Acellular allogeneic nerve grafting combined with bone marrow mesenchymal stem cell transplantation for the repair of long-segment sciatic nerve defects: biomechanics and validation of mathematical models
2016-12-01YajunLiBaolinZhaoHaozeLvZhigangQinMinLuoSchoolofMathematicsJilinUniversityChangchunJilinProvinceChinaDepartmentofOrthopedicsChinaJapanUnionHospitalofJilinUniversityChangchunJilinProvinceChinaDepartmentofClinicalMedi
Ya-jun Li, Bao-lin Zhao, Hao-ze Lv, Zhi-gang Qin, Min Luo School of Mathematics, Jilin University, Changchun, Jilin Province, China Department of Orthopedics, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China Department of Clinical Medicine, School of Clinical Medicine, Jilin University, Changchun, Jilin Province, China4 Department of Neurosurgery, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China Department of Pain, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
Acellular allogeneic nerve grafting combined with bone marrow mesenchymal stem cell transplantation for the repair of long-segment sciatic nerve defects: biomechanics and validation of mathematical models
Ya-jun Li1, Bao-lin Zhao2, Hao-ze Lv3, Zhi-gang Qin4,*, Min Luo5
1 School of Mathematics, Jilin University, Changchun, Jilin Province, China
2 Department of Orthopedics, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
3 Department of Clinical Medicine, School of Clinical Medicine, Jilin University, Changchun, Jilin Province, China
4 Department of Neurosurgery, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
5 Department of Pain, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
How to cite this article: Li YJ, Zhao BL, Lv HZ, Qin ZG, Luo M (2016) Acellular allogeneic nerve grafting combined with bone marrow mesenchymal stem cell transplantation for the repair of long-segment sciatic nerve defects∶ biomechanics and validation of mathematical models. Neural Regen Res 11(8)∶1322-1326.
Funding: This study was supported by the Science and Technology Development Plan Project Fund of Jilin Province in China, No. 20110492.
Zhi-gang Qin, M.D.,
lyj@jlu.edu.cn.
orcid:
0000-0002-8080-5241
(Ya-jun Li)
Accepted: 2016-05-27
Graphical Abstract
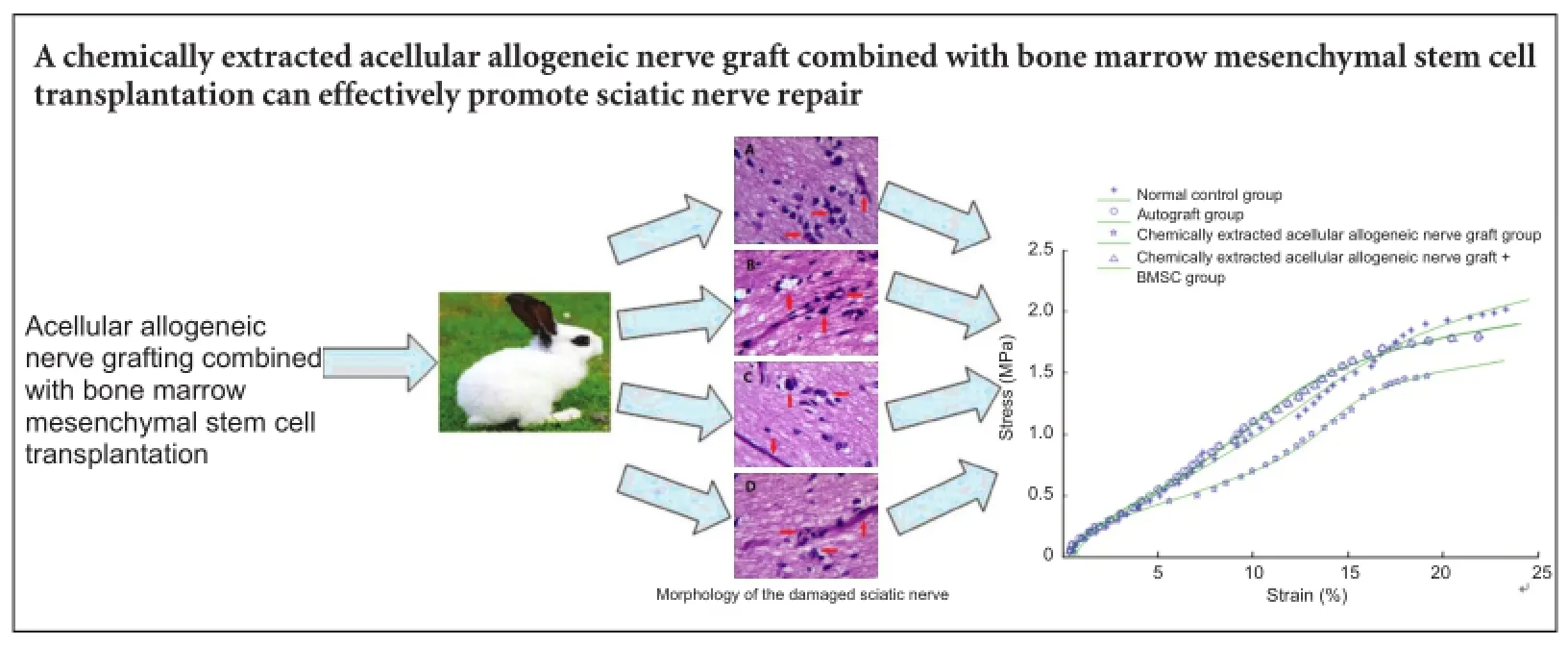
We hypothesized that a chemically extracted acellular allogeneic nerve graft used in combination with bone marrow mesenchymal stem cell transplantation would be an effective treatment for long-segment sciatic nerve defects. To test this, we established rabbit models of 30 mm sciatic nerve defects, and treated them using either an autograft or a chemically decellularized allogeneic nerve graft with or without simultaneous transplantation of bone marrow mesenchymal stem cells. We compared the tensile properties, electrophysiological function and morphology of the damaged nerve in each group. Sciatic nerves repaired by the allogeneic nerve graft combined with stem cell transplantation showed better recovery than those repaired by the acellular allogeneic nerve graft alone, and produced similar results to those observed with the autograft. These findings confirm that a chemically extracted acellular allogeneic nerve graft combined with transplantation of bone marrow mesenchymal stem cells is an effective method of repairing long-segment sciatic nerve defects.
nerve regeneration; chemically extracted acellular allogeneic nerve graft; autograft; bone marrow mesenchymal stem cells; sciatic nerve defects; biomechanics; electrophysiology; morphology; neural regeneration
Introduction
Autologous nerve transplantation is considered the gold standard for repairing peripheral nerve damage; however, because of the inevitable damage to other (relatively minor) nerve branches that occurs using this method, allografts are the preferred method used in the clinic (Yu et al., 2014). Although allografts are readily obtainable, immunological rejection is common (Jensen et al., 2005; Zhang et al., 2014a, b). Several techniques have been used to minimize rejection of grafts, including the development of acellular nerves (Zhang et al., 2015a). Zhang et al. (2012) prepared acellular allogeneic nerves by chemical processing, effectively eliminating the immunogenic components of the allograft while retaining Schwann cells, the basement membrane and the integrityof the acellular nerve structure. Zhou et al. (2015) repaired sciatic nerve defects using bone marrow mesenchymal stem cells (BMSCs) combined with grafting of tissue-engineered artificial nerves. The recovery rates of sciatic functional index, nerve conduction and wet weight of triceps muscle were markedly improved, indicating that this technique effectively promotes nerve regeneration and functional recovery. Zhao et al. (2011) demonstrated that chemically extracted acellular nerve allografts (CEANAs) with BMSCs embedded in fibrin glue successfully repaired transected sciatic nerves.
However, many studies have focused only on the mechanical properties of CEANAs, without evaluating the biomechanical properties after transplantation. We hypothesized that a CEANA would restore the mechanical properties of injured sciatic nerves and thus provide a biomechanical basis for the repair of a sciatic nerve defect.
Materials and Methods
Ethical approval
The experiment was approved by the Animal Ethics Committee of the China-Japan Union Hospital of Jilin University, China. Precautions were taken to minimize suffering and the number of animals used in each experiment.
Animals
Seventy-one clean, healthy, female Japanese rabbits, aged 5 months and weighing 2.8-3.1 kg, were provided by the Changchun High-tech Medical Animal Experimental Center, China (licence No. SCXK (Ji) 2003-0004). Rabbits were housed in individual cages at 22-24°C and relative humidity of 56-69%, with air circulation and natural lighting. Rabbits were allowed free access to food (nutritionally complete pellet feeds) and water in their home cages.
Harvesting sciatic nerves for allogeneic grafts
Of the 71 rabbits, 20 were selected at random, anesthetized with 10% chloral hydrate (3 mL/kg intraperitoneally), and secured on a surgical table in the supine position. A median incision was made along the posterior part of the left femur. The skin and subcutaneous tissue were cut to dissociate the semimembranosus and semitendinosus muscles and expose the sciatic nerves. A 30-mm segment of sciatic nerve was collected from each rat, bilaterally, at the level of the lower edge of the piriformis (40 segments in total). Specimen dimensions were measured with a reading microscope (CGH-3; Changchun Third Optical Instrument Factory, Changchun, Jilin Province, China). All samples were 30 mm in length and 1.48-1.52 mm in diameter.
Chemical decellularization of allogeneic nerve
In accordance with a previous study (Dachtler et al., 2011), sciatic nerve samples were rinsed in distilled water for 1.5 hours, gently rocked in 0.3% Triton X-100 solution for 1.5 hours, and washed three times with distilled water. Sodium deoxycholate solution (0.4%; Shanghai Mingbo Biological Technology Co., Ltd., Shanghai, China) was then added and gentle rocking continued for 1.5 hours. After three further washes with distilled water, the samples were placed in sterile phosphate-buffered saline (PBS; pH 7.4), irradiated with60Co (25 kGy) for 12 hours, and stored at 4°C.
BMSC culture
Third and fourth passage mouse BMSCs (Shanghai Yiyan Biological Technology Co., Ltd., Shanghai, China) were placed in basic medium (Shanghai Yiyan Biological Technology Co., Ltd.) containing 20% fetal bovine serum and 50 mL double monoclonal antibodies (penicillin and streptomycin, each 1 × 104U/mL), and incubated at 5% CO2, 37°C, and saturated humidity.
Preparation of animal models of sciatic nerve defect
The remaining 51 rabbits were equally and randomly allocated to three groups: autograft, CEANA, and CEANA + BMSCs (n = 17 per group). Rabbits in each group were anesthetized with 6% chloral hydrate (6 mL/kg intraperitoneally) and secured on a surgical table. A median incision was made along the posterior part of the left femur. The skin and subcutaneous tissue were cut to dissociate the semimembranosus and semitendinosus muscles and expose the sciatic nerves bilaterally. A 30-mm segment was excised from each side, 3 mm from the lower edge of the piriformis. The right sciatic nerve from all animals comprised the normal control group.
Nerve graft repair
In the autograft group, under an operating microscope (Shanghai Anxin Optical Instrument Co., Ltd., Shanghai, China), the autologous sciatic nerve was turned over and inserted back into the defect, and the epineurium sutured using four 9-0 noninvasive sutures (Qingdao Nesco Medical Co., Ltd., Qingdao, Shandong Province, China) at each end of the graft. Muscle and skin were then sutured.
The anastomotic stoma was washed with gentamicin and the incision was closed in each group. No external fixation was given after surgery. When the rabbits regained consciousness, they were placed back in individual cages with food and water freely available, and injected intraperitoneally with penicillin (1 × 104U/kg) twice a day for 7 consecutive days. The incision was disinfected with 75% ethanol once a day during this period.
Electrophysiology
从表6可以看出,HRD体系无论静失水和动失水都不大,说明进入储层的滤液少。在岩心静失水和动失水过程中,岩心可形成薄而密的滤饼,表明具有良好的造壁性。
Twenty-four weeks after surgery, electrical activity was evaluated by electromyography with a NIM-Neuro 2.0 Nerve Monitor (Medtronic, Minneapolis, MN, USA). Seventeen rabbits from each group were anesthetized with 10% chloral hydrate (400 mg/kg intraperitoneally). In the prone position, the sciatic nerve trunk was exposed bilaterally. The soleus muscle belly was punctured with concentric needleelectrodes, used as recording electrodes. An alligator clip fastened to the skin at the edge of the wound served as the ground wire. Parallel stimulating electrodes were placed at the level of sciatic nodules proximal to the anastomotic stoma and at the sciatic nerve branch distal to the anastomotic stoma to evoke two motor potentials at 50 mA. Electromyography was used to display the amplitude and latency of the action potential. The distance between two stimulating electrodes was measured with a vernier caliper (Shanghai Measuring & Cutting Tool Works Co., Ltd., Shanghai, China). Motor nerve conduction velocity (MNCV) was calculated.
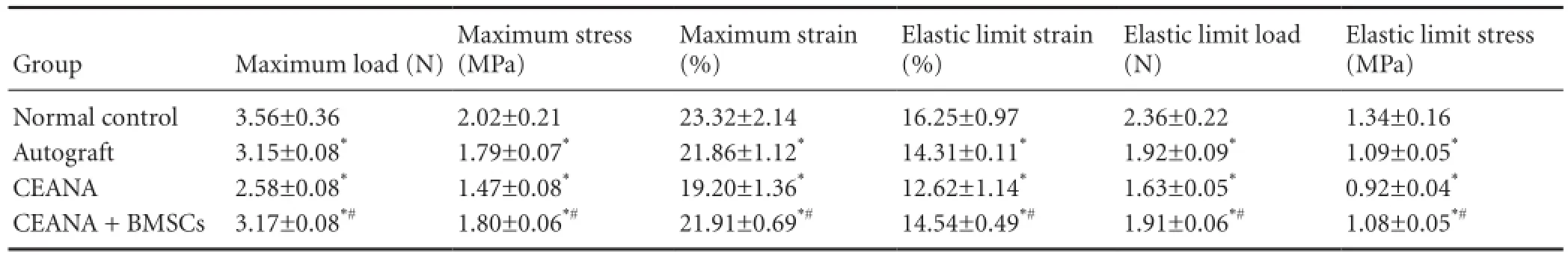
Table 2 Effects of CEANA combined with BMSC transplantation on tensile properties of sciatic nerves after long-segment damage

Figure 1 Effects of CEANA combined with BMSC transplantation on sciatic nerve morphology after long-segment damage (hematoxylin-eosin staining, × 400).
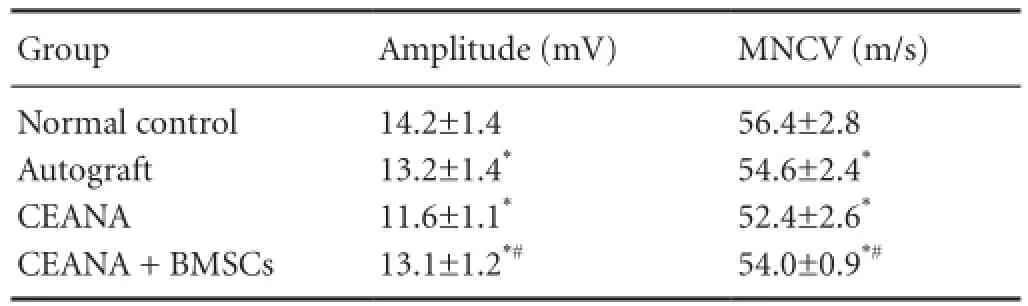
Table 1 Effects of CEANA combined with BMSC transplantation on electrophysiological function of sciatic nerve after long-segment damage
Sample collection
After electromyography, a 20 mm length of sciatic nerve was collected from each group (using the anastomotic stoma as the midpoint), and placed in a glass trough containing physiological saline. Fifteen samples from each group were used for tensile testing, and two samples from each group for microstructural observation.
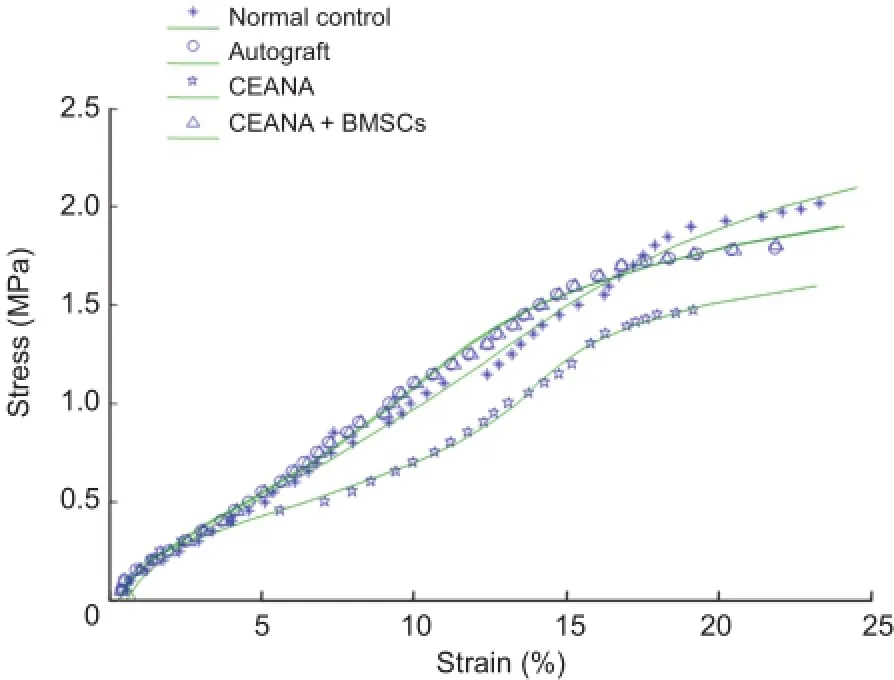
Figure 2 Tensile stress-strain curves of sciatic nerves in each group. CEANA: Chemically extracted acellular nerve allografts; BMSCs: bone marrow mesenchymal stem cells.
Hematoxylin-eosin staining
The sciatic nerve of two rabbits from each group was frozen and cut into 0.8-mm-thick sections, which were fixed in paraformaldehyde for 5 minutes, stained with hematoxylin for 2-5 minutes, treated with HCl-ethanol and then with NaOH, and counterstained with eosin for 20 seconds to 3 minutes; the sections were washed under running tap water after each step. Sections were then dehydrated through a graded alcohol series, permeabilized with xylene, mounted with neutral resin, and observed under a light microscope (Olympus, Tokyo, Japan).
Tensile testing
In accordance with previous studies (Jin et al., 2015; Wang et al., 2015; Zhang et al., 2015b), after presetting (loading and unloading were repeated 20 times in each sample),tensile testing was performed in 15 rabbits from each group with an electronic universal testing machine (MODEL55100; Changchun Testing Machine Institute, Changchun, Jilin Province, China). Samples in each group were tested at 36.5 ± 1°C and 2 mm/min. To keep the samples wet, they were sprayed with physiological saline. Tensile stressstrain curves and tensile test data were output by the machine.
Statistical analysis
Data are expressed as the mean ± SD and were analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). The differences in intergroup data were compared with one-way analysis of variance followed by Scheffe’s method. A value of P < 0.05 was considered statistically significant.
Results
Action potential amplitude and MNCV were not significantly different between the autograft and CEANA + BMSCs groups (P > 0.05), but were higher in both of these groups than in the CEANA group (P < 0.05; Table 1).
CEANA combined with BMSC transplantation improved morphological recovery from long-segment sciatic nerve defect
Hematoxylin-eosin staining revealed distinct axons surrounded by myelin sheath, and regularly distributed nerve fibers, in the normal control group (Figure 1A). The CEANA + BMSCs group showed good myelinization and regular nerve fibers (Figure 1B). In the CEANA group, most sciatic nerve fibers were regularly arranged, although a few were not (Figure 1C). In the autograft group, nerve fibers were also regularly distributed, and a large amount of myelin sheath was seen in the distal stump of the injured nerve (Figure 1D).
CEANA combined with BMSC transplantation improved the tensile properties of sciatic nerve after injury
Tensile testing demonstrated that the elastic limit load, stress and strain, and maximum load, stress and strain, in the CEANA + BMSCs group were higher than in the CEANA group (P < 0.05), but not significantly different from the autograft group (P > 0.05; Table 2).
Stress-strain curves and functions for stress-strain relationship
Stress-strain curves for sciatic nerve samples in each group were drawn (Figure 2), and curve fitting was conducted on tensile testing data. Stress-strain curves showed exponential changes when sciatic nerve strain increased from 0 to 7.06%, 6.32%, 5.11% and 6.41% in the normal control, CEANA, CEANA + BMSCs, and autograft groups, respectively. A linear stress-strain relationship was observed when sciatic nerve strain increased from 7.07% to 14.86%, from 6.33% to 13.01.%, from 5.12% to 11.31%, and from 6.42% to 13.32% in the normal control, CEANA, CEANA + BMSCs and autograft groups, respectively. When sciatic nerve strain increased from 14.87% to 23.23%, from 13.02% to 21.9%, from 11.32% to 19.20%, and from 13.33% to 21.86% in the normal control, CEANA, CEANA + BMSCs, and autograft groups, respectively, samples showed marked deformation, near loss of bearing capacity, and damage.
Functions for sciatic nerve stress-strain relationships (σ(ε)) were constructed by linear regression analysis in each group, as follows: normal control group, σ(ε) = 0.0988e5+ 0.7307e4+ 2.6155e3- 0.1431e2; CEANA + BMSCs group, σ(ε) = 0.0745e5+ 0.5970e4+ 2.4617e3- 0.0907e2; autograft group, σ(ε) = 0.1739e5+ 1.344e4+ 0.7573e3+ 0.6122e2; CEANA group, σ(ε) = 0.09324e5+ 0.8136e4+ 0.5798e3+ 0.2637e2.
Discussion
The biomechanical properties of peripheral nerves are maintained by surrounding connective tissue, of which collagen fiber is the main component. Collagen is tough, with high tensile strength, and can withstand a certain amount of mechanical stimulation. Its quantity and distribution determine the biomechanical properties of peripheral nerves (Eather et al., 1986). Chemically extracted acellular allogeneic nerve is a new tissue-engineered material with low immunogenicity and a three-dimensional structure (Sondell et al., 1998; Hudson et al., 2004). CEANAs make use of this material to guide Schwann cell migration and promote axonal regeneration, and are a promising substitute for autologous nerve transplantation. Borschel et al. (2003) confirmed that nerve decellularization processes may remove one or more collagen components, leading to changes in the mechanical properties of the nerve. He et al. (2009) found that tissue engineered nerves constructed with BMSCs had better reparative effects in 10 mm sciatic nerve defects than did CEANAs. BMSCs can differentiate into neural cells, replace apoptotic nerve cells, secrete neurotrophic factors, and promote axonal regeneration. BMSCs can also regulate Schwann cells and promote peripheral nerve regeneration, and are ideal seed cells (Lin et al., 2008; Wang et al., 2009; Zheng et al., 2010).
Greater CMAP and MNCV are associated with better recovery of tissue morphology and stronger nerves after repair. In addition, the injury mechanism and functional recovery of the sciatic nerve were strongly associated with its mechanical properties. The present findings indicate that CEANA combined with BMSC transplantation markedly improved sciatic nerve recovery compared with CEANA alone, to a degree similar to that after an autograft. CEANA combined with BMSC transplantation is a promising treatment for the repair of peripheral nerve damage in the clinic. Further research to improve the method and its introduction in the clinic will identify additional applications for this technique.
The present results demonstrate that CEANA used in combination with BMSC transplantation for the repair of sciatic nerve defects restores damaged collagen and improves the biomechanical properties of the sciatic nerve. Furthermore, CEANA combined with BMSC transplantation enhanced the electrophysiological properties of the sciatic nerve after injury. We also calculated the stress-strain function in thedamaged nerves using regression analysis. Experimental data were evaluated using mathematical and statistical models, to better understand the mechanical properties of the repaired nerve.
Because of individual differences and the limited number of experimental animals, there is a large amount of dispersion among the experimental data. However, the present data provide a valuable reference for further investigation into the treatment of sciatic nerve injury.
Author contributions: YJL and ZGQ conceived and designed the study. HZL provided the data. BLZ and ZGQ ensured the integrity of the data and analyzed data. YJL wrote the paper and served as a principle investigator. ZGQ was in charge of manuscript authorization. BLZ undertook the statistical analysis. ML obtained funding. ML and BLZ provided technical or data support. All authors performed the experiments, and approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Borschel GH, Kia KF, Kuzon WM Jr, Dennis RG (2003) Mechanical properties of acellular peripheral nerve. J Surg Res 114:133-139.
Dachtler J, Hardingham NR, Glazewski S, Wright NF, Blain EJ, Fox K (2011) Experience-dependent plasticity acts via GluR1 and a novel αNOS1 dependent synaptic mechanism in adult cortex. J Neurosci 31:11220-11230.
Eather TF, Pollock M, Myers DB (1986) Proximal and distal changes in collagen content of peripheral nerve that follow transection and crush lesions. Exp Neurol 92:299-310.
He HY, Deng YH, Tong XJ, Cheng JM, Du ZK (2009) Repair of sciatic nerve defects with tissue engineered nerves constructed with marrow stromal cells. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu 13:5662-5566.
Hudson TW, Liu SY, Schmidt CE (2004) Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng 10:1346-1358.
Jensen JN, Brenner MJ, Tung TH, Hunter DA, Mackinnon SE (2005) Effect of FK506 on peripheral nerve regeneration through long grafts in inbred swine. Ann Plast Surg 54:420-427.
Jin H, Yang Q, Ji F, Zhang YJ, Zhao Y, Luo M (2015) Human amniotic epithelial cell transplantation for the repair of injured brachial plexus nerve: evaluation of nerve viscoelastic properties. Neural Regen Res 10:260-265.
Lin W, Chen X, Wang X, Liu J, Gu X (2008) Adult rat bone marrow stromal cells differentiate into Schwann cell-like cells in vitro. In Vitro Cell Dev Biol Anim 44:31-40.
Ma XL, Yang ZB, Li XL, Ma JX, Zhang Y, Guo HG, Sun XL (2010) A study on biomechanical properties of chemically extracted acellular peripheral nerve. Zhongguo Xiufu Chongjian Waike Zazhi 24:1293-1297.
Sondell M, Lundborg G, Kanje M (1998) Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res 795:44-54.
Wang J, Ding F, Gu Y, Liu J, Gu X (2009) Bone marrow mesenchymal stem cells promote cell proliferation and neurotrophic function of Schwann cells in vitro and in vivo. Brain Res 1262:7-15.
Wang Y, Li ZW, Luo M, Li YJ, Zhang KQ (2015) Biological conduits combining bone marrow mesenchymal stem cells and extracellular matrix to treat long-segment sciatic nerve defects. Neural Regen Res 10:965-971.
Yang Z, Ma XL, Li XL, Ma JX, Zhang Y, Guo HG, Sun XL (2011) Effects of different acellular methods on biomechanical properties of peripheral nerve. Zhongguo Shengwu Yixue Gongcheng Xuebao 30:155-159.
Yu GM, Wang W, Zhang L, Zhang DL (2014) Repairing sciatic nerve in rats by acellular allogeneic nerve transplantation treated with chondroitinase ABC-PLGA microspheres. Jiefangjun Yixueyuan Xuebao 35:858-862.
Zhang Y, Zhang H, Katiella K, Huang W (2014a) Chemically extracted acellular allogeneic nerve graft combined with ciliary neurotrophic factor promotes sciatic nerve repair. Neural Regen Res 9:1358-1364. Zhang Y, Zhang H, Zhang G, Ka K, Huang W (2014b) Combining acellular nerve allografts with brain-derived neurotrophic factor transfected bone marrow mesenchymal stem cells restores sciatic nerve injury better than either intervention alone. Neural Regen Res 9:1814-1819.
Zhang YR, Yao JN, Zhou MW, Li YB, Wang YS (2012) Human placenta amniotic membrane wrap chemically extracted acellular nerve allograft repair of canine nerves. Zhengzhou Daxue Xuebao: Yixue Ban 47:509-511.
Zhang YR, Ka K, Zhang GC, Zhang H, Shang Y, Zhao GQ, Huang WH (2015a) Repair of peripheral nerve defects with chemically extracted acellular nerve allografts loaded with neurotrophic factors-transfected bone marrow mesenchymal stem cells. Neural Regen Res 10:1498-1506.
Zhang ZJ, Li YJ, Liu XG, Huang FX, Liu TJ, Jiang DM, Lv XM, Luo M (2015b) Human umbilical cord blood stem cells and brain-derived neurotrophic factor for optic nerve injury: a biomechanical evaluation. Neural Regen Res 10:1134-1138.
Zhao Z, Wang Y, Peng J, Zhao B, Zhao Q, Liu Y, Ren ZW, Zhan SF, Zhang L, Xu WJ, Lu SB (2011) Effect of chemical extracted acellular nerve allograft supplementing with bone marrow mesenchymal stem cells embedded in fibrin glue on functional recovery of transected sciatic nerves. Zhongguo Xiufu Chongjian Waike Zazhi 25:488-493.
Zheng W, Honmou O, Miyata K, Harada K, Suzuki J, Liu H, Houkin K, Hamada H, Kocsis JD (2010) Therapeutic benefits of human mesenchymal stem cells derived from bone marrow after global cerebral ischemia. Brain Res 1310:8-16.
Zhou LN, Cui XJ, Zu KX, Wang XH, Cai XY, Guo JH, Na QQ (2015) Repairment of 1 cm sciatic nerve defect by bone marrow mesenchymal stem cell of adult rat combined with tissue-engineered artificial nerve. Qiguan Yizhi 6:157-160.
Copyedited by Slone-Murphy J, Haase R, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.189198
The CEANA group an acellular graft, which was also turned over and sutured. In the CEANA + BMSCs group, fifth passage mouse BMSCs (1 mL; approximately 5 × 105) were infused into the CEANA conduit, and the sciatic nerve tissue was turned over and sutured.
*Correspondence to:
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Secondary parkinsonism induced by hydrocephalus after subarachnoid and intraventricular hemorrhage
- Prospects for bone marrow cell therapy in amyotrophic lateral sclerosis: how far are we from a clinical treatment?
- Uncoupling protein 2 in the glial response to stress: implications for neuroprotection
- Selective neuronal PTEN deletion: can we take the brakes off of growth without losing control?
- TRPV1 may increase the effectiveness of estrogen therapy on neuroprotection and neuroregeneration
- Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery
