Differing roles for parvalbumin neurons after nerve injury
2016-12-01PeterJ.Shortland,DavidA.Mahns
Differing roles for parvalbumin neurons after nerve injury
Neuropathic pain is abnormal, persistent pain that is caused by injury to the somatosensory system. It commonly involves damage to the peripheral nerves as a result of physical trauma, metabolic diseases such as diabetes, infections such as HIV or shingles, or toxicity induced neuropathies as a result of cancer treatments. Neuropathic pain is common, with epidemiological studies suggesting that around 18-30% of American, European and Australian adults suffer from chronic pain. This leads to significant disabilities and suffering. People with chronic pain complain of spontaneous pains that may be electric-shock like or continuous. Often coexisting with these are abnormal, evoked sensations such as tactile allodynia (pain in response to innocuous stimuli e.g., light brush or pressure) and cold allodynia (pain in response to innocuous cooling), or hyperalgesia (increase pain in response to an already noxious stimulus), as well as ataxia and proprioceptive deficits. The management of these conditions is often suboptimal for two main reasons: firstly, an incomplete knowledge of the mechanisms that are involved (and which of them are the most important). Secondly, patients often have compliance issues with pharmacological medications (Attal, 2012). Both peripheral and central neuronal mechanisms contribute to the pathophysiology and since the turn of the century particular emphasis has focussed on the role of glial cells in pain (West et al., 2015).
Primary sensory afferents convey information about peripheral stimuli to the central nervous system (CNS). Their cell bodies are located in dorsal root ganglia (DRGs) and constitute a heterogeneous population that has been classified in several ways. Initially, they were split into the small dark and large light groups based on neurofilament (and myelination) content that correlated well with conduction velocities. With the advent of immunohistochemistry, and the discovery of different trophic factors, these groups were further subdivided into peptide-rich and peptide-poor nociceptors, low threshold mechanoreceptor and proprioceptor subgroups (Scott, 1992). Moreover, it is now known that different DRG neurons can express in > 30 different neurotransmitters or receptors associated with a wide variety of stimuli and that they show considerable phenotypic plasticity associated with peripheral injury (Fukuoka and Noguchi, 2002; West et al., 2015). Most of the attention has focussed on the changes associated with small DRG cells that primarily innervate skin, since these are presumed to be responsible for evoked pain sensations after injury. However, less attention has been paid to the effects of injury on proprioceptors in general, and muscle afferents in particular. This is, in part, due to the paucity of histological markers associated with such fibres.
The most commonly used marker of proprioceptive (muscle) afferents is parvalbumin (PV) (Medici and Shortland, 2015). PV is a calcium binding protein that is found in subsets of sensory neurons, inhibitory interneurons and motoneurons. Studies that have explored the effects of nerve injury on muscle afferents reported no changes in its staining patterns after injury. However, those early studies used nerve injury models where injured and non-injured cell bodies coexist within the same DRG. Therefore, plasticity could occur without changing the absolute number of positive cells (downregulation in injured and upregulation in spared neurons). Now there are neuronal markers, such as activating transcription factor 3 (ATF3), which can identify damaged afferents and nerve injury models where the injured and non-injured cell bodies are spatially segregated in adjacent spinal ganglia (Shortland et al., 2006). This prompted us to reinvestigate whether or not PV positive cells show phenotypic plasticity (Medici and Shortland, 2015).
We first determined that around 25% of L4-5DRG cells innervating the sciatic nerve express PV. Following axotomy of the sciatic nerve, this number did not significantly change over 1-28 days, confirming earlier studies. Co-expression with the neuronal injury marker ATF3 showed that approximately half the PV cells were axotomised suggesting that both injured and uninjured neurons express PV after sciatic nerve injury. However, around 50-60% of the L4& L5DRG neurons contribute axons to the sciatic nerve, so by itself this result did not really answer whether or not phenotypic plasticity was happening, since for switching to occur, one might predict that little or no colocalisation with ATF3 should occur. Therefore, we switched to the spinal nerve ligation model and selectively lesioned the L5spinal nerve, whilst being careful not to injure the adjacent L4spinal nerve. In this model, essentially all DRG neurons were injured and expressed ATF3 (Shortland et al., 2006).
The percentage of PV staining in the axotomized L5DRG was unchanged compared to the adjacent L4DRG, the contralateral L5DRG and naïve L5DRG. On the other hand, calcitonin gene related peptide (CGRP) staining, which is an accepted marker of peptidergic nociceptor afferents, including muscle afferents, was completely abolished in the ipsilateral L5DRG. Thus, these results unequivocally showed that nerve injury did not produce a phenotypic change in PV positive afferents. They also highlighted a clear difference between phenotypic plasticity in proprioceptors versus nociceptors in response to nerve injury.
There is now increasing knowledge of the molecular and biochemical repertoire of proprioceptors. Proprioceptors contain several calcium binding proteins such as calcineurin, calretinin and calbindin. They also contain metabolic markers such as carbonic anhydrase and cytochrome oxidase and the enzyme α3Na+/K+ATPase. Several ion channels are associated with proprioceptors such as Piezo 2, acid sensing ion channel-3 (ASIC3), transient receptor potential V2 (TRPV2), vesicular glutamate transporter-1 (vglut1) and other markers such as the PDZ scaffold protein whirlin. Piezo2, α3Na+/ K+ATPase and whirlin are specific markers for muscle spindles. Following nerve injury, muscle afferents become spontaneously active and can change their neurotransmitter content. They can upregulate galanin, neuropeptide tyrosine (NPY) and brain derived neurotrophic factor (BDNF), but many of the markers do not change e.g., TRPV2, ASIC3, carbonic anhydrase, whilst the response to others such as Piezo 2 or whirlin are unknown. A key player in contributing to chronic pain appears to be the de novo expression of BDNF in muscle afferents (Michael et al., 1999; Zhou et al., 2010). Recent evidence also implicates changes in the sodium channel Nav1.7 in proprioceptors as an important contributor to chronic pain (Fukuoka et al., 2015), as this is increased in muscle afferents after nerve injury. Yet the afferents retain their normal levels of PV. PV is important for regulating calcium homeostasis in a neuron. After nerve injury, muscle afferents have a reduced and redistributed presynaptic input onto homonymous motoneurons. Before injury, synapses were on the proximal dendrites, whilst after injury they were fewer in number and located further away from the soma. This explained the observations of weak excitatory post synaptic potentials to motoneurons and disappearance of stretch reflexes (Vincent et al., 2015). PV is found in the cell body and peripheral endings, but not in the central terminals of afferents in the spinal cord (Petitjean et al., 2015), suggesting PV is more important in physiological process such as speed of muscle contraction rather than neurotransmitter release. Nerve injury causes a selective loss of small, but not large-sized, DRG neurons and so PV may have a role in cell survival. Support for this idea comes from studies in motoneuron disease models, where PV is expressed in motoneuron groups that are resistant to amyotrophic lateral sclerosis (ALS) and over-expression studies where PV prevents excitotoxic cell death (Medici and Shortland, 2015).
Although PV is not present in the central terminals of afferents in the spinal cord, PV staining is clearly present in the dorsal horn. This is associated with the cell bodies and dendrites of a subset of inhibitory interneurons located in lamina IIi-III. Recent pharmacogenetic and selective ablation studies have shown that these PV neurons play a key gate keeping role in mechanical allodynia associated with nerve injury (Petitjean et al., 2015). These neurons receive input from low threshold cutaneous afferents and in turn provide presynaptic inhibition of A-fibres as well as postsynapticinhibition to PKCγ excitatory interneurons. Nerve injury induces a 50% loss of synaptic appositions between PV interneurons and PKCγ interneurons resulting in disinhibition of pain circuits, without causing any death of PV neurons. Moreover, selective pharmacological blockade of PKCγ neurons attenuated the injury-induced mechanical allodynia (Petitjean et al., 2015). Two other circuits have also been proposed for regulating nociceptive transmission: one involves the inhibition of somatostatin (SOM)-positive excitatory interneurons by dynorphin (Dyn)-positive inhibitory interneurons. The second involves vesicular glutamate transport 3 (vGlut3)-positive excitatory interneurons which regulate activity in PKCγ or calretinin-positive excitatory interneurons (Gangadharan and Kuner, 2015). Under normal conditions, the vGlut3 interneurons are under powerful inhibitory control but after nerve injury they can drive mechanical allodynia (Arcourt and Lechner, 2015; Gangadharan and Kuner, 2015). Future experiments should determine whether PV interneurons also gate connections with these two neuronal subpopulations.
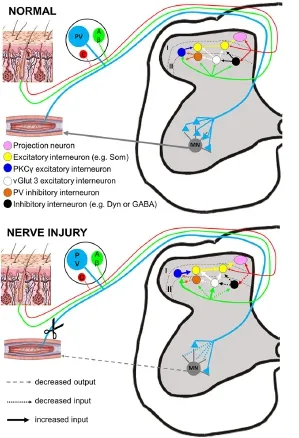
Figure 1 Overview of the different roles of PV neurons in pain circuits of the spinal cord.
A final layer of complexity is added by the presence of another DRG subpopulation that can cause allodynia after nerve injury. C-low threshold mechanoreceptors (CLTMs) are attributed to conveying sensations of pleasant touch in normal circumstances but produce allodynia after injury (Nagi et al., 2011; Samour et al., 2015). These fibres have a unique molecular repertoire and terminate in lamina IIi of the cord onto central, stalked and vertical cells (Arcourt and Lechner, 2015). Since PV cells have these morphologies (Hughes et al., 2012) they are ideally placed to receive input from such afferents, although this speculation requires confirmation.
Taken together, the results of our and other studies suggest that PV is an important protein related to sensory pathways and the plasticity that occurs after injury (Figure 1). We demonstrated that in primary afferents, PV did not change and so can still be used as an anatomical marker of proprioceptors to explore changes in other transmitters, ion channels or transcription factors after peripheral nerve injury. In the CNS, PV may confer preferential survival to excitotoxic insults and importantly gates abnormal sensory information after injury.
Peter J. Shortland*, David A. Mahns
School of Science & Health, School of Medicine, Western Sydney
University, Campbelltown, NSW, Australia (Shortland PJ)
三是加强社会治理创新,广泛动员社会力量参与社区矫正,切实“打造共建共治共享的社会治理格局”。加强区域执法协作,按照京津冀社区矫正执法协作启动仪式上所提出的《关于社区矫正区域执法协作工作的指导意见》,深入推进京津冀三地区域协作,实现更全面地信息互通、资源共享,维护京津冀三地社会和谐稳定,更好地服务于京津冀一体化建设。同时,继续坚持专群相结合的工作模式,明确不同类型工作人员在社区矫正心理矫治工作中的定位和职责。
School of Medicine, Western Sydney University, Campbelltown, NSW, Australia (Mahns DA)
*Correspondence to: Peter J. Shortland, Ph.D.,
p.shortland@westernsydney.edu.au.
Accepted: 2016-07-15
orcid: 0000-0002-7598-9744 (Peter J. Shortland)
How to cite this article: Shortland PJ, Mahns DA (2016) Differing roles for parvalbumin neurons after nerve injury. Neural Regen Res 11(8):1241-1242.
References
Arcourt A, Lechner SG (2015) Peripheral and spinal circuits involved in mechanical allodynia. Pain 156:220-221.
Fukuoka T, Noguchi K (2002) Contribution of spared primary afferent neurons to the pathomechanisms of neuropathic pain. Mol Neurobiol 26:57-67.
Fukuoka T, Miyoshi K, Noguchi K (2015) De novo expression of Nav1.7 in injured putative proprioceptive afferents: multiple tetrodotoxin-sensitive sodium channels are retained in the rat dorsal root after spinal nerve ligation. Neuroscience 284:693-706.
Gangadharan V, Kuner R (2015) Unravelling spinal circuits of pain and mechanical allodynia. Neuron 87:673-675.
Hughes DI, Sikander S, Kinnon CM, Boyle KA, Watanabe M, Callister RJ, Graham BA (2012) Morphological, neurochemical and electrophysiological features of parvalbumin-expressing cells: a likely source of axo-axonic inputs in the mouse spinal dorsal horn. J Physiol (Lond) 590: 3927-3951.
Medici T, Shortland PJ (2015) Effects of peripheral nerve injury on parvalbumin expression in adult rat dorsal root ganglion neurons. BMC Neurosci 16:93 DOI: 10.1186/s12868-015-0232-9
Michael GJ, Averill S, Shortland PJ, Yan Q, Priestley JV (1999) Axotomy results in major changes in BDNF expression by dorsal root ganglion cells: BDNF expression in large trkB and trkC cells, in pericellular baskets, and in projections to deep dorsal horn and dorsal column nuclei. Eur J Neurosci 11:3539-3551.
Nagi SS, Rubin TK, Chelvanayagam DK, Macefield VG, Mahns DA (2011) Allodynia mediated by C-tactile afferents in human hairy skin. J Physiol (Lond) 589:4065-4075.
Petitjean H, Pawloski SA, Fraine SL, Hamad D, Fatima T, Berg, J, Brown CM, Jan LY Ribeiro-da-Silva A, Braz JM, Basbaum AI, Sharif-Naeini R (2015) Dorsal horn parvalbumin neurons are gate-keepers of touch evoked pain after nerve injury. Cell Rep 13:1246-1257.
Samour MS, Nagi SS, Mahns DA (2015) Cav3.2-expressing low-threshold C fibres in human hairy skin contribute to cold allodynia--a non-TRPV1- and non-TRPM8-dependent phenomenon. Pain 156:1566-1578.
Scott SA (1992) Sensory neurons: diversity, development and plasticity. Oxford University Press Chapters 2-4.
Shortland PJ, Baytug B, Krzyzanowska A, McMahon SB, Priestley JV, Averill S (2006) Injury to L4 dorsal root ganglion cells after L5 spinal nerve transection. Eur J Neurosci 23:365-373.
Vincent JA, Nardelli P, Gabriel HM, Deardorf AS, Cope TC (2015) Complex impairment of IA muscle proprioceptors following traumatic or neurotoxic injuries. J Anat 227:221-230.
West SJ, Bannister K, Dickenson AH, Bennett DL (2015) Circuitry and plasticity of the dorsal horn - toward a better understanding of neuropathic pain. Neuroscience 300:254-275.
10.4103/1673-5374.189179
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Secondary parkinsonism induced by hydrocephalus after subarachnoid and intraventricular hemorrhage
- Prospects for bone marrow cell therapy in amyotrophic lateral sclerosis: how far are we from a clinical treatment?
- Uncoupling protein 2 in the glial response to stress: implications for neuroprotection
- Selective neuronal PTEN deletion: can we take the brakes off of growth without losing control?
- TRPV1 may increase the effectiveness of estrogen therapy on neuroprotection and neuroregeneration
- Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery
