Gal receptors in the DRG and SDH may play important roles after nerve injury. We found that GalR1 was downregulated in the SDH of diabetic rats and further decreased after sciatic nerve pinch injury. This phenomenon was more obvious when exogenous Gal was applied, unlike in the DRG where GalR1 was downregulated after sciatic nerve pinch injury and was further depressed by exogenous Gal application. Based on the observation that GalR1 activation produces hyperpolarization via Gi/o and inhibits adenylyl cyclase (Lang et al., 2007; Kongand Yu, 2013) GalR1 expression in the DRG and SDH might inhibit pre- or/and post-synaptic abnormal nerve excitability in diabetes. GalR1 expression in diabetic rats was lower than that in normal rats after sciatic nerve pinch injury. This may be another explanation for the more severe pain seen in diabetic rats. We found that although exogenous Gal application further reduced GalR1 levels in the DRG and SDH, diabetic neuropathic pain could still be effectively reduced by daily intrathecal injection of Gal. GalR1 is still a valid treatment target for diabetic neuropathic pain.
Many studies have shown that Gal plays an important anti-apoptotic role and promotes axonal growth through the activation of GalR2 in multiple neurons, including in the DRG, in various types of neuropathy, such as mechanical, degenerative, and metabolic injuries (Xu et al., 2012b; Li et al., 2013; Weyne et al., 2014; Barreda-Gomez et al., 2015). Our current data show that exogenous Gal administration improves neuropathic pain after nerve pinch injury in both diabetic and normal animals. Daily injections of Gal daily should be valued as a treatment that can lessen nerve damage in diabetic rats through GalR2 and reduce neuropathic pain. A recent study found that Gal stimulates large-conductance Ca2+-activated K+(BK) channels via GalR2 in human embryonic kidney cells (Pan et al., 2014). BK channels are distributed in DRG and SDH neurons and can reduce neuronal excitability via K+outward flow (Furukawa et al., 2008; Zhang et al., 2010). Thus, the analgesic effect of Gal on diabetic neuropathic pain functions partly by activating BK channels through GalR2. We found that GalR2 levels were significantly upregulated in DRG and SDH in both diabetic and normal rats after sciatic nerve pinch injury. This phenomenon was more obvious in diabetic rats when exogenous Gal was applied daily. Although a minor reduction in GalR2 expression was found in diabetic rats in our previous study (Xu et al., 2012a), the upregulation of GalR2 in this study was not significantly different between diabetic and normal rats after nerve injury, unlike that of Gal and GalR1. This might indicate that the upregulation of GalR2 expression is an important and basic mechanism after nerve injury, and is not seriously disrupted by diabetic neuropathy. The increase in GalR2 corresponds to Gal upregulation, which seems to be a reactive regeneration mechanism after nerve injury. Exogenous Gal application could increase the expression of GalR2 in DRG and SDH neurons in diabetic rats, which may relate to the better status of these neurons due to the nutritive effects of Gal. In this study, we also observed the very interesting phenomenon that GalR2 level is significant higher in diabetic rats than normal rats after sciatic nerve pinch injury with daily exogenous Gal application. Though the causes of this phenomenon and its mechanisms are still unclear, the upregulation of GalR2 enhances the neurotrophic effects of Gal.
Although our results are based on an animal model, early research suggests that Gal is involved in glucose metabolism and diabetes in humans. Plasma galanin concentrations are significantly lower in type 1 diabetes patients with autonomic neuropathy than in those without (Sundkvist et al., 1992). However, significantly higher plasma Gal concentrations have been detected in type 2 diabetes patients, women with gestational diabetes, and healthy persons during an oral glucose tolerance test (Legakis et al., 2005; Legakis et al., 2007; Zhang et al., 2014). Whether the varying levels of plasma Gal in humans is a cause or a result of diabetes is still unclear. Many studies have shown that Gal may improve insulin resistance and carbohydrate utilization in diabetes by regulating glucose transporter 4 (Zhang et al., 2012; Bu et al., 2014; Fang et al., 2015). As it remains poorly understood whether glucose metabolism in peripheral neurons is influenced by Gal, thereby improving nerve function, this area needs further study. Our present study provides data that may help determine the therapeutic effect of Gal in human diabetic peripheral neuropathy.
In conclusion, Gal and its receptor signaling system are involved in diabetic neuropathy. The expression of endogenous Gal and its receptors (GalR1 and GalR2) in the DRG and SDH showed great plasticity in diabetic rats with sciatic nerve pinch injury. Exogenous Gal treatment significantly improved hyperalgesia and promoted nerve regeneration in sciatic nerve pinch diabetic rats. These findings provide a rationale and experimental evidence for the development of further investigations of Gal therapeutic strategies to alleviate diabetic neuropathy.
Author contributions: XFX, DDZ, JCL, and LX researched and compiled data, and contributed to discussion. WQ provided technical assistance and statistical analysis and reviewed and edited the paper. QW contributed to discussion, and reviewed and edited the paper. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Ali S, Driscoll HE, Newton VL, Gardiner NJ (2014) Matrix metalloproteinase-2 is downregulated in sciatic nerve by streptozotocin induced diabetes and/or treatment with minocycline: implications for nerve regeneration. Exp Neurol 261:654-665.
Barreda-Gomez G, Lombardero L, Giralt MT, Manuel I, Rodriguez-Puertas R (2015) Effects of galanin subchronic treatment on memory and muscarinic receptors. Neuroscience 293:23-34.
Brumovsky P, Hygge-Blakeman K, Villar MJ, Watanabe M, Wiesenfeld-Hallin Z, Hokfelt T (2006) Phenotyping of sensory and sympathetic ganglion neurons of a galanin-overexpressing mouse-possible implications for pain processing. J Chem Neuroanat 31:243-262.
Bu L, Yao Q, Liu Z, Tang W, Zou J, Qu S (2014) Combined galanin with insulin improves insulin sensitivity of diabetic rat muscles. J Endocrinol 221:157-165.
Chen YS, Chung SS, Chung SK (2010) Aldose reductase deficiency improves Wallerian degeneration and nerve regeneration in diabetic thy1-YFP mice. J Neuropathol Exp Neurol 69:294-305.
Cordero-Llana O, Rinaldi F, Brennan PA, Wynick D, Caldwell MA (2014) Galanin promotes neuronal differentiation from neural progenitor cells in vitro and contributes to the generation of new olfactory neurons in the adult mouse brain. Exp Neurol 256:93-104.
Fang P, Shi M, Zhu Y, Zhang Z, Bo P (2015) Central injection of GalR1 agonist M617 facilitates GLUT4 expression in cardiac muscle of type 2 diabetic rats. Exp Gerontol 65:85-89.
Freeman OJ, Unwin RD, Dowsey AW, Begley P, Ali S, Hollywood KA, Rustogi N, Petersen RS, Dunn WB, Cooper GJ, Gardiner NJ (2016) Metabolic dysfunction is restricted to the sciatic nerve in experimental diabetic neuropathy. Diabetes 65:228-238.
Furukawa N, Takasusuki T, Fukushima T, Hori Y (2008) Presynaptic large-conductance calcium-activated potassium channels control synaptic transmission in the superficial dorsal horn of the mouse. Neurosci Lett 444:79-82.
Hirose K, Iwakura N, Orita S, Yamashita M, Inoue G, Yamauchi K, Eguchi Y, Ochiai N, Kishida S, Nakamura J, Takaso M, Ishikawa T, Arai G, Miyagi M, Kamoda H, Aoki Y, Hiwatari R, Kakizaki J, Kunishi T, Kono M, Suzuki T, Toyone T, Takahashi K, Kuniyoshi K, Ohtori S (2010) Evaluation of behavior and neuropeptide markers of pain in a simple, sciatic nerve-pinch pain model in rats. Eur Spine J 19:1746-1752.
Hobson SA, Bacon A, Elliot-Hunt CR, Holmes FE, Kerr NC, Pope R, Vanderplank P, Wynick D (2010) Galanin acts as a trophic factor to the central and peripheral nervous systems. EXS 102:25-38.
Hokfelt T, Wiesenfeld-Hallin Z, Villar M, Melander T (1987) Increase of galanin-like immunoreactivity in rat dorsal root ganglion cells after peripheral axotomy. Neurosci Lett 83:217-220.
Hua XY, Salgado KF, Gu G, Fitzsimmons B, Kondo I, Bartfai T, Yaksh TL (2005) Mechanisms of antinociception of spinal galanin: how does galanin inhibit spinal sensitization? Neuropeptides 39:211-216. Hulse RP, Wynick D, Donaldson LF (2011) Activation of the galanin receptor 2 in the periphery reverses nerve injury-induced allodynia. Mol Pain 7:26.
King RH, Llewelyn JG, Thomas PK, Gilbey SG, Watkins PJ (1989) Diabetic neuropathy: abnormalities of Schwann cell and perineurial basal laminae. Implications for diabetic vasculopathy. Neuropathol Appl Neurobiol 15:339-355.
Kong Q, Yu LC (2013) Antinociceptive effects induced by intra-periaqueductal grey injection of the galanin receptor 1 agonist M617 in rats with morphine tolerance. Neurosci Lett 550:125-128.
Landry M, Liu HX, Shi TJ, Brumovsky P, Nagy F, Hokfelt T (2005) Galaninergic mechanisms at the spinal level: focus on histochemical phenotyping. Neuropeptides 39:223-231.
Lang R, Gundlach AL, Kofler B (2007) The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther 115:177-207.
Legakis I, Mantzouridis T, Mountokalakis T (2005) Positive correlation of galanin with glucose in type 2 diabetes. Diabetes Care 28:759-760.
Legakis IN, Mantzouridis T, Mountokalakis T (2007) Positive correlation of galanin with glucose in healthy volunteers during an oral glucose tolerance test. Horm Metab Res 39:53-55.
Li L, Yu L, Kong Q (2013) Exogenous galanin attenuates spatial memory impairment and decreases hippocampal beta-amyloid levels in rat model of Alzheimer’s disease. Int J Neurosci 123:759-765.
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402-408.
Mahoney SA, Hosking R, Farrant S, Holmes FE, Jacoby AS, Shine J, Iismaa TP, Scott MK, Schmidt R, Wynick D (2003) The second galanin receptor GalR2 plays a key role in neurite outgrowth from adult sensory neurons. J Neurosci 23:416-421.
Menichella DM, Abdelhak B, Ren D, Shum A, Frietag C, Miller RJ (2014) CXCR4 chemokine receptor signaling mediates pain in diabetic neuropathy. Mol Pain 10:42.
Pan NC, Bai YF, Yang Y, Hokfelt T, Xu ZQ (2014) Activation of galanin receptor 2 stimulates large conductance Ca(2+)-dependent K(+) (BK) channels through the IP3 pathway in human embryonic kidney (HEK293) cells. Biochem Biophys Res Commun 446:316-321.
Pope RJ, Holmes FE, Kerr NC, Wynick D (2010) Characterisation of the nociceptive phenotype of suppressible galanin overexpressing transgenic mice. Mol Pain 6:67.
Said G, Goulon-Goeau C, Slama G, Tchobroutsky G (1992) Severe early-onset polyneuropathy in insulin-dependent diabetes mellitus. A clinical and pathological study. N Engl J Med 326:1257-1263.
Shi TJ, Hua XY, Lu X, Malkmus S, Kinney J, Holmberg K, Wirz S, Ceccatelli S, Yaksh T, Bartfai T, Hokfelt T (2006) Sensory neuronal phenotype in galanin receptor 2 knockout mice: focus on dorsal root ganglion neurone development and pain behaviour. Eur J Neurosci 23:627-636.
Shi TJ, Zhang MD, Zeberg H, Nilsson J, Grunler J, Liu SX, Xiang Q, Persson J, Fried KJ, Catrina SB, Watanabe M, Arhem P, Brismar K, Hokfelt TG (2013) Coenzyme Q10 prevents peripheral neuropathy and attenuates neuron loss in the db-/db- mouse, a type 2 diabetes model. Proc Natl Acad Sci U S A 110:690-695.
Srinivasan S, Stevens M, Wiley JW (2000) Diabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunction. Diabetes 49:1932-1938.
Stevens MJ, Obrosova I, Cao X, Van Huysen C, Greene DA (2000) Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes 49:1006-1015.
Suarez V, Guntinas-Lichius O, Streppel M, Ingorokva S, Grosheva M, Neiss WF, Angelov DN, Klimaschewski L (2006) The axotomy-induced neuropeptides galanin and pituitary adenylate cyclase-activating peptide promote axonal sprouting of primary afferent and cranial motor neurones. Eur J Neurosci 24:1555-1564.
Sundkvist G, Bramnert M, Bergström B, Manhem P, Lilja B, Ahrén B (1992) Plasma neuropeptide Y (NPY) and galanin before and during exercise in type 1 diabetic patients with autonomic dysfunction. Diabetes Res Clin Pract 15:219-226.
Thomas PK, Beamish NG, Small JR, King RH, Tesfaye S, Ward JD, Tsigos C, Young RJ, Boulton AJ (1996) Paranodal structure in diabetic sensory polyneuropathy. Acta Neuropathol 92:614-620.
Villar MJ, Cortes R, Theodorsson E, Wiesenfeld-Hallin Z, Schalling M, Fahrenkrug J, Emson PC, Hokfelt T (1989) Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin. Neuroscience 33:587-604.
Wang XL, Zhang Q, Zhang YZ, Liu YT, Dong R, Wang QJ, Guo YX. (2011) Downregulation of GABAB receptors in the spinal cord dorsal horn in diabetic neuropathy. Neurosci Lett 490:112-115.
Weyne E, Albersen M, Hannan JL, Castiglione F, Hedlund P, Verbist G, De Ridder D, Bivalacqua TJ, Van der Aa F (2014) Increased expression of the neuroregenerative peptide galanin in the major pelvic ganglion following cavernous nerve injury. J Sex Med 11:1685-1693.
Wu W, Xu X, Hao J (2004) Chronic lumbar catheterization of the spinal subarachnoid space in mice. J Neurosci Methods 133:65-69.
Xu X, Liu Z, Liu H, Yang X, Li Z (2012a) The effects of galanin on neuropathic pain in streptozotocin-induced diabetic rats. Eur J Pharmacol 680:28-33.
Xu X, Yang X, Zhang P, Chen X, Liu H, Li Z (2012b) Effects of exogenous galanin on neuropathic pain state and change of galanin and its receptors in DRG and SDH after sciatic nerve-pinch injury in rat. PLoS One 7:e37621.
Xu XJ, Hökfelt T, Wiesenfeld-Hallin Z (2010) Galanin and spinal pain mechanisms: past, present, and future. EXS 102:39-50.
Yasuda H, Terada M, Maeda K, Kogawa S, Sanada M, Haneda M, Kashiwagi A, Kikkawa R (2003) Diabetic neuropathy and nerve regeneration. Prog Neurobiol 69:229-285.
Zhang Q, Fang D, Cai J, Wan Y, Han JS, Xing GG (2012) Enhanced excitability of small dorsal root ganglion neurons in rats with bone cancer pain. Mol Pain 8:24.
Zhang XL, Mok LP, Katz EJ, Gold MS (2010) BKCa currents are enriched in a subpopulation of adult rat cutaneous nociceptive dorsal root ganglion neurons. Eur J Neurosci 31:450-462.
Zochodne DW, Ramji N, Toth C (2008) Neuronal targeting in diabetes mellitus: a story of sensory neurons and motor neurons. Neuroscientist 14:311-318.
Zhang Z, Gu C, Fang P, Shi M, Wang Y, Peng Y, Bo P, Zhu Y (2014) Endogenous galanin as a novel biomarker to predict gestational diabetes mellitus. Peptides 54:186-189.
Zhang Z, Sheng S, Guo L, Li G, Zhang L, Zhang L, Shi M, Bo P, Zhu Y (2012) Intracerebroventricular administration of galanin antagonist sustains insulin resistance in adipocytes of type 2 diabetic trained rats. Mol Cell Endocrinol 361:213-218.
Zochodne DW, Sun HS, Cheng C, Eyer J (2004) Accelerated diabetic neuropathy in axons without neurofilaments. Brain 127:2193-2200.
Copyedited by Brooks W, Norman C, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.191228
*Correspondence to:
杂志排行
中国神经再生研究(英文版)的其它文章
- Recovery of corticospinal tract injured by traumatic axonal injury at the subcortical white matter: a case report
- Stem Cell Ophthalmology Treatment Study (SCOTS):improvement in serpiginous choroidopathy following autologous bone marrow derived stem cell treatment
- Changes in microtubule-associated protein tau during peripheral nerve injury and regeneration
- Does crossover innervation really affect the clinical outcome? A comparison of outcome between unilateral and bilateral digital nerve repair
- Effects of triptolide on hippocampal microglial cells and astrocytes in the APP/PS1 double transgenic mouse model of Alzheimer's disease
- Can long-term thiamine treatment improve the clinical outcomes of myotonic dystrophy type 1?